Consumption of Different Egg-Based Diets Alters Clinical Metabolic and Hematological Parameters in Young, Healthy Men and Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
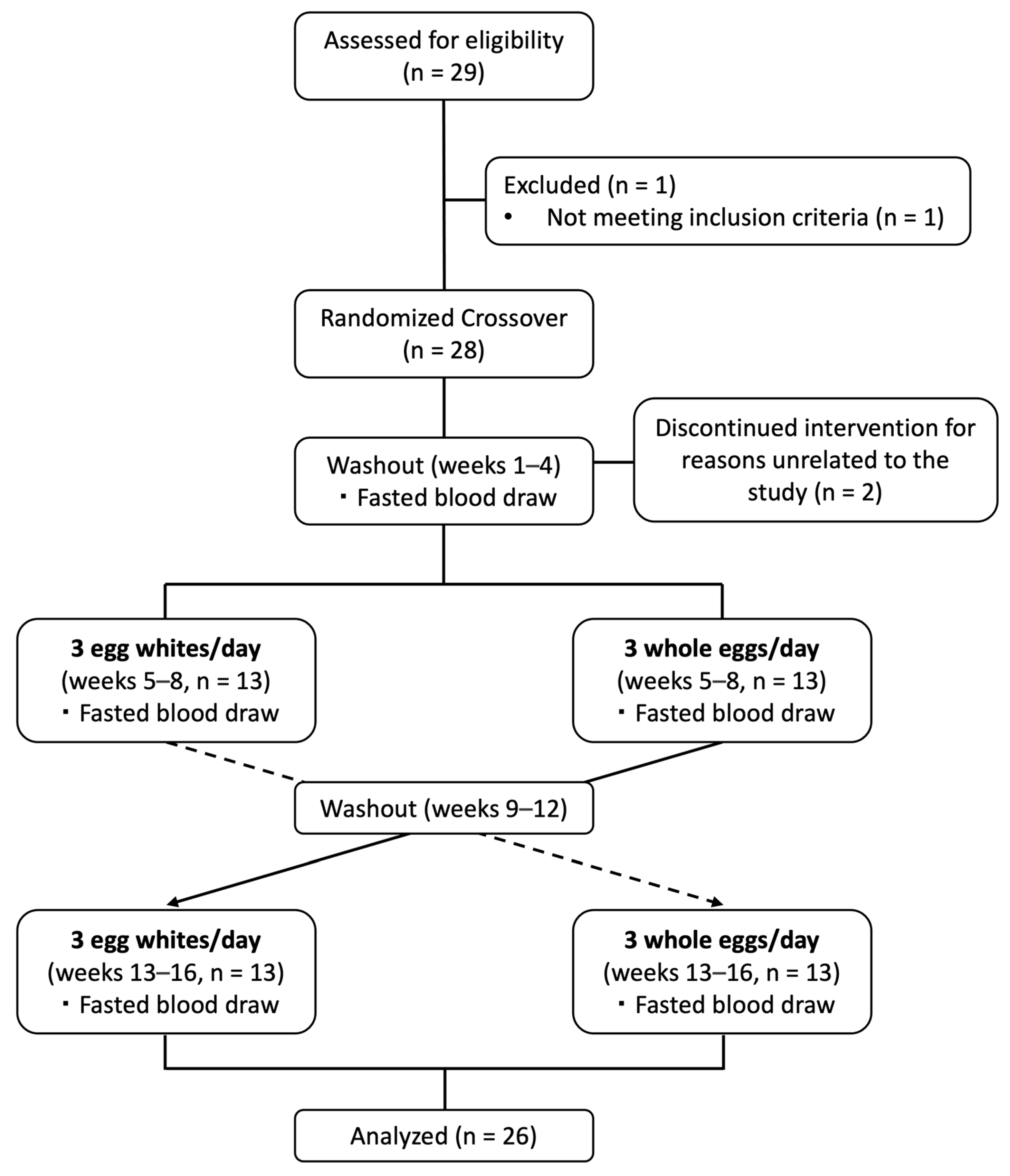
2.2. Study Design and Dietary Intervention
2.3. Dietary Intake Analysis
2.4. Body Composition Analysis
2.5. Blood Collection
2.6. Clinical Metabolic Parameters and Measures of Choline Status
2.7. Lipoprotein Size Profiles
2.8. Serum Amino Acid and Insulin Resistance Measures
2.9. Complete Blood Cell Counts
2.10. Statistical Analysis
3. Results
3.1. Average Daily Nutrient Intake Differed across Egg Diet Periods
3.2. Effect of Egg Intake on Body Composition and Clinical Metabolic Profiles
3.3. Effects of Egg Intake on Serum Lipid Profiles
3.4. Effects of Egg Intake on Lipoprotein Particle Profiles
3.5. Effects of Egg Intake on Markers of Insulin Sensitivity
3.6. Effects of Egg Intake on Clinical Immune Profiles
3.7. Effects of Egg Intake on Clinical Erythrocyte and Platelet Profiles
3.8. Egg-Induced Changes in Lipoprotein and Glucose Sensitivity Measures Differentially Correlate with Changes in Immune Cell Subset Counts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, C.J. Bioactive Egg Components and Inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Andersen, C.J. The good egg, the forgotten benefits: Protein, carotenoids, choline and glycemic index. In Handbook of Eggs in Human Function; Watson, R.R., De Meester, F., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; Volume 9, pp. 17–34. [Google Scholar]
- Blesso, C.N.; Andersen, C.J.; Fernandez, M.L. Eggs Effects on HDL-C Metabolism, Inflammation, and Insulin Resistance. In Nutritional Intervention in Metabolic Syndrome; Dichi, I., Colado Simao, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Andersen, C.J. Eggs: Composition and Health Effects. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 2, pp. 470–475. [Google Scholar]
- Rehault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.W.; Fernandez, M.L. Egg intake improves carotenoid status by increasing plasma HDL cholesterol in adults with metabolic syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am. J. Clin. Nutr. 2010, 91, 1095S–1101S. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Fulgoni, V.L., 3rd. Patterns of Egg Consumption Can Help Contribute to Nutrient Recommendations and Are Associated with Diet Quality and Shortfall Nutrient Intakes. Nutrients 2021, 13, 4094. [Google Scholar] [CrossRef]
- Andersen, C.J.; Ragonesi, N.; Cintron-Rivera, L.; Murray, K.; Cerrito, B.; Melville, J.; McCabe, M. Food Pantry Inventories Vary in Food Group Availability, Diversity, and Nutritional Composition Across City Districts: A Pilot Study. J. Hunger Environ. Nutr. 2022. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metab. Clin. Exp. 2013, 62, 400–410. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of Carbohydrate Restriction and Dietary Cholesterol Provided by Eggs on Clinical Risk Factors in Metabolic Syndrome. