Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Sample Collection
2.3. Serum Bio-Parameter Analysis
2.4. Tissue Histology
2.4.1. Hematoxylin–Eosin Staining
2.4.2. Oil Red O Staining
2.5. Real-Time PCR
2.6. Western Blotting
2.7. Cell Culture
2.8. Statistical Analysis
3. Results
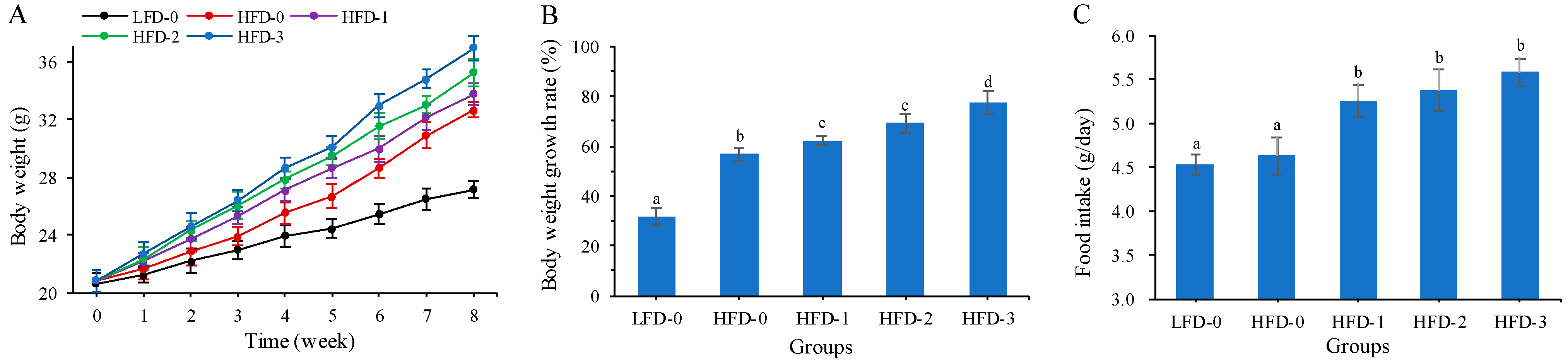
3.1. Body Weight and Food Intake of Mice
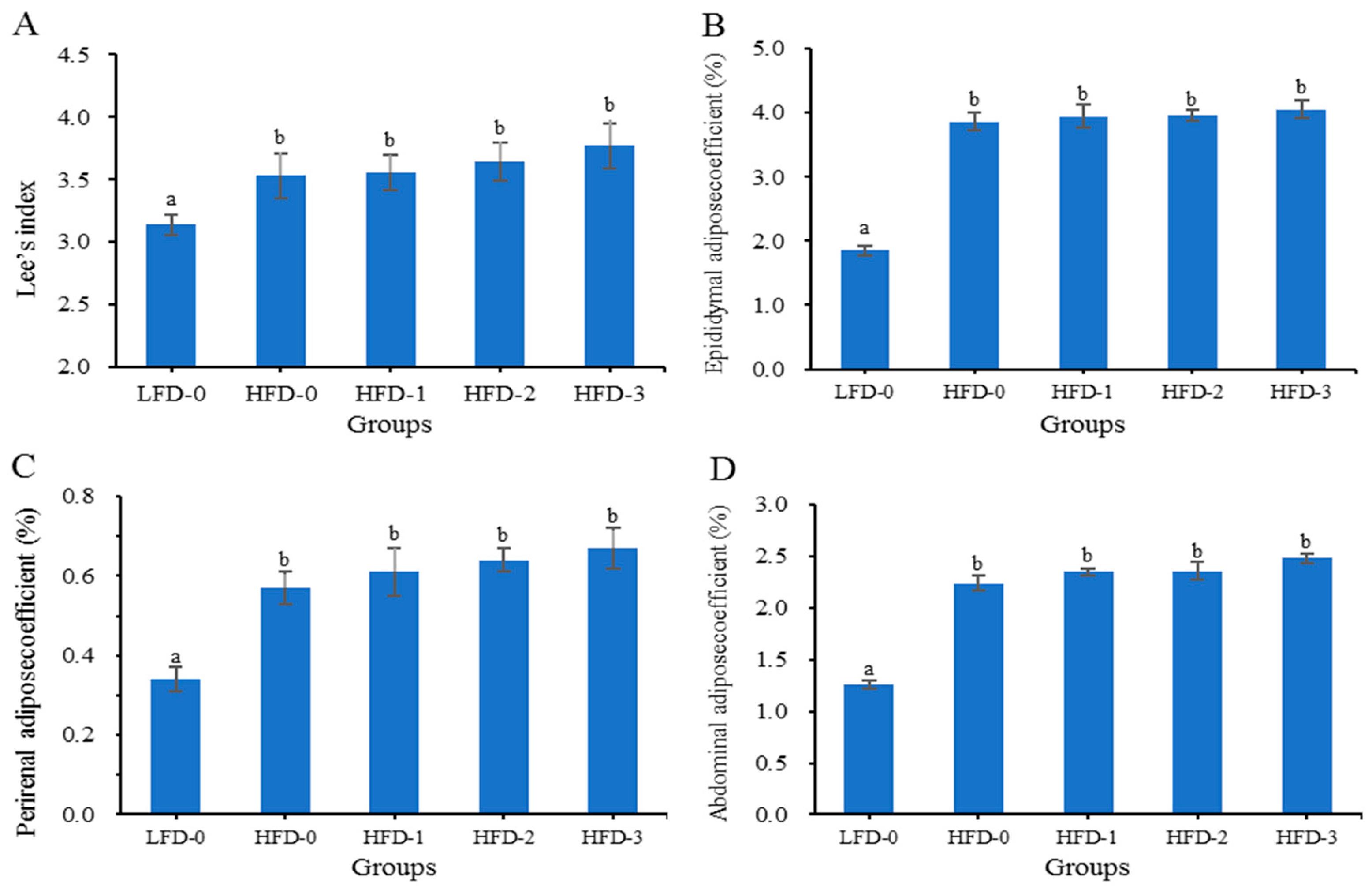
3.2. Lee’s Index and Adipose Tissue Coefficient of Mice
3.3. Organ Index of Mice
3.4. Serum Bio-Parameter Analysis
3.4.1. Analysis of Blood Lipid Level
3.4.2. Analysis of Liver Function Indicator Levels
3.4.3. Analysis of Inflammatory Cytokine Levels
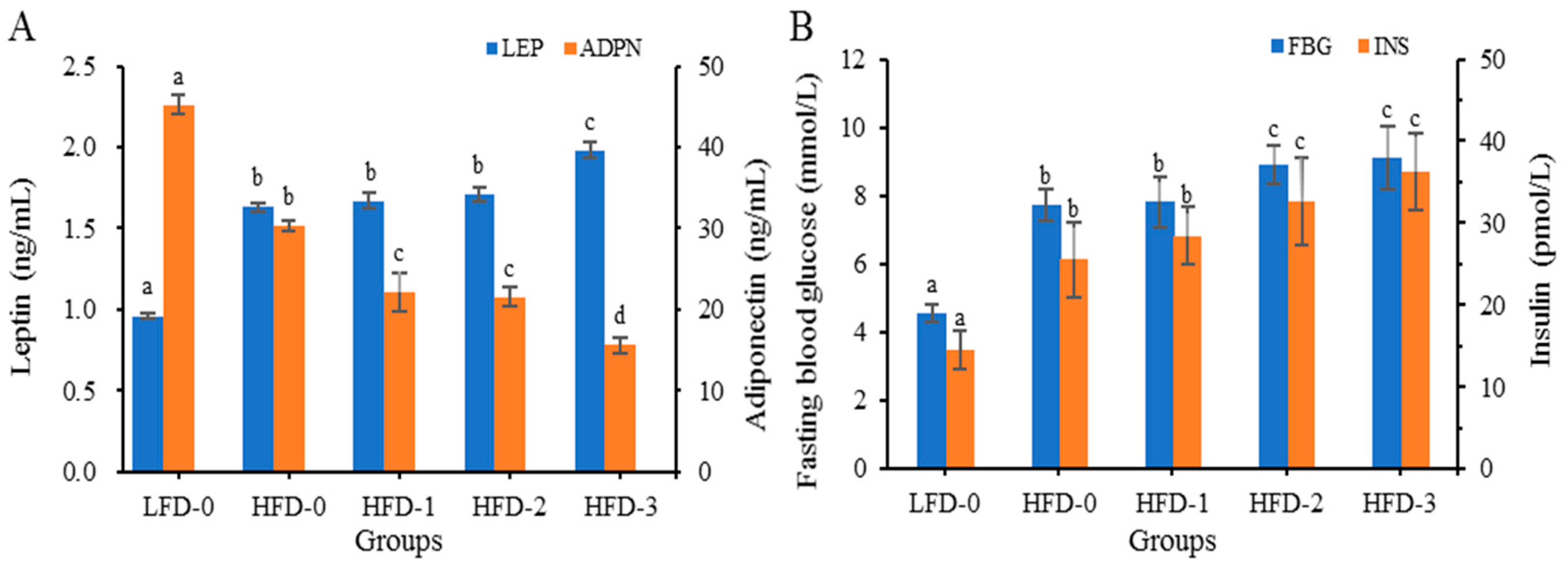
3.4.4. Analysis of Adipose Cytokine Levels
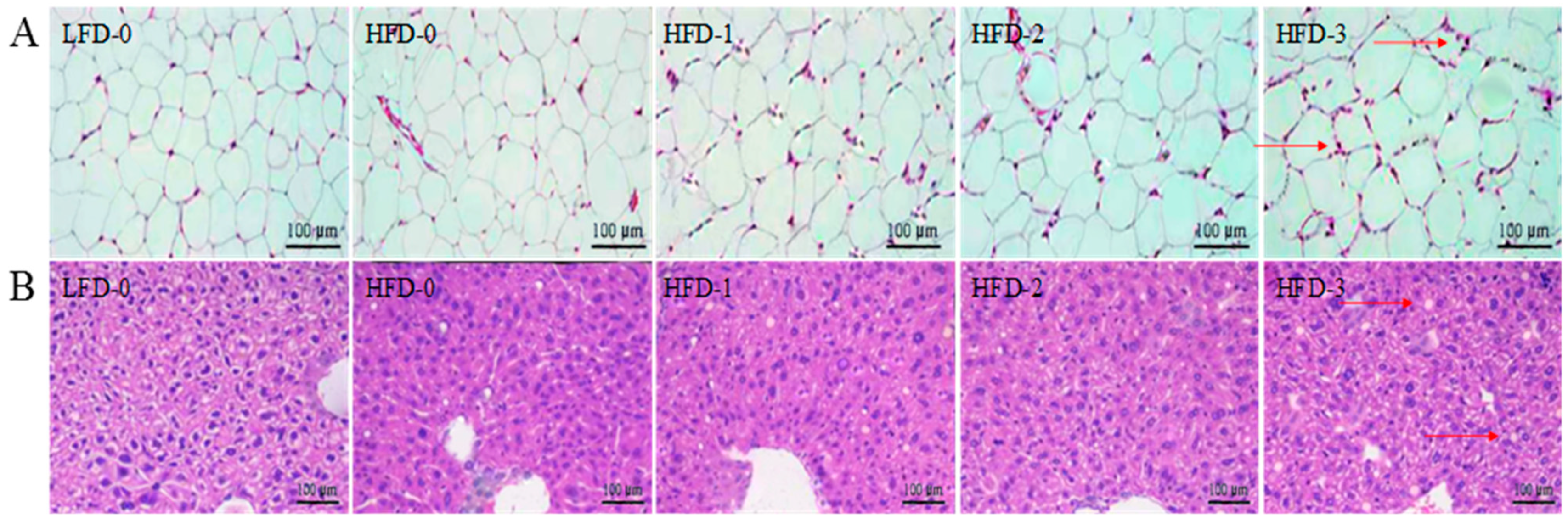
3.5. Analysis of Tissue Histology
3.6. Analysis of Relative mRNA Expression Levels of Lipid Metabolism-Related Genes in Adipose Tissue
3.7. Analysis of Protein Expression Levels of Lipid Metabolism-Related Genes in Adipose Tissue
3.8. Effect of Acetate on the Differentiation of Mouse Adipose Mesenchymal Stem Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, W.; Zhang, J.; Zhou, H.; Zhang, A.; Wang, Y.; Tian, X.; Wen, D.; Wang, Y. Obesity intervention efforts in China and the 2022 World Obesity Day. Glob. Health J. 2022, 6, 118–121. [Google Scholar] [CrossRef]
- Hu, G. More vigorous efforts are needed to fight obesity, a serious public health problem in China. Obesity 2021, 29, 1580–1581. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Mack, A.; Gibson, G.R. Review article: Emulsifiers in the food supply and implications for gastrointestinal disease. Aliment. Pharmacol. Ther. 2019, 49, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.P.; Singh, A.K.; Jha, R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim. Nutr. 2019, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Yu, X.; Liu, Y.; Sun, L.; Cai, K. The Baseline Gut Microbiota Directs Dieting-Induced Weight Loss Trajectories. Gastroenterology 2021, 160, 2029–2042. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Li, S.; Yi, X.; Shao, S.; Yu, W.; Li, E. Biological factors controlling starch digestibility in human digestive system. Food Sci. Hum. Wellness 2023, 12, 351–358. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91–106. [Google Scholar] [CrossRef]
