Update on In Vitro Diagnostic Tools and Treatments for Food Allergies
Abstract
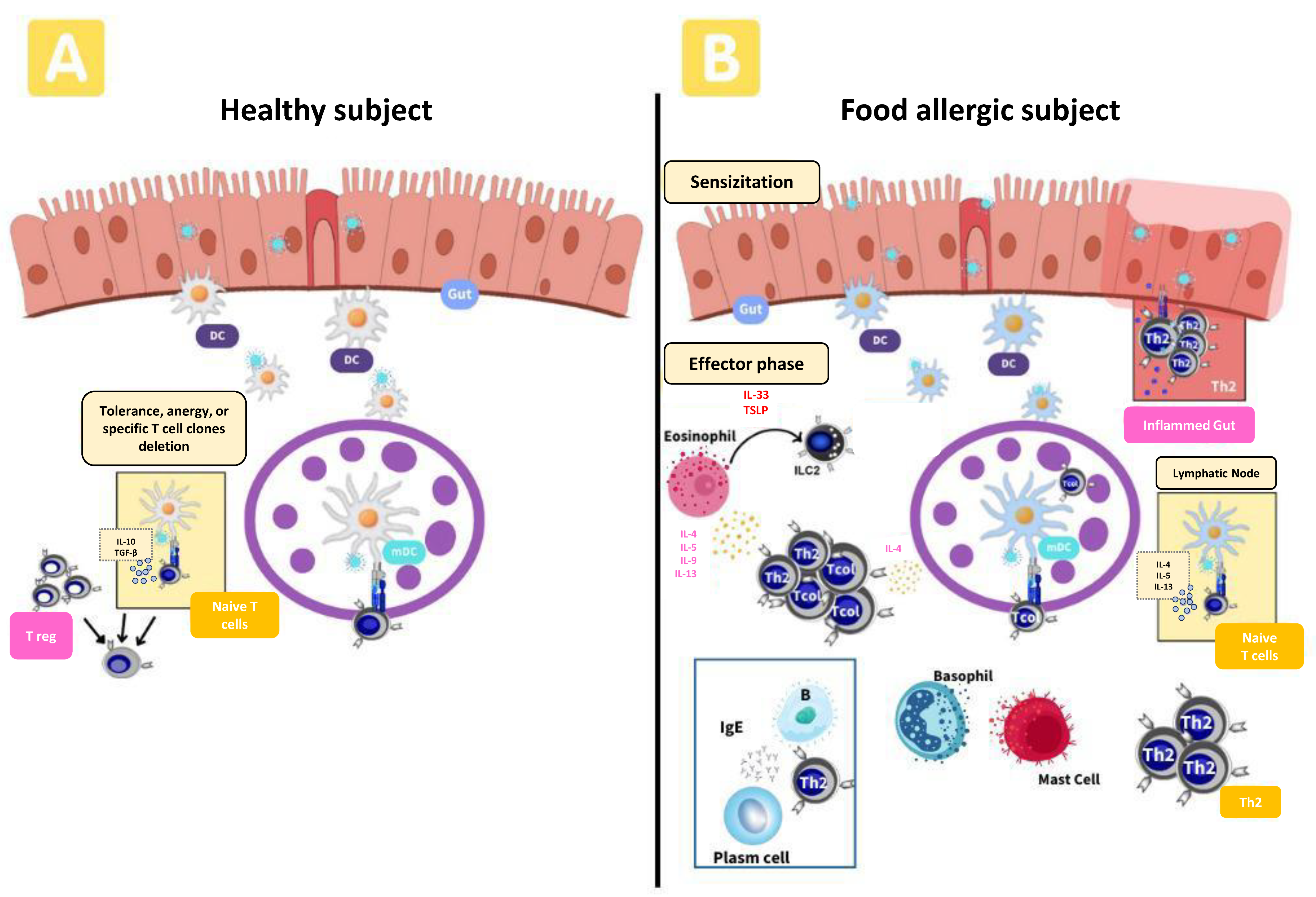
:1. Introduction
2. Immune Mechanism in FA
2.1. IgE-Mediated Reactions
2.2. Non-IgE-Mediated Reactions
2.3. Mixed Reactions
3. FA In Vitro Supporting Diagnostic Tools
3.1. Allergen-Specific IgE In Vitro Testing
3.2. The Activation Test (BAT)
3.3. Mast Cell Activation (MAT)
3.4. T Cells Assay
| Diagnostic Tools | FA | Advantages | Limitations |
|---|---|---|---|
Specific IgE | Sesame [32] Hazelnut [33] Peanut allergic children [35] Kiwi [36] LTP (Multiple Food) [38] Egg white and yolk [39] | Standardized High throughput Can be automatized | Poor in detection of sensitization from clinically reactive FA. |
BAT | Peanut [44] LTP (Multiple Food) [45] Cow’s milk [47] | High sensitivity | Lack of standardization. Requires fresh blood. Its specificity is variable. |
MAT | Peanut [51,52] LTP (Multiple Food) [53] | Requires plasma. Its specificity is stable. | Lack of standardization. Low sensitivity. |
T cells | Peanut [55] Milk [56] | High throughput High sensitivity and specificity Concordant with clinical results | Need to improve the development of molecular techniques, and the characterization of allergens. Larger amounts of blood. |
4. FA Immunotherapy Treatment
4.1. Allergen Immunotherapy
4.2. Nanoparticles: Platform AIT
4.3. Hypoallergenic Proteins: Product AIT
4.4. Monoclonal Antibodies (Anti-IgE): Adjuvant AIT
| Immunotherapy | Treatment Type/FA | Advantages | Limitations |
|---|---|---|---|
| Allergen immunotherapy | OIT: Peanut [60,61,62,63] EPIT: Peanut [65] SLIT: Peanut, Apple, Egg and LTP (Multiple Food) [66,67,68,69,70] | -AIT is specific treatment that can address underlying cause of the allergic reaction. -Long-term effects, reducing the severity of allergic reactions. -ATI eliminates the need for other medications to control allergic reactions | -Lack of clinical studies to evaluate its safety and efficacy -Risks of suffering severe allergic reactions during treatment -Prolonged duration of treatment |
| Nanoparticles | PLG and LNP: Peanut [77,78,79] T cells epitopes + CpG: Shrimp and CM [80,81,82] Glycoparticles: Peach [86,87,88] | -Controlled delivery of allergens. -Improved absorption and bioavailability | -More studies are needed to evaluate their long-term safety and effectiveness -Technical complexity -Lack of regulation and approval for its implementation as therapies |
| Hypoallergenic proteins | Hydrolyzed protein formulation and B5M: CMA. [90,91,92,94] mtTM, mtMLC: Crab [95] GTM: Shrimp [96,97] 1BS-18H: Wheat [98,99] r Mal d 1: Apple [68] | -Reduced risk of allergic reactions. | -Potential nutrient deficiency -Cost and availability -Altered taste and texture |
| Monoclonal antibody (anti-IgE) | Monotherapy: Multiple Food and CM [102,104] Multiple therapy: Multiple Food and peanut [105,106,107] | -Reduced risk of allergic reactions (IgE inhibition). -Increasing food intake. -Improves the quality of life of patients. | -Cost -Side effects -Duration of treatment |
5. New Therapeutic Approaches
5.1. Probiotics and Symbiotics
5.2. Herbal Medicine
5.3. Dietary Supplements
| Therapeutic Approaches | Model/FA | Achievements |
|---|---|---|
| Probiotics and symbiotics | Clostridium butyricum, Lactobacillus gasseri and Bifidobacterium species [111,112,113] and Lactobacillus vaginalis [114]: FA animal model. Lactobacillus paracasei L9: CM [135] Leuconostoc citreum: FA animal model [116] Akkermansia muciniphila: FA animal model [136]. Lactobacillus rhamnosus: Penaut [117] Synbiotic-containing fructooligosaccharides and Bifidobacterium breve M-16V:CM [121,122]. | -Increase the ratio of effector Treg cells and enhance the secretion of regulatory cytokines. -Modulation of Th1/Th2 balance and attenuation of allergic reaction. -IgE reduction -Transformation of gut microbial to improve the healthy infants. |
| Herbal medicine | E-B-FAHF-2 and B-FAHF-2: Peanut [125,126]. Berberine: peanut and cholera toxine animal model [126]. Oleuropein: FA animal model [128]. | -Reduction in anaphylaxis symptoms. -Reduction in histamine and IgE plasma levels. -Reduction in B cells in spleen and modification of gut microbiota |
| Dietary supplements | Ginger: CACO2 cells [131]. Olive oil: FA animal model [132]. Arachidonic and docosahexaenoic acids (PUFAs): mother during suckling period levels [133]. Omega-3 supplementation: mothers during pregnancy [134]. | -Suppression of FA inflammation. -Reduction in allergic symptoms. -Th2 cells reduction and increase in Treg. -Increase in oral tolerance in children. -Increase in Th1 cytokines levels. -Reduction in the risk of FA in children. |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATI | Allergen immunotherapy |
| BAT | Basophil activation test |
| CMPA | Cow milk protein |
| EGID | Eosinophilic gastrointestinal disorders |
| EoE | Eosinophilic esophagitis |