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Blesso, C.N.; Lee, J.; Barona, J.; Shah, D.; Thomas, M.J.; Fernandez, M.L. Egg Consumption Modulates HDL Lipid Composition and Increases the Cholesterol-Accepting Capacity of Serum in Metabolic Syndrome. Lipids 2013, 48, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Missimer, A.; DiMarco, D.; Murillo, G.; Creighton, B.; Andersen, C.J.; Ketzmer, R.; Fernandez, M.L. Intake of 2 Eggs or Oatmeal for Breakfast does not Increase Biomarkers for Heart Disease while Eggs improve Liver Enzymes and raise HDL Cholesterol in Young Healthy Individuals. J. Fed. Am. Soc. Exp. Biol. 2015, 29, 274.2. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Chen, S.; Li, Y.; Schwab, A.L.; Stampfer, M.J.; Sacks, F.M.; Rosner, B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Egg consumption and risk of cardiovascular disease: Three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ 2020, 368, m513. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Egg consumption and risk of type 2 diabetes in men and women. Diabetes Care 2009, 32, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Little, M.; Zivot, C.; Humphries, S.; Dodd, W.; Patel, K.; Dewey, C. Burden and Determinants of Anemia in a Rural Population in South India: A Cross-Sectional Study. Anemia 2018, 2018, 7123976. [Google Scholar] [CrossRef]
- Blesso, C.N.; Fernandez, M.L. Dietary Cholesterol, Serum Lipids, and Heart Disease: Are Eggs Working for or Against You? Nutrients 2018, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Corbin, K.D.; da Costa, K.A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef]
- DiMarco, D.M.; Missimer, A.; Murillo, A.G.; Lemos, B.S.; Malysheva, O.V.; Caudill, M.A.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids 2017, 52, 255–263. [Google Scholar] [CrossRef]
- Li, D.; Lu, Y.; Yuan, S.; Cai, X.; He, Y.; Chen, J.; Wu, Q.; He, D.; Fang, A.; Bo, Y.; et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: An umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 2022, 116, 230–243. [Google Scholar] [CrossRef]
- Godos, J.; Micek, A.; Brzostek, T.; Toledo, E.; Iacoviello, L.; Astrup, A.; Franco, O.H.; Galvano, F.; Martinez-Gonzalez, M.A.; Grosso, G. Egg consumption and cardiovascular risk: A dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2021, 60, 1833–1862. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J. Egg consumption is associated with a lower risk of type 2 diabetes in middle-aged and older men. Nutr. Res. Pract. 2018, 12, 396–405. [Google Scholar] [CrossRef]
- Missimer, A.; DiMarco, D.M.; Andersen, C.J.; Murillo, A.G.; Vergara-Jimenez, M.; Fernandez, M.L. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin while Maintaining the LDL/HDL Ratio. Nutrients 2017, 9, 89. [Google Scholar] [CrossRef]