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. J. Lab. Clin. Med. 2013, 161, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Fan, X.Q.; Lu, Y.Y.; Fan, C.N.; Li, P.; Qi, K.M. Acetate Can Treat Obesity by Enhancing Fatty Acid β-oxidation in Mice. Food Nutr. China 2017, 23, 69–72. [Google Scholar]
- Li, Q.; Jing, S.S.; Zhu, D. Weight reducing effect of ge lan decoction on nutritional obesity mice. J. Guangxi Med. Univ. 2014, 31, 909–911. [Google Scholar]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef]
- Notarte, K.I.R.; Quimque, M.T.J.; Macaranas, I.T.; Khan, A.; Pastrana, A.M.; Villaflores, O.B.; Arturo, H.C.P.; Iv, D.Y.H.P.; Tan, S.M.M.; Wei, D.Q. Attenuation of Lipopolysaccharide-Induced Inflammatory Responses through Inhibition of the NF-κB Pathway and the Increased NRF2 Level by a Flavonol-Enriched n-Butanol Fraction from Uvaria alba. ACS Omega 2023, 8, 5377–5392. [Google Scholar] [CrossRef]
- Winkler, S.; Hempel, M.; Brückner, S.; Mallek, F.; Weise, A.; Liehr, T.; Tautenhahn, H.M.; Bartels, M.; Christ, B. Mouse white adipose tissue-derived mesenchymal stem cells gain pericentral and periportal hepatocyte features after differentiation invitro, which are preserved invivo after hepatic transplantation. Acta Physiol. 2015, 215, 89–104. [Google Scholar] [CrossRef]
- Kuang, Y.; Zheng, X.; Zhang, L.; Ai, X.; Doeppner, T.R. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J. Extracell. Vesicles 2020, 10, e12024. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Ge, S.J. Establishment of high-fat diet-induced obesity and sleeve gastrectomy in C57BL/6J mice. Fudan Univ. J. Ofmedical Sci. 2022, 49, 88–93. [Google Scholar]
- Chen, R.H.; Chen, C.; Shen, H.Z. Effect of water quality on obesity-related indicators in mice. Chin. J. Public Health 2014, 30, 454–456. [Google Scholar]