| EMA | European Medicines Agency |
| FA | Food allergy |
| FPIES | Food protein-induced enterocolitis syndrome |
| FPIAP | Food protein-induced allergic proctocolitis |
| FPE | Food protein-induced allergic enteropathy |
| FDA | Food and Drug Administration |
| GORD | Gastro-esophageal reflux disorder |
| MAT | Mast cell activation test |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OFC | Oral food challenge |
| OIT | Oral immunotherapy |
| SPT | Skin prick test |
| SMPA | Soja’s milk protein allergy |
| sIgE | Specific immunoglobulin E |
| TSLP | Thymic stromal lymphopoietin |
References
- Ballegaard, A.R.; Bogh, K.L. Intestinal protein uptake and IgE-mediated food allergy. Food Res. Int. 2023, 163, 112150. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, C.E.; Andreae, D.A. Food Allergy. Prim. Care 2023, 50, 205–220. [Google Scholar] [CrossRef]
- Feng, H.; Liu, Y.; Xiong, X.; Xu, Q.; Zhang, Z.; Wu, Y.; Lu, Y. Epidemiological survey of self-reported food allergy among university students in China. Medicine 2022, 101, e29606. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Cecilia Berin, M.; Sicherer, S. Update on Food Protein-Induced Enterocolitis Syndrome (FPIES). Curr. Allergy Asthma Rep. 2022, 22, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Arasi, S.; Ballmer-Weber, B.; Baseggio Conrado, A.; Deschildre, A.; Gerdts, J.; Halken, S.; Muraro, A.; Patel, N.; Van Ree, R.; et al. Risk factors for severe reactions in food allergy: Rapid evidence review with meta-analysis. Allergy 2022, 77, 2634–2652. [Google Scholar] [CrossRef]
- Munoz-Cano, R.; San Bartolome, C.; Casas-Saucedo, R.; Araujo, G.; Gelis, S.; Ruano-Zaragoza, M.; Roca-Ferrer, J.; Palomares, F.; Martin, M.; Bartra, J.; et al. Immune-Mediated Mechanisms in Cofactor-Dependent Food Allergy and Anaphylaxis: Effect of Cofactors in Basophils and Mast Cells. Front. Immunol. 2020, 11, 623071. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food allergy across the globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef]
- Paranjape, A.; Tsai, M.; Mukai, K.; Hoh, R.A.; Joshi, S.A.; Chinthrajah, R.S.; Nadeau, K.C.; Boyd, S.D.; Galli, S.J. Oral Immunotherapy and Basophil and Mast Cell Reactivity in Food Allergy. Front. Immunol. 2020, 11, 602660. [Google Scholar] [CrossRef]
- Fernandez-Rivas, M.; Vereda, A.; Vickery, B.P.; Sharma, V.; Nilsson, C.; Muraro, A.; Hourihane, J.O.; DunnGalvin, A.; du Toit, G.; Blumchen, K.; et al. Open-label follow-on study evaluating the efficacy, safety, and quality of life with extended daily oral immunotherapy in children with peanut allergy. Allergy 2022, 77, 991–1003. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Mayorga, C.; Palomares, F.; Canas, J.A.; Perez-Sanchez, N.; Nunez, R.; Torres, M.J.; Gomez, F. New Insights in Therapy for Food Allergy. Foods 2021, 10, 1037. [Google Scholar] [CrossRef]
- Zhang, S.; Sicherer, S.; Berin, M.C.; Agyemang, A. Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021, 10, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.C.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.U.A.; Santoro, A.; et al. Non-IgE- or Mixed IgE/Non-IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, Y.; Jimenez-Saiz, R.; Spill, P.; Chu, D.K.; Waserman, S.; Jordana, M. The Initiation of Th2 Immunity towards Food Allergens. Int. J. Mol. Sci. 2018, 19, 1447. [Google Scholar] [CrossRef] [PubMed]
- Palomares, F.; Gomez, F.; Bogas, G.; Maggi, L.; Cosmi, L.; Annunziato, F.; Nunez, R.; Perez, N.; Munoz-Cano, R.; Torres, M.J.; et al. Innate lymphoid cells type 2 in LTP-allergic patients and their modulation during sublingual immunotherapy. Allergy 2021, 76, 2253–2256. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Pan, J.; Liu, N.; Qin, Y.; Qiu, L.; Liu, M.; Wang, T. The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases. Front. Immunol. 2021, 12, 586078. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Layhadi, J.A.; Golebski, K.; Istvan Komlosi, Z.; Peng, Y.; Sekerel, B.; Durham, S.R.; Brough, H.; Morita, H.; Akdis, M.; et al. Innate lymphoid cells: The missing part of a puzzle in food allergy. Allergy 2021, 76, 2002–2016. [Google Scholar] [CrossRef]
- Benede, S.; Tordesillas, L.; Berin, C. Demonstration of distinct pathways of mast cell-dependent inhibition of Treg generation using murine bone marrow-derived mast cells. Allergy 2020, 75, 2088–2091. [Google Scholar] [CrossRef]
- Locke, A.; Hung, L.; Upton, J.E.M.; O’Mahony, L.; Hoang, J.; Eiwegger, T. An update on recent developments and highlights in food allergy. Allergy 2023. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.F.; Cabanillas, B. Recent advances in cellular and molecular mechanisms of IgE-mediated food allergy. Food Chem. 2023, 411, 135500. [Google Scholar] [CrossRef]