- Ding, C.; Egli, L.; Bosco, N.; Sun, L.; Goh, H.J.; Yeo, K.K.; Yap, J.J.L.; Actis-Goretta, L.; Leow, M.K.; Magkos, F. Plasma Branched-Chain Amino Acids Are Associated With Greater Fasting and Postprandial Insulin Secretion in Non-diabetic Chinese Adults. Front. Nutr. 2021, 8, 664939. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Miguel, M.; Mesas, A.E.; Fernandez-Rodriguez, R.; Fernandez-Franco, S.; Pozuelo-Carrascosa, D.P.; Lopez-Gil, J.F.; Martinez-Vizcaino, V. The role of protein intake in the relationship between egg consumption and body composition in young adults. A mediation analysis. Clin. Nutr. 2022, 41, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.E.; Seo, Y.G. Relationship between Egg Consumption and Body Composition as Well as Serum Cholesterol Level: Korea National Health and Nutrition Examination Survey 2008–2011. J. Clin. Med. 2021, 10, 5918. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Ross, G.W.; Chen, R.; Abbott, R.D.; Bell, C.; Willcox, B.; Launer, L.; Petrovitch, H.; Kaya, B.; Masaki, K. Total and differential white blood cell counts in late life predict 8-year incident stroke: The Honolulu Heart Program. J. Am. Geriatr. Soc. 2015, 63, 439–446. [Google Scholar] [CrossRef]
- Jee, S.H.; Park, J.Y.; Kim, H.S.; Lee, T.Y.; Samet, J.M. White blood cell count and risk for all-cause, cardiovascular, and cancer mortality in a cohort of Koreans. Am. J. Epidemiol. 2005, 162, 1062–1069. [Google Scholar] [CrossRef]
- Andersen, C.J.; Lee, J.Y.; Blesso, C.N.; Carr, T.P.; Fernandez, M.L. Egg intake during carbohydrate restriction alters peripheral blood mononuclear cell inflammation and cholesterol homeostasis in metabolic syndrome. Nutrients 2014, 6, 2650–2667. [Google Scholar] [CrossRef]
- Perez-Guzman, C.; Vargas, M.H.; Quinonez, F.; Bazavilvazo, N.; Aguilar, A. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005, 127, 643–651. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Zhang, X.; Zeng, Q.; Huang, Q.; Sheng, L.; Ahn, D.U.; Ciai, Z. Restoration of immunity by whole egg was superior to egg white or egg yolk in a cyclophosphamide-induced immunocompromised mouse model. Food Biosci. 2022, 50, 102013. [Google Scholar] [CrossRef]
- Azarcoya-Barrera, J.; Wollin, B.; Veida-Silva, H.; Makarowski, A.; Goruk, S.; Field, C.J.; Jacobs, R.L.; Richard, C. Egg-Phosphatidylcholine Attenuates T-Cell Dysfunction in High-Fat Diet Fed Male Wistar Rats. Front. Nutr. 2022, 9, 811469. [Google Scholar] [CrossRef]
- Asare, G.A.; Santa, S.; Ngala, R.A.; Asiedu, B.; Afriyie, D.; Amoah, A.G. Effect of hormonal contraceptives on lipid profile and the risk indices for cardiovascular disease in a Ghanaian community. Int. J. Womens Health 2014, 6, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Endrikat, J.; Klipping, C.; Cronin, M.; Gerlinger, C.; Ruebig, A.; Schmidt, W.; Dusterberg, B. An open label, comparative study of the effects of a dose-reduced oral contraceptive containing 20 microg ethinyl estradiol and 100 microg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception 2002, 65, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wynn, V.; Niththyananthan, R. The effect of progestins in combined oral contraceptives on serum lipids with special reference to high-density lipoproteins. Am. J. Obstet. Gynecol. 1982, 142, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Tekle, E.; Gelaw, Y.; Asrie, F. Hematological Profile Changes among Oral Contraceptive Users: A Narrative Review. J. Blood Med. 2022, 13, 525–536. [Google Scholar] [CrossRef]