- Cui, J.; Wang, R.; Gao, S.; Chen, L.; Xu, Y.; Liu, X. Effects of Aerobic Exercise on Lipid Metabolism-related Enzymes and Neuropeptide Yin High-fat Diet Mice. J. Xinxiang Univ. 2022, 39, 26–31. [Google Scholar]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Eduard, W.J.; Marcus, C.; Levin, E.; Nieuwdorp, M. Recent insights into the role of microbiome in the pathogenesis of obesity. Ther. Adv. Gastroenterol. 2022, 15. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, Y.; Yang, Y. The Clinical Significance of Determining Blood Lipid,Serum cTnI and Hcy Levels in Critically ill Patients in the Diagnosis. J. Radioimmunol. 2013, 26, 53–56. [Google Scholar]
- Yi, L.; Rui, H. The blood lipids and its clinical significance. Clin. Focus 2018, 33, 461–464. [Google Scholar]
- Hua, S.; Qi, Q.; Kizer, J.R.; Guyen, J.W.; Isasi, C.R. Association of liver enzymes with incident diabetes in US Hispanic/Latino adults. Diabet. Med. 2021, 38, e14522. [Google Scholar] [CrossRef] [PubMed]
- El-Wakkad, A.; Hassan, E.M.; Sibaii, H.; El-Zayat, S.R. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013, 61, 682–687. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Zhang, W.; Li, J.; Zhu, T. Thrombin induces pro-inflammatory and anti-inflammatory cytokines secretion from human mast cell line (HMC-1) via protease-activated receptors. Mol. Immunol. 2022, 141, 60–69. [Google Scholar]
- Słabuszewska-Jóźwiak, A.; Lukaszuk, A.; Janicka-Kośnik, M.; Wdowiak, A.; Jakiel, G. Role of Leptin and Adiponectin in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 5307. [Google Scholar] [CrossRef]
- Friedman, M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Xu, K.X.; Yu, T.Y.; Guan, L.J.; Wang, X.R.; Xia, Y.K.; Wu, D. The disruption of leptin and leptin receptor’ s function in the development of hypothalamus leads to obesity in the adulthood: A new mechanism of obesity. Chin. J. Dis. Control. Prev. 2015, 19, 949–954. [Google Scholar]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, X.; Huang, C.; Chen, Y.; Zhao, F.; Hao, R.; Wang, N.; Liao, W.; Xia, H.; Yang, L. The effects of a low carbohydrate diet combined with partial meal replacement on obese individuals. Nutr. Metab. 