- Al-Iede, M.; Sarhan, L.; Alshrouf, M.A.; Said, Y. Perspectives on Non-IgE-Mediated Gastrointestinal Food Allergy in Pediatrics: A Review of Current Evidence and Guidelines. J. Asthma Allergy 2023, 16, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Non-IgE Mediated Food Allergy. Curr. Pediatr. Rev. 2020, 16, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Martorell-Aragones, A.; Echeverria-Zudaire, L.; Alonso-Lebrero, E.; Bone-Calvo, J.; Martin-Munoz, M.F.; Nevot-Falco, S.; Piquer-Gibert, M.; Valdesoiro-Navarrete, L.; Food allergy committee of SEICAP. Position document: IgE-mediated cow’s milk allergy. Allergol. Immunopathol. 2015, 43, 507–526. [Google Scholar] [CrossRef]
- Cronin, C.; Ramesh, Y.; De Pieri, C.; Velasco, R.; Trujillo, J. ‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management. Nutrients 2023, 15, 1397. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Viscido, A.; Ginaldi, L. Food Allergy Insights: A Changing Landscape. Arch. Immunol. Ther. Exp. 2020, 68, 8. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Suppa, M.; Ginaldi, L.; De Martinis, M. Does Allergy Break Bones? Osteoporosis and Its Connection to Allergy. Int. J. Mol. Sci. 2020, 21, 712. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef]
- Arasi, S.; Barni, S.; Mastrorilli, C.; Comberiati, P.; Chiera, F.; Pelosi, U.; Paravati, F.; Caimmi, D. Role of in vitro testing in food allergy. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S26), 36–38. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gomez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.R.; Appel, M.Y.; Nachshon, L.; Holmqvist, M.; Epstein-Rigbi, N.; Levy, M.B.; Lidholm, J.; Elizur, A. Combinatorial advantage of Ses i 1-specific IgE and basophil activation for diagnosis of sesame food allergy. Pediatr. Allergy Immunol. 2021, 32, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Berthold, M.; Mascialino, B.; Orme, M.; Sjolander, S.; Hamilton, R. Allergen components in diagnosing childhood hazelnut allergy: Systematic literature review and meta-analysis. Pediatr. Allergy Immunol. 2020, 31, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Maesa, J.M.; Dobrzynska, A.; Banos-Alvarez, E.; Isabel-Gomez, R.; Blasco-Amaro, J.A. ImmunoCAP ISAC in food allergy diagnosis: A systematic review of diagnostic test accuracy. Clin. Exp. Allergy 2021, 51, 778–789. [Google Scholar] [CrossRef]
- Brand, H.K.; Schreurs, M.W.J.; Emons, J.A.M.; Gerth van Wijk, R.; de Groot, H.; Arends, N.J.T. Peanut components measured by ISAC: Comparison with ImmunoCap and clinical relevance in peanut allergic children. Clin. Mol. Allergy 2021, 19, 14. [Google Scholar] [CrossRef]
- D’Amelio, C.M.; Bernad, A.; Garcia-Figueroa, B.E.; Garrido-Fernandez, S.; Azofra, J.; Beristain, A.; Bueno-Diaz, C.; Garrido-Arandia, M.; Gastaminza, G.; Ferrer, M.; et al. Unraveling the Diagnosis of Kiwifruit Allergy: Usefulness of Current Diagnostic Tests. J. Investig. Allergol. Clin. Immunol. 2022, 32, 206–212. [Google Scholar] [CrossRef]
- Blazowski, L.; Majak, P.; Kurzawa, R.; Kuna, P.; Jerzynska, J. Food allergy endotype with high risk of severe anaphylaxis in children-Monosensitization to cashew 2S albumin Ana o 3. Allergy 2019, 74, 1945–1955. [Google Scholar] [CrossRef]
- Sara, B.V.; Ulrike, F.; Bettina, B.; Yvonne, W.; Teresa, P.; Clara, S.B.; Giovanna, A.S.; Rocio, C.S.; Maria, T.; Rocio, L.; et al. Improving In Vitro Detection of Sensitization to Lipid Transfer Proteins: A New Molecular Multiplex IgE Assay. Mol. Nutr. Food Res. 2023, 67, e2200906. [Google Scholar] [CrossRef]
- Ehlers, A.M.; Otten, H.G.; Wierzba, E.; Flugge, U.; Le, T.M.; Knulst, A.C.; Suer, W. Detection of specific IgE against linear epitopes from Gal d 1 has additional value in diagnosing hen’s egg allergy in adults. Clin. Exp. Allergy 2020, 50, 1415–1423. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Alpan, O.; Wasserman, R.L.; Kim, T.; Darter, A.; Shah, A.; Jones, D.; McNeil, D.; Li, H.; Ispas, L.; Rathkopf, M.; et al. Towards an FDA-cleared basophil activation test. Front. Allergy 2022, 3, 1009437. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bridts, C.H.; Mertens, C.H.; Sabato, V. Principles, potential, and limitations of ex vivo basophil activation by flow cytometry in allergology: A narrative review. J. Allergy Clin. Immunol. 2021, 147, 1143–1153. [Google Scholar] [CrossRef]
- Santos, A.F.; Du Toit, G.; O’Rourke, C.; Becares, N.; Couto-Francisco, N.; Radulovic, S.; Khaleva, E.; Basting, M.; Harris, K.M.; Larson, D.; et al. Biomarkers of severity and threshold of allergic reactions during oral peanut challenges. J. Allergy Clin. Immunol. 2020, 146, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Bergmann, M.; Brough, H.A.; Couto-Francisco, N.; Kwok, M.; Panetta, V.; Haddad, D.; Lack, G.; Eigenmann, P.; Caubet, J.C. Basophil Activation Test Reduces Oral Food Challenges to Nuts and Sesame. J. Allergy Clin. Immunol. Pract. 2021, 9, 2016–2027. [Google Scholar] [CrossRef]