- Fooddata Central [Internet]. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 2 July 2023).
- Lorenzo, C.; Festa, A.; Hanley, A.J.; Rewers, M.J.; Escalante, A.; Haffner, S.M. Novel Protein Glycan-Derived Markers of Systemic Inflammation and C-Reactive Protein in Relation to Glycemia, Insulin Resistance, and Insulin Secretion. Diabetes Care 2017, 40, 375–382. [Google Scholar] [CrossRef]
- Kim, S.; Eliot, M.; Koestler, D.C.; Wu, W.C.; Kelsey, K.T. Association of Neutrophil-to-Lymphocyte Ratio With Mortality and Cardiovascular Disease in the Jackson Heart Study and Modification by the Duffy Antigen Variant. JAMA Cardiol. 2018, 3, 455–462. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, T.; Zheng, E.; Deng, X.; Wang, Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: An up-to-date meta-analysis. Medicine (Baltim.) 2017, 96, e7051. [Google Scholar] [CrossRef]
- Oh, S.Y.; Miller, L.T. Effect of Dietary Egg on Variability of Plasma Cholesterol Levels and Lipoprotein Cholesterol. Am. J. Clin. Nutr. 1985, 42, 421–431. [Google Scholar] [CrossRef]
- Djousse, L.; Kamineni, A.; Nelson, T.L.; Carnethon, M.; Mozaffarian, D.; Siscovick, D.; Mukamal, K.J. Egg consumption and risk of type 2 diabetes in older adults. Am. J. Clin. Nutr. 2010, 92, 422–427. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Schwab, A.L.; Chen, S.; Li, Y.; Sacks, F.M.; Rosner, B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; et al. Egg consumption and risk of type 2 diabetes: Findings from 3 large US cohort studies of men and women and a systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 112, 619–630. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Andersen, C.J. Effects of dietary cholesterol in diabetes and cardiovascular disease. Clin. Lipidol. 2014, 9, 607–616. [Google Scholar] [CrossRef]
- Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients 2015, 7, 3449–3463. [Google Scholar] [CrossRef] [PubMed]
- Shalaurova, I.; Connelly, M.A.; Garvey, W.T.; Otvos, J.D. Lipoprotein insulin resistance index: A lipoprotein particle-derived measure of insulin resistance. Metab. Syndr. Relat. Disord. 2014, 12, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Gruppen, E.G.; Connelly, M.A.; Shalaurova, I.; Otvos, J.D.; Garcia, E.; Bakker, S.J.L.; Dullaart, R.P.F. A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. J. Clin. Med. 2020, 9, 2781. [Google Scholar] [CrossRef]
- DiBella, M.; Thomas, M.S.; Alyousef, H.; Millar, C.; Blesso, C.; Malysheva, O.; Caudill, M.A.; Fernandez, M.L. Choline Intake as Supplement or as a Component of Eggs Increases Plasma Choline and Reduces Interleukin-6 without Modifying Plasma Cholesterol in Participants with Metabolic Syndrome. Nutrients 2020, 12, 3120. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Okba, A.M.; Amin, M.M.; Abdelmoaty, A.S.; Ebada, H.E.; Kamel, A.H.; Allam, A.S.; Sobhy, O.M. Neutrophil/lymphocyte ratio and lymphocyte/monocyte ratio in ulcerative colitis as non-invasive biomarkers of disease activity and severity. Auto. Immun. Highlights 2019, 10, 4. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-kappaB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef]