2023, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Crozet, L.; Holtman, I.R.; Loyher, P.L.; Lazarov, T.; White, J.B.; Mass, E.; Stanley, E.R.; Elemento, O.; Glass, C.K.; et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science 2021, 373, eabe9383. [Google Scholar] [CrossRef]
- Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Inflammatory and Oxidative Stress Markers in Skeletal Muscle of Obese Subjects. In Obesity-Oxidative Stress and Dietary Antioxidants; Chapter 8; Academic Press: New York, NY, USA, 2018; pp. 163–189. [Google Scholar]
- Tang, P.; Virtue, S.; Goie, J.Y.; Png, C.W.; Guo, J.; Li, Y.; Jiao, H.; Chua, Y.L.; Campbell, M.; Moreno-Navarrete, J.M.; et al. Regulation of adipogenic differentiation and adipose tissue inflammation by interferon regulatory factor 3. Cell Death Differ. 2021, 28, 3022–3035. [Google Scholar] [CrossRef]
- Baumard, L.; Weerts, Z.Z.R.M.; Masclee, A.A.M.; Keszthelyi, D.; Michaeltitus, A.T.; Peiris, M. Effect of Obesity on the Expression of Nutrient Receptors and Satiety Hormones in the Human Colon. Nutrients 2021, 13, 1271. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Salehi, A.; Flodgren, E.; Nilsson, N.E.; Jimenez-Feltstrom, J.; Miyazaki, J.; Owman, C.; Olde, B. Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion. Cell. Tissue. Res. 2005, 322, 207–215. [Google Scholar] [CrossRef]
- Chambers, E.; Morrison, D.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef]
- Naser, E.M.S.; Eissa, M.T.; Mansour, M.T.; Abdelmonem, H.A.; Ahmed, A.A.; Ali, N.O.; Abd El-haleem, M.A.; Sayed, S.A.; Emara, S.M.; Yousif, M.M. Green Synthesis of Purslane Seeds-Selenium Nanoparticles and Evaluate its Cytokines Inhibitor in Lead Acetate Induced Toxicity in Rats. Asian J. Chem. Int. Q. Res. J. Chem. 2022, 34, 894–900. [Google Scholar]