- Duan, L.; Celik, A.; Hoang, J.A.; Schmidthaler, K.; So, D.; Yin, X.; Ditlof, C.M.; Ponce, M.; Upton, J.E.M.; Lee, J.S.; et al. Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study. Allergy 2021, 76, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Canas, J.A.; Perez-Sanchez, N.; Lopera-Doblas, L.; Palomares, F.; Molina, A.; Bartra, J.; Torres, M.J.; Gomez, F.; Mayorga, C. Basophil Activation Test Utility as a Diagnostic Tool in LTP Allergy. Int. J. Mol. Sci. 2022, 23, 4979. [Google Scholar] [CrossRef] [PubMed]
- Ruinemans-Koerts, J.; Schmidt-Hieltjes, Y.; Jansen, A.; Savelkoul, H.F.J.; Plaisier, A.; van Setten, P. The Basophil Activation Test reduces the need for a food challenge test in children suspected of IgE-mediated cow’s milk allergy. Clin. Exp. Allergy 2019, 49, 350–356. [Google Scholar] [CrossRef]
- Krawiec, M.; Radulovic, S.; Foong, R.X.; Marques-Mejias, A.; Bartha, I.; Kwok, M.; Jama, Z.; Harrison, F.; Ricci, C.; Lack, G.; et al. Diagnostic utility of allergy tests to predict baked egg and lightly cooked egg allergies compared to double-blind placebo-controlled food challenges. Allergy 2023. [Google Scholar] [CrossRef]
- Bahri, R.; Custovic, A.; Korosec, P.; Tsoumani, M.; Barron, M.; Wu, J.; Sayers, R.; Weimann, A.; Ruiz-Garcia, M.; Patel, N.; et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J. Allergy Clin. Immunol. 2018, 142, 485–496. [Google Scholar] [CrossRef]
- Peters, R.L.; Krawiec, M.; Koplin, J.J.; Santos, A.F. Update on food allergy. Pediatr. Allergy Immunol. 2021, 32, 647–657. [Google Scholar] [CrossRef]
- Santos, A.F.; Couto-Francisco, N.; Becares, N.; Kwok, M.; Bahnson, H.T.; Lack, G. A novel human mast cell activation test for peanut allergy. J. Allergy Clin. Immunol. 2018, 142, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Huang, Y.; Yeung, L.H.; Hemmings, O.; Jama, Z.; Kwok, M.; Lack, G.; Santos, A.F. Ara h 2-Specific IgE Presence Rather Than Its Function Is the Best Predictor of Mast Cell Activation in Children. J. Allergy Clin. Immunol. Pract. 2023, 11, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, J.A.; Lebron-Martin, C.; Garcia-Oton, R.; Delgado, M.J.; Martin-Astorga, M.D.C.; Perez-Sanchez, N.; Gomez, F.; Torres, M.J.; Canas, J.A.; Aranda, C.J.; et al. In vitro supporting diagnostic tools in plant-food allergy. Allergy 2023. Online ahead of print.. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Peters, B. T-cell epitope discovery and single-cell technologies to advance food allergy research. J. Allergy Clin. Immunol. 2023, 151, 15–20. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, X.; Dunham, D.; Lyu, S.C.; Manohar, M.; Zhang, W.; Zhao, F.; Davis, M.M.; Nadeau, K. Allergen-specific CD8(+) T cells in peanut-allergic individuals. J. Allergy Clin. Immunol. 2019, 143, 1948–1952. [Google Scholar] [CrossRef]
- Morgan, D.M.; Ruiter, B.; Smith, N.P.; Tu, A.A.; Monian, B.; Stone, B.E.; Virk-Hundal, N.; Yuan, Q.; Shreffler, W.G.; Love, J.C. Clonally expanded, GPR15-expressing pathogenic effector T(H)2 cells are associated with eosinophilic esophagitis. Sci. Immunol. 2021, 6, eabi5586. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, B.; Smith, N.P.; Monian, B.; Tu, A.A.; Fleming, E.; Virkud, Y.V.; Patil, S.U.; Whittaker, C.A.; Love, J.C.; Shreffler, W.G. Expansion of the CD4(+) effector T-cell repertoire characterizes peanut-allergic patients with heightened clinical sensitivity. J. Allergy Clin. Immunol. 2020, 145, 270–282. [Google Scholar] [CrossRef]
- Sampath, V.; Sindher, S.B.; Alvarez Pinzon, A.M.; Nadeau, K.C. Can food allergy be cured? What are the future prospects? Allergy 2020, 75, 1316–1326. [Google Scholar] [CrossRef]
- Vickery, B.P.; Vereda, A.; Nilsson, C.; du Toit, G.; Shreffler, W.G.; Burks, A.W.; Jones, S.M.; Fernandez-Rivas, M.; Blumchen, K.; Hourihane, J.O.B.; et al. Continuous and daily oral immunotherapy for peanut allergy: Results from a 2-year open-label follow-on study. J. Allergy Clin. Immunol. Pract. 2021, 9, 1879–1889. [Google Scholar] [CrossRef]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): A randomised placebo-controlled study. Lancet 2022, 399, 359–371. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Long, A.; O’Laughlin, K.L.; Lyu, S.C.; Manohar, M.; Boyd, S.D.; Tibshirani, R.; Maecker, H.; et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): A large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019, 394, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; James, L.K.; Kwok, M.; McKendry, R.T.; Anagnostou, K.; Clark, A.T.; Lack, G. Peanut oral immunotherapy induces blocking antibodies but does not change the functional characteristics of peanut-specific IgE. J. Allergy Clin. Immunol. 2020, 145, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Mukai, K.; Chinthrajah, R.S.; Nadeau, K.C.; Galli, S.J. Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J. Allergy Clin. Immunol. 2020, 145, 885–896.e6. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Greenhawt, M.; Sussman, G.; Begin, P.; Nowak-Wegrzyn, A.; Petroni, D.; Beyer, K.; Brown-Whitehorn, T.; Hebert, J.; Hourihane, J.O.; et al. Effect of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Ingestion among Children with Peanut Allergy: The PEPITES Randomized Clinical Trial. JAMA 2019, 321, 946–955. [Google Scholar] [CrossRef]