- Andersen, C.J. Impact of Dietary Cholesterol on the Pathophysiology of Infectious and Autoimmune Disease. Nutrients 2018, 10, 764. [Google Scholar] [CrossRef]
- Andersen, C.J.; Dupree, L.; Murray, K.; Ragonesi, N.; McMullen, K.; Cintron-Rivera, L.; Doerr, A. Low-Density Lipoproteins, High-Density Lipoproteins (HDL), and HDL-Associated Proteins Differentially Modulate Chronic Myelogenous Leukemia Cell Viability. Lipids 2020, 55, 615–626. [Google Scholar] [CrossRef]
- Meurs, I.; Hoekstra, M.; van Wanrooij, E.J.; Hildebrand, R.B.; Kuiper, J.; Kuipers, F.; Hardeman, M.R.; Van Berkel, T.J.; Van Eck, M. HDL cholesterol levels are an important factor for determining the lifespan of erythrocytes. Exp. Hematol. 2005, 33, 1309–1319. [Google Scholar] [CrossRef]
- Andersen, C.J.; Fernandez, M.L. Dietary strategies to reduce metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.C.; Andersen, C.J. Assessment of Dietary Patterns Represents a Potential, Yet Variable, Measure of Inflammatory Status: A Review and Update. Dis. Mark. 2019, 2019, 3102870. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Fulgoni, V.L., 3rd. Egg Consumption in U.S. Children is Associated with Greater Daily Nutrient Intakes, including Protein, Lutein + Zeaxanthin, Choline, alpha-Linolenic Acid, and Docosahexanoic Acid. Nutrients 2019, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, J.; Leite, J.O.; de Ogburn, R.; Puglisi, M.J.; VanHeest, J.; Fernandez, M.L. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men. Nutr. Res. 2010, 30, 96–103. [Google Scholar] [CrossRef]
- B Keogh, J.; M Clifton, P. Energy Intake and Satiety Responses of Eggs for Breakfast in Overweight and Obese Adults-A Crossover Study. Int. J. Environ. Res. Public Health 2020, 17, 5583. [Google Scholar] [CrossRef]
- Melough, M.M.; Chung, S.J.; Fernandez, M.L.; Chun, O.K. Association of eggs with dietary nutrient adequacy and cardiovascular risk factors in US adults. Public Health Nutr. 2019, 22, 2033–2042. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, J.V.; Schooling, C.M. The associations of plasma phospholipid arachidonic acid with cardiovascular diseases: A Mendelian randomization study. EBioMedicine 2021, 63, 103189. [Google Scholar] [CrossRef]
- Saande, C.J.; Webb, J.L.; Curry, P.E.; Rowling, M.J.; Schalinske, K.L. Dietary Whole Egg Reduces Body Weight Gain in a Dose-Dependent Manner in Zucker Diabetic Fatty Rats. J. Nutr. 2019, 149, 1766–1775. [Google Scholar] [CrossRef]
- Saande, C.J.; Bries, A.E.; Pritchard, S.K.; Nass, C.A.; Reed, C.H.; Rowling, M.J.; Schalinske, K.L. Whole Egg Consumption Decreases Cumulative Weight Gain in Diet-Induced Obese Rats. J. Nutr. 2020, 150, 1818–1823. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Li, Q.; Dang, S.; Yan, H. Body Fat Mass, Fat Distribution and Egg Consumption: A Population-Based Study in Chinese