- Huang, Y.Q.; Tang, Y.X.; Qiu, B.H.; Talukder, M.; Li, X.N.; Li, J.L. Di-2-ethylhexyl phthalate (DEHP) induced lipid metabolism disorder in liver via activating the LXR/SREBP-1c/PPARα/γ and NF-κB signaling pathway. Food Chem. Toxicol. Int. J. Public Br. Ind. Biol. Res. Assoc. 2022, 165, 113119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pini, M.; Yao, T.; Zhou, Z.; Sun, C.; Fantuzzi, G.; Song, Z. Homocysteine suppresses lipolysis in adipocytes by activating the AMPK pathway. Am. J. Physiol. 2011, 301, 703–712. [Google Scholar] [CrossRef]
- Hong, Y.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.; Feng, D.D.; Chen, C.; Lee, H.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.N.; Gabrielli, M.; Bonifacino, G.; Codesido, M.M.; Vila, M.D.C. Lead enhancement of 3T3-L1 fibroblasts differentiation to adipocytes involves ERK, C/EBPβ and PPARγ activation. Mol. Cell. Biochem. 2018, 437, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chu, X.; Wang, M.; Xin, Y.; Liu, S. MiR-146a-5p, targeting ErbB4, promotes 3T3-L1 preadipocyte differentiation through the ERK1/2/PPAR-γ signaling pathway. Lipids Health Dis. 2022, 21, 54. [Google Scholar] [CrossRef]
- Ang, Z.; Er, J.Z.; Tan, N.S.; Lu, J.; Liou, Y.C.; Grosse, J.; Ding, J.L. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci. Rep. 2016, 6, 34145. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, M.; Jager, J.; Laurent, K.; Gonzalez, T.; Cormont, M.; Binétruy, B.; Marchand-Brustel, L.; Tanti, J.F.; Bost, F. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 2007, 581, 5591–5596. [Google Scholar] [CrossRef]
- Kwon, M.S.; Karadeniz, F.; Kim, J.A.; Seo, Y.; Kong, C.S. Adipogenesis inhibitory effects of Limonium tetragonum in mouse bone marrow stromal D1 cells. Food Sci. Biotechnol. 2016, 25, 125–131. [Google Scholar] [CrossRef]
- Chakrabarti, P.; English, T.; Shi, J.; Smas, C.M.; Kandror, K.V. Mammalian Target of Rapamycin Complex 1 Suppresses Lipolysis, Stimulates Lipogenesis, and Promotes Fat Storage. Diabetes 2010, 59, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Kandror, K.V. The role of mTOR in lipid homeostasis and diabetes progression. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Park, J.; Park, H.; Lee, H. Endrin potentiates early-stage adipogenesis in 3T3-L1 cells by activating the mammalian target of rapamycin. Life Sci. 2021, 288, 120151. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward (5′~3′) | Reverse (3′~5′) |