- Scurlock, A.M.; Burks, A.W.; Sicherer, S.H.; Leung, D.Y.M.; Kim, E.H.; Henning, A.K.; Dawson, P.; Lindblad, R.W.; Berin, M.C.; Cho, C.B.; et al. Epicutaneous immunotherapy for treatment of peanut allergy: Follow-up from the Consortium for Food Allergy Research. J. Allergy Clin. Immunol. 2021, 147, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Schworer, S.A.; Kim, E.H. Sublingual immunotherapy for food allergy and its future directions. Immunotherapy 2020, 12, 921–931. [Google Scholar] [CrossRef]
- DuBuske, L. Efficacy and safety of sublingual allergen immunotherapy. Allergy Asthma Proc. 2022, 43, 272–280. [Google Scholar] [CrossRef]
- Sanchez Acosta, G.; Kinaciyan, T.; Kitzmuller, C.; Mobs, C.; Pfutzner, W.; Bohle, B. IgE-blocking antibodies following SLIT with recombinant Mal d 1 accord with improved apple allergy. J. Allergy Clin. Immunol. 2020, 146, 894–900.e2. [Google Scholar] [CrossRef]
- Sagara, N.; Fujita, S.; Suzuki, R.; Aota, A.; Akashi, K.; Katsunuma, T. Successful sublingual immunotherapy for severe egg allergy in children: A case report. Allergy Asthma Clin. Immunol. 2021, 17, 2. [Google Scholar] [CrossRef]
- Beitia, J.M.; Vega Castro, A.; Cardenas, R.; Pena-Arellano, M.I. Pru p 3 Sublingual Immunotherapy in Patients with Lipid Transfer Protein Syndrome: Is It Worth? Int. Arch. Allergy Immunol. 2021, 182, 447–454. [Google Scholar] [CrossRef]
- Kanagaratham, C.; El Ansari, Y.S.; Lewis, O.L.; Oettgen, H.C. IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front. Immunol. 2020, 11, 603050. [Google Scholar] [CrossRef]
- McKendry, R.T.; Kwok, M.; Hemmings, O.; James, L.K.; Santos, A.F. Allergen-specific IgG show distinct patterns in persistent and transient food allergy. Pediatr. Allergy Immunol. 2021, 32, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tang, L.F.; Cheng, L.; Wang, H.Y. The clinical significance of allergen-specific IgG4 in allergic diseases. Front. Immunol. 2022, 13, 1032909. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.E.; Keshavarz, B.; Wilson, J.M.; Li, R.C.; Heymann, P.W.; Gold, D.R.; McGowan, E.C.; Erwin, E.A. An Overview of the Relevance of IgG4 Antibodies in Allergic Disease with a Focus on Food Allergens. Children 2021, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, J.M.; Baloh, C.; Lim, N.; Larson, D.; Qin, T.; Wheatley, L.; Kim, E.H.; Jones, S.M.; Burks, A.W.; Kulis, M.D. Peanut-Specific IgG4 and IgA in Saliva Are Modulated by Peanut Oral Immunotherapy. J. Allergy Clin. Immunol. Pract. 2022, 10, 3270–3275. [Google Scholar] [CrossRef]
- Sindher, S.B.; Long, A.; Chin, A.R.; Hy, A.; Sampath, V.; Nadeau, K.C.; Chinthrajah, R.S. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy 2022, 77, 2937–2948. [Google Scholar] [CrossRef]
- Hughes, K.R.; Saunders, M.N.; Landers, J.J.; Janczak, K.W.; Turkistani, H.; Rad, L.M.; Miller, S.D.; Podojil, J.R.; Shea, L.D.; O’Konek, J.J. Masked Delivery of Allergen in Nanoparticles Safely Attenuates Anaphylactic Response in Murine Models of Peanut Allergy. Front. Allergy 2022, 3, 829605. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Liao, Y.P.; Luo, L.; Xia, T.; Nel, A.E. Use of a Liver-Targeting Immune-Tolerogenic mRNA Lipid Nanoparticle Platform to Treat Peanut-Induced Anaphylaxis by Single- and Multiple-Epitope Nucleotide Sequence Delivery. ACS Nano 2023, 17, 4942–4957. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Liao, Y.P.; Chang, C.H.; Li, J.; Xia, T.; Nel, A.E. Use of a Liver-targeting Nanoparticle Platform to Intervene in Peanut-induced anaphylaxis through delivery of an Ara h2 T-cell Epitope. Nano Today 2022, 42, 101370. [Google Scholar] [CrossRef]
- Hong, J.; Gao, Q.; Xiao, X.; Cao, H.; Yuan, R.; Liu, Z.; Chen, T. T cell epitope of arginine kinase with CpG co-encapsulated nanoparticles attenuates a shrimp allergen-induced Th2-bias food allergy. Biosci. Biotechnol. Biochem. 2020, 84, 804–814. [Google Scholar] [CrossRef]
- Liu, M.; Thijssen, S.; Hennink, W.E.; Garssen, J.; van Nostrum, C.F.; Willemsen, L.E.M. Oral pretreatment with beta-lactoglobulin derived peptide and CpG co-encapsulated in PLGA nanoparticles prior to sensitizations attenuates cow’s milk allergy development in mice. Front. Immunol. 2022, 13, 1053107. [Google Scholar] [CrossRef]
- Liu, M.; Thijssen, S.; van Nostrum, C.F.; Hennink, W.E.; Garssen, J.; Willemsen, L.E.M. Inhibition of cow’s milk allergy development in mice by oral delivery of beta-lactoglobulin-derived peptides loaded PLGA nanoparticles is associated with systemic whey-specific immune silencing. Clin. Exp. Allergy 2022, 52, 137–148. [Google Scholar] [CrossRef]
- Palomares, F.; Ramos-Soriano, J.; Gomez, F.; Mascaraque, A.; Bogas, G.; Perkins, J.R.; Gonzalez, M.; Torres, M.J.; Diaz-Perales, A.; Rojo, J.; et al. Pru p 3-Glycodendropeptides Based on Mannoses Promote Changes in the Immunological Properties of Dendritic and T-Cells from LTP-Allergic Patients. Mol. Nutr. Food Res. 2019, 63, e1900553. [Google Scholar] [CrossRef]
- Palomares, F.; Gomez, F.; de la Fuente, M.C.; Perez-Sanchez, N.; Torres, M.J.; Mayorga, C.; Rojo, J.; Ramos-Soriano, J. Fucodendropeptides induce changes in cells of the immune system in food allergic patients via DC-SIGN receptor. Carbohydr. Res. 2022, 517, 108580. [Google Scholar] [CrossRef]
- Losada Mendez, J.; Palomares, F.; Gomez, F.; Ramirez-Lopez, P.; Ramos-Soriano, J.; Torres, M.J.; Mayorga, C.; Rojo, J. Immunomodulatory Response of Toll-like Receptor Ligand-Peptide Conjugates in Food Allergy. ACS Chem. Biol. 2021, 16, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Nunez, R.; Rodriguez, M.J.; Palomares, F.; Gomez, F.; Jabato, F.M.; Cordoba-Caballero, J.; Seoane, P.; Losada, J.; Rojo, J.; Torres, M.J.; et al. Transcriptional changes in dendritic cells underlying allergen specific induced tolerance in a mouse model. Sci. Rep. 2022, 12, 2797. [Google Scholar] [CrossRef] [PubMed]
- Nunez, R.; Rodriguez, M.J.; Lebron-Martin, C.; Martin-Astorga, M.D.C.; Palomares, F.; Ramos-Soriano, J.; Rojo, J.; Torres, M.J.; Canas, J.A.; Mayorga, C. Methylation changes induced by a glycodendropeptide immunotherapy and associated to tolerance in mice. Front. Immunol. 2022, 13, 1094172. [Google Scholar] [CrossRef]
- Nunez, R.; Rodriguez, M.J.; Lebron-Martin, C.; Martin-Astorga, M.D.C.; Ramos-Soriano, J.; Rojo, J.; Torres, M.J.; Canas, J.A.; Mayorga, C. A synthetic glycodendropeptide induces methylation changes on regulatory T cells linked to tolerant responses in anaphylactic-mice. Front. Immunol. 2023, 14, 1165852. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kulis, M. Hypoallergenic Proteins for the Treatment of Food Allergy. Curr. Allergy Asthma Rep. 2019, 19, 15. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Brough, H.A.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the Management of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2021, 13, 2762. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Barrio-Torres, J.; Dupont, C.; Howells, H.E.; Shamir, R.; Venter, C.; Meyer, R. Hydrolyzed rice formula for dietary management of infants with cow’s milk allergy. World Allergy Organ. J. 2022, 15, 100717. [Google Scholar] [CrossRef]
- Paparo, L.; Picariello, G.; Bruno, C.; Pisapia, L.; Canale, V.; Sarracino, A.; Nocerino, R.; Carucci, L.; Cosenza, L.; Cozzolino, T.; et al. Tolerogenic Effect Elicited by Protein Fraction Derived from Different Formulas for Dietary Treatment of Cow’s Milk Allergy in Human Cells. Front. Immunol. 2020, 11, 604075. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, Y.; Lin, S.; Xu, L.; Li, L.; Cai, C.; Li, H. Alteration of Allergen Fold of Bos d 5 into a Hypoallergenic Vaccine for Immunotherapy of Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2022, 183, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Xia, F.; Liu, Q.M.; Chen, Y.Y.; Yun, X.; Liu, M.; Chen, G.X.; Wang, L.; Cao, M.J.; Liu, G.M. Hypoallergenic derivatives of Scylla paramamosain heat-stable allergens alleviated food allergy symptoms in Balb/c mice. Food Funct. 2022, 13, 11518–11531. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Lin, H. Reducing the Allergenicity of Shrimp Tropomyosin and Allergy Desensitization Based on Glycation Modification. J. Agric. Food Chem. 2021, 69, 14742–14750. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.M.; Li, Z.; Lin, H. Investigation of glycated shrimp tropomyosin as a hypoallergen for potential immunotherapy. Food Funct. 2021, 12, 2750–2759. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, T.; Chen, K.; Wang, H.; Meng, X. Assessment of the effect of glycation on the allergenicity of sesame proteins. Food Res. Int. 2023, 168, 112771. [Google Scholar] [CrossRef]
- Yamada, Y.; Yokooji, T.; Kunimoto, K.; Inoguchi, K.; Ogino, R.; Taogoshi, T.; Morita, E.; Matsuo, H. Hypoallergenic Wheat Line (1BS-18H) Lacking omega5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy. Foods 2022, 11, 2181. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Nestl, B.; Richter, S.M.; Emperle, M.; Buchweitz, M. Purification and Characterization of Recombinant Expressed Apple Allergen Mal d 1. Methods Protoc. 2020, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, J.A.; Wood, R.A. Omalizumab as an adjuvant in food allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Artesani, M.C.; Riccardi, C.; Mennini, M.; Pecora, V.; Fierro, V.; Calandrelli, V.; Dahdah, L.; Valluzzi, R.L. Impact of Omalizumab on Food Allergy in Patients Treated for Asthma: A Real-Life Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Chinuki, Y.; Yagami, A.; Adachi, A.; Matsunaga, K.; Ugajin, T.; Yokozeki, H.; Hayashi, M.; Katayama, I.; Kohno, K.; Shiwaku, K.; et al. In vitro basophil activation is reduced by short-term omalizumab treatment in hydrolyzed wheat protein allergy. Allergol. Int. 2020, 69, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Badina, L.; Belluzzi, B.; Contorno, S.; Bossini, B.; Benelli, E.; Barbi, E.; Berti, I. Omalizumab effectiveness in patients with a previously failed oral immunotherapy for severe milk allergy. Immun. Inflamm. Dis. 2022, 10, 117–120. [Google Scholar] [CrossRef]