|---|---|---|
| β-Actin | F: GACCTCTATGCCAACACAGT | R: CACCAATCCACACAGAGTAC |
| PPAR-γ | F: GATGCACTGCTATGAGCACTT | R: AGAGGTCCACAGAGCTGATCC |
| C/EBP-α | F: GTCTACGCTCCACCACCATT | R: CCAAACCAGAAGGAAAGAGG |
| SREBP-1c | F: GATGTGCGAACTGGACA | R: CATAGGGGGCGTCAAACAG |
| AFABP | F: CAGATGACAGGAAAGGCAAG | R: TCCACCACCAGTTTATCACC |
| HSL | F: CATCCGGTCAGAGAGTACTTTT | R: TAGGGTTATGATGCTCTTCACC |
| LPL | F: CTGTTCCTCCAGTCGGTCAG | R: CGTCGCAGGAGGGAAGTTAG |
| FAS | F: CGGCGAGTCTATGCCACTAT | R: ACACAGGGACCGAGTAATGC |
| ACC-1 | F: GGGAATACCTGTGGGAGTAGT | R: GCTGGATTATCTTGGCTTCA |
| CPT-1 | F: ATGGTGGGCGACTAACT | R: TGCCTGCTGTCTGTGAG |
| CPT-2 | F: ATGACCGTTTCTGCCATCC | R: AAGGTGTTGGTGTCGCTTCT |
| SCD-1 | F: GCCATCATTATGAGTGCCAATT | R: AGGGATAAGAACGCTGAGAATT |
| AMPK-α | F: CCCTGTGTATGTGGCTCTG | R: GTGGGTGAACCTCTGCTT |
| AdipoR1 | F: CGGCTCATCTACCTCTCCAT | R: ACACACCTGCTCTTGTCTGC |
| AdipoR2 | F: CTGGCTCAAGGATAACGACTT | R: ATGTTGCCTGTCTCTGTGTG |
| LEPR | F: AAGAACAGAGATGAGGTGGTGC | R: CCAGTGTGGCGTATTTCACG |
| GPR41 | F: CCATCTATCTCACCTCCCTGTTC | R: AACCAGCAGAGCCCACTGAC |
| GPR43 | F: CGTCCAACTTCCGCTGGTA | R: CTTGTACTGCACGGGGTAGG |
| Groups | Heart (%) | Liver (%) | Spleen (%) | Lungs (%) | Kidneys (%) |
|---|---|---|---|---|---|
| LFD-0 | 0.55 ± 0.01 a | 4.23 ± 0.21 a | 0.36 ± 0.01 a | 0.63 ± 0.01 a | 1.33 ± 0.08 a |
| HFD-0 | 0.58 ± 0.02 a | 5.35 ± 0.26 b | 0.38 ± 0.02 a | 0.65 ± 0.01 a | 1.35 ± 0.07 a |
| HFD-1 | 0.60 ± 0.01 a | 5.53 ± 0.32 b | 0.37 ± 0.01 a | 0.64 ± 0.03 a | 1.37 ± 0.05 a |
| HFD-2 | 0.61 ± 0.01 a | 5.84 ± 0.24 b | 0.39 ± 0.02 a | 0.65 ± 0.02 a | 1.36 ± 0.06 a |
| HFD-3 | 0.62 ± 0.01 a | 6.16 ± 0.27 b | 0.40 ± 0.02 a | 0.66 ± 0.02 a | 1.38 ± 0.08 a |
| Groups | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
|---|---|---|---|---|
| LFD-0 | 2.94 ± 0.01 a | 0.94 ± 0.01 a | 0.95 ± 0.01 a | 2.53 ± 0.03 a |
| HFD-0 | 4.79 ± 0.02 b | 2.15 ± 0.03 b | 1.93 ± 0.01 b | 1.33 ± 0.01 b |
| HFD-1 | 4.86 ± 0.04 b | 2.18 ± 0.01 b | 1.96 ± 0.02 b | 1.27 ± 0.01 b |