- Andorf, S.; Purington, N.; Kumar, D.; Long, A.; O’Laughlin, K.L.; Sicherer, S.; Sampson, H.; Cianferoni, A.; Whitehorn, T.B.; Petroni, D.; et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. EClinicalMedicine 2019, 7, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.K.; Albuhairi, S.; Noh, E.; El-Khoury, K.; Rezaei, S.; Abdel-Gadir, A.; Umetsu, D.T.; Burke-Roberts, E.; LeBovidge, J.; Schneider, L.; et al. Long-Term Outcome of Peanut Oral Immunotherapy Facilitated Initially by Omalizumab. J. Allergy Clin. Immunol. Pract. 2019, 7, 451–461. [Google Scholar] [CrossRef]
- Brandstrom, J.; Vetander, M.; Sundqvist, A.C.; Lilja, G.; Johansson, S.G.O.; Melen, E.; Sverremark-Ekstrom, E.; Nopp, A.; Nilsson, C. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin. Exp. Allergy 2019, 49, 1328–1341. [Google Scholar] [CrossRef]
- Azzano, P.; Paquin, M.; Langlois, A.; Morin, C.; Parizeault, G.; Lacombe-Barrios, J.; Samaan, K.; Graham, F.; Paradis, L.; Des Roches, A.; et al. Determinants of omalizumab dose-related efficacy in oral immunotherapy: Evidence from a cohort of 181 patients. J. Allergy Clin. Immunol. 2021, 147, 233–243. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Y.; Gao, Y.; Sun, S.; Wu, R.; Wu, J. Research Progress on the Correlation between the Intestinal Microbiota and Food Allergy. Foods 2022, 11, 2913. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, G.; Wang, Y.; Wei, X.; Liu, H.; Zhang, L.; Zhang, L. A Review on Maternal and Infant Microbiota and Their Implications for the Prevention and Treatment of Allergic Diseases. Nutrients 2023, 15, 2483. [Google Scholar] [CrossRef]
- Zhang, J.; Su, H.; Li, Q.; Wu, H.; Liu, M.; Huang, J.; Zeng, M.; Zheng, Y.; Sun, X. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits beta-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog. 2017, 9, 11. [Google Scholar] [CrossRef]
- Aoki-Yoshida, A.; Yamada, K.; Hachimura, S.; Sashihara, T.; Ikegami, S.; Shimizu, M.; Totsuka, M. Enhancement of Oral Tolerance Induction in DO11.10 Mice by Lactobacillus gasseri OLL2809 via Increase of Effector Regulatory T Cells. PLoS ONE 2016, 11, e0158643. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the effect of probiotics supplementation on intestinal microbiota of food allergic mice. Am. J. Transl. Res. 2017, 9, 376–385. [Google Scholar]
- Tian, X.; Liang, X.; He, H.; Cui, Q.; Liu, Q.; Fan, R.; Liu, T.; Yi, H.; Gong, P.; Wang, Q.; et al. Probiotics Alleviate Food Protein Allergy in Mice by Activating TLR4 Signaling Pathway. Mol. Nutr. Food Res. 2023, 67, e2200579. [Google Scholar] [CrossRef] [PubMed]
- Neau, E.; Delannoy, J.; Marion, C.; Cottart, C.H.; Labellie, C.; Holowacz, S.; Butel, M.J.; Kapel, N.; Waligora-Dupriet, A.J. Three Novel Candidate Probiotic Strains with Prophylactic Properties in a Murine Model of Cow’s Milk Allergy. Appl. Environ. Microbiol. 2016, 82, 1722–1733. [Google Scholar] [CrossRef]
- Miranda, V.C.; Souza, R.O.; Quintanilha, M.F.; Gallotti, B.; Assis, H.C.; Faria, A.M.C.; Nicoli, J.R.; Cara, D.C.; Martins, F.S. A Next-Generation Bacteria (Akkermansia muciniphila BAA-835) Presents Probiotic Potential Against Ovalbumin-Induced Food Allergy in Mice. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Ponsonby, A.L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J. Allergy Clin. Immunol. 2015, 135, 737–744.e8. [Google Scholar] [CrossRef] [PubMed]
- Dunn Galvin, A.; Lloyd, M.; Hsiao, K.C.; Tang, M.L.K. Long-term benefit of probiotic peanut oral immunotherapy on quality of life in a randomized trial. J. Allergy Clin. Immunol. Pract. 2021, 9, 4493–4495.E1. [Google Scholar] [CrossRef]
- Loke, P.; Orsini, F.; Lozinsky, A.C.; Gold, M.; O’Sullivan, M.D.; Quinn, P.; Lloyd, M.; Ashley, S.E.; Pitkin, S.; Axelrad, C.; et al. Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): A multicentre, randomised, phase 2b trial. Lancet Child Adolesc. Health 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Sato, M.; Toyokuni, K.; Irahara, M.; Hiraide-Kotaki, E.; Harima-Mizusawa, N.; Morita, H.; Matsumoto, K.; Ohya, Y. Combination of heat-killed Lactiplantibacillus plantarum YIT 0132 (LP0132) and oral immunotherapy in cow’s milk allergy: A randomised controlled trial. Benef. Microbes 2023, 14, 17–29. [Google Scholar] [CrossRef]