| HFD-2 | 5.31 ± 0.02 bc | 2.41 ± 0.02 b | 2.42 ± 0.01 c | 0.97 ± 0.01 c |
| HFD-3 | 5.94 ± 0.01 c | 2.57 ± 0.04 b | 2.54 ± 0.03 c | 0.82 ± 0.01 c |
| Groups | AST (U/L) | ALT (U/L) | AKP (U/L) | GGT (U/L) |
|---|---|---|---|---|
| LFD-0 | 28.76 ± 2.64 a | 32.25 ± 2.37 a | 55.47 ± 3.43 a | 13.22 ± 1.42 a |
| HFD-0 | 67.43 ± 2.15 b | 64.27 ± 3.45 b | 80.15 ± 2.18 b | 44.28 ± 2.76 b |
| HFD-1 | 83.28 ± 3.35 b | 74.58 ± 2.36 b | 93.49 ± 4.75 b | 62.21 ± 3.19 b |
| HFD-2 | 135.47 ± 5.17 c | 86.41 ± 4.42 bc | 113.24 ± 4.36 c | 73.28 ± 4.35 bc |
| HFD-3 | 153.43 ± 5.72 c | 95.78 ± 5.63 c | 135.28 ± 5.62 c | 86.45 ± 3.21 c |
| Groups | IL-1β (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) | IL-4 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|---|
| LFD-0 | 13.53 ± 1.87 a | 15.17 ± 2.38 a | 78.64 ± 4.26 a | 51.36 ± 3.52 a | 93.57 ± 6.74 a |
| HFD-0 | 30.72 ± 2.69 b | 27.61 ± 3.41 b | 138.15 ± 5.85 b | 30.15 ± 3.18 b | 45.19 ± 3.25 b |
| HFD-1 | 31.24 ± 3.53 b | 28.54 ± 2.47 b | 141.63 ± 6.28 b | 23.55 ± 3.78 c | 24.43 ± 4.35 c |
| HFD-2 | 37.67 ± 3.45 c | 35.53 ± 2.48 c | 145.47 ± 5.17 b | 21.47 ± 2.66 c | 23.25 ± 3.35 c |
| HFD-3 | 39.26 ± 2.74 c | 36.29 ± 3.57 c | 166.43 ± 5.72 c | 12.24 ± 1.62 d | 11.36 ± 1.21 d |
| Time | Genes | 0 mmol/L | 3 mmol/L | 6 mmol/L | 9 mmol/L |
|---|---|---|---|---|---|
| 6 h | PPAR-γ | 1.00 ± 0.03 a | 1.51 ± 0.07 b | 1.45 ± 0.08 b | 1.43 ± 0.13 b |
| C/EBP-α | 1.00 ± 0.07 a | 1.34 ± 0.05 b | 1.63 ± 0.08 c | 1.69 ± 0.15 c | |
| SREBP-1 c | 1.00 ± 0.08 a | 1.23 ± 0.05 b | 1.58 ± 0.09 c | 1.28 ± 0.10 b | |
| FAS | 1.00 ± 0.05 a | 0.70 ± 0.04 b | 0.93 ± 0.06 a | 1.05 ± 0.03 a | |
| ACC-1 | 1.00 ± 0.03 a | 0.73 ± 0.03 b | 1.06 ± 0.08 a | 1.07 ± 0.06 a | |
| CPT-1 | 1.00 ± 0.04 a | 0.83 ± 0.05 b | 1.06 ± 0.07 a | 1.08 ± 0.09 a | |
| GPR41 | 1.00 ± 0.05 a | 1.09 ± 0.06 a | 1.33 ± 0.12 b | 1.36 ± 0.13 b | |
| GPR43 | 1.00 ± 0.08 a | 1.26 ± 0.15 b | 1.57 ± 0.13 c | 1.87 ± 0.18 d | |
| AMPK-α | 1.00 ± 0.06 a | 0.77 ± 0.03 b | 1.03 ± 0.07 a | 1.05 ± 0.06 a | |