- Fox, A.T.; Wopereis, H.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Candy, D.C.A.; Shah, N.; West, C.E.; et al. A specific synbiotic-containing amino acid-based formula in dietary management of cow’s milk allergy: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 5. [Google Scholar] [CrossRef]
- Candy, D.C.A.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; et al. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr. Res. 2018, 83, 677–686. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Atwal, K.; Graham, L.; Narayanan, S.; Cooke, L.; Casewell, C.; Denton, S.A.; Gavin, J.; Browne, R.M.; Kinnear, F.J.; et al. Synbiotic containing extensively hydrolyzed formula improves gastrointestinal and atopic symptom severity, growth, caregiver quality of life, and hospital-related healthcare use in infants with cow’s milk allergy. Immun. Inflamm. Dis. 2022, 10, e636. [Google Scholar] [CrossRef]
- Wang, J.; Wood, R.A.; Raymond, S.; Suarez-Farinas, M.; Yang, N.; Sicherer, S.H.; Sampson, H.A.; Li, X.M. Double-Blind, Placebo-Controlled Study of E-B-FAHF-2 in Combination with Omalizumab-Facilitated Multiallergen Oral Immunotherapy. J. Allergy Clin. Immunol. Pract. 2023, 11, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.Z.; Geliebter, J.; Tiwari, R.; Li, X.M. Traditional Chinese medicine for food allergy and eczema. Ann. Allergy Asthma Immunol. 2021, 126, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Maskey, A.R.; Srivastava, K.; Kim, M.; Wang, Z.; Musa, I.; Shi, Y.; Gong, Y.; Fidan, O.; Wang, J.; et al. Inhibition of pathologic immunoglobulin E in food allergy by EBF-2 and active compound berberine associated with immunometabolism regulation. Front. Immunol. 2023, 14, 1081121. [Google Scholar] [CrossRef]
- Cao, T.W.; Xie, C.L.; Chen, C.Q.; He, Z.H.; Yan, Q.X.; Xu, G.; Yang, X.W. Anti-Food Allergic Alkaloids from the Lotus Seed Pot. Chem. Biodivers. 2021, 18, e2100770. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Y.; Ma, L.; Guo, Z.; Xiao, Y.; Liu, Y.; Li, J.; Wang, S.; Liu, Y. Oleuropein Prevents OVA-Induced Food Allergy in Mice by Enhancing the Intestinal Epithelial Barrier and Remodeling the Intestinal Flora. Mol. Nutr. Food Res. 2022, 66, e2200455. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nagata, Y.; Hayashi, S.; Kadowaki, M. Isoflavones Suppress Cyp26b1 Expression in the Murine Colonic Lamina Propria. Biol. Pharm. Bull. 2020, 43, 1945–1949. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.; Groetch, M.; Vassilopoulou, E.; Meyer, R.; Laitinen, K.; Swain, A.; Durban, R.; Benjamin, O.; Bottse, R.; Grimshaw, K.; et al. The immune-supportive diet in allergy management: A narrative review and proposal. Allergy 2023, 78, 1441–1458. [Google Scholar] [CrossRef]
- Nagata, Y.; Yamamoto, T.; Kadowaki, M. Ginger Increases ALDH1A1 Expression and Enhances Retinoic Acid Signaling in a Human Colonic Epithelial Cell Line. J. Nutr. Sci. Vitaminol. 2020, 66, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Guo, Y.; Ma, L.; Liu, Y.; Kuang, H.; Han, B.; Xiao, Y.; Wang, Y. Dietary olive oil enhances the oral tolerance of the food allergen ovalbumin in mice by regulating intestinal microecological homeostasis. J. Food Biochem. 2022, 46, e14297. [Google Scholar] [CrossRef]
- Patel, D.; Goruk, S.; Richard, C.; Field, C.J. Combined Supplementation with Arachidonic and Docosahexaenoic Acids in T Helper Type-2 Skewed Brown Norway Rat Offspring is Beneficial in the Induction of Oral Tolerance toward Ovalbumin and Immune System Development. J. Nutr. 2022, 152, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.B.P.; Nguyen, N.N.; Fan, H.Y.; Huang, S.Y.; Huang, C.H.; Chen, Y.C. Maternal Omega-3 Supplementation during Pregnancy, but not Childhood Supplementation, Reduces the Risk of Food Allergy Diseases in Offspring. J. Allergy Clin. Immunol. Pract. 2023, in press. [CrossRef]
- Deng, M.; Wu, X.; Duan, X.; Xu, J.; Yang, X.; Sheng, X.; Lou, P.; Shao, C.; Lv, C.; Yu, Z. Lactobacillus paracasei L9 improves colitis by expanding butyrate-producing bacteria that inhibit the IL-6/STAT3 signaling pathway. Food Funct. 2021, 12, 10700–10713. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Lopes, M.F.P.; Lamosa, P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brasal-Prieto, M.; Fernández-Prades, L.; Dakhaoui, H.; Sobrino, F.; López-Enríquez, S.; Palomares, F. Update on In Vitro Diagnostic Tools and Treatments for Food Allergies. Nutrients 2023, 15, 3744. https://doi.org/10.3390/nu15173744
Brasal-Prieto M, Fernández-Prades L, Dakhaoui H, Sobrino F, López-Enríquez S, Palomares F. Update on In Vitro Diagnostic Tools and Treatments for Food Allergies. Nutrients. 2023; 15(17):3744. https://doi.org/10.3390/nu15173744
Chicago/Turabian StyleBrasal-Prieto, Mariano, Laura Fernández-Prades, Hala Dakhaoui, Francisco Sobrino, Soledad López-Enríquez, and Francisca Palomares. 2023. "Update on In Vitro Diagnostic Tools and Treatments for Food Allergies" Nutrients 15, no. 17: 3744. https://doi.org/10.3390/nu15173744