| 12 h | PPAR-γ | 1.11 ± 0.04 a | 1.31 ± 0.05 b | 1.55 ± 0.07 c | 1.63 ± 0.09 c |
| C/EBP-α | 1.05 ± 0.08 a | 1.25 ± 0.04 b | 1.48 ± 0.06 c | 1.67 ± 0.05 d | |
| SREBP-1c | 1.08 ± 0.06 a | 1.23 ± 0.05 b | 1.58 ± 0.09 c | 1.78 ± 0.10 d | |
| FAS | 0.97 ± 0.05 a | 0.75 ± 0.06 b | 1.04 ± 0.09 a | 1.28 ± 0.07 c | |
| ACC-1 | 0.96 ± 0.04 a | 0.73 ± 0.03 b | 1.28 ± 0.04 c | 1.26 ± 0.06 c | |
| CPT-1 | 0.98 ± 0.04 a | 0.98 ± 0.04 a | 1.12 ± 0.07 a | 1.28 ± 0.08 b | |
| GPR41 | 1.02 ± 0.06 a | 1.05 ± 0.06 a | 1.13 ± 0.12 a | 1.16 ± 0.13 a | |
| GPR43 | 1.13 ± 0.07 a | 1.66 ± 0.15 b | 1.87 ± 0.11 c | 1.97 ± 0.16 c | |
| AMPK-α | 1.00 ± 0.06 a | 0.92 ± 0.07 a | 1.11 ± 0.07 a | 1.35 ± 0.04 b | |
| 24 h | PPAR-γ | 1.12 ± 0.05 a | 1.26 ± 0.04 b | 1.35 ± 0.07 b | 1.78 ± 0.10 c |
| C/EBP-α | 1.08 ± 0.07 a | 1.18 ± 0.07 b | 2.63 ± 0.08 c | 3.69 ± 0.15 d | |
| SREBP-1c | 1.10 ± 0.09 a | 1.23 ± 0.08 b | 4.48 ± 0.10 c | 3.52 ± 0.12 d | |
| FAS | 0.94 ± 0.05 a | 0.78 ± 0.05 b | 0.67 ± 0.03 b | 0.55 ± 0.09 b | |
| ACC-1 | 0.91 ± 0.05 a | 0.73 ± 0.03 b | 1.06 ± 0.08 a | 1.09 ± 0.06 a | |
| CPT-1 | 0.93 ± 0.04 a | 0.76 ± 0.05 b | 1.26 ± 0.07 c | 1.37 ± 0.11 c | |
| GPR41 | 1.04 ± 0.06 a | 1.09 ± 0.09 a | 1.13 ± 0.12 a | 1.15 ± 0.13 a | |
| GPR43 | 1.18 ± 0.04 a | 1.68 ± 0.14 b | 1.96 ± 0.13 c | 2.07 ± 0.18 c | |
| AMPK-α | 0.92 ± 0.06 a | 0.77 ± 0.03 b | 0.73 ± 0.04 b | 0.53 ± 0.06 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Li, A.; Wang, H.; Ma, J.; Hou, J. Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice. Nutrients 2023, 15, 3736. https://doi.org/10.3390/nu15173736
Sun C, Li A, Wang H, Ma J, Hou J. Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice. Nutrients. 2023; 15(17):3736. https://doi.org/10.3390/nu15173736
Chicago/Turabian StyleSun, Changbao, Ang Li, Huan Wang, Jiage Ma, and Juncai Hou. 2023. "Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice" Nutrients 15, no. 17: 3736. https://doi.org/10.3390/nu15173736
APA StyleSun, C., Li, A., Wang, H., Ma, J., & Hou, J. (2023). Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice. Nutrients, 15(17), 3736. https://doi.org/10.3390/nu15173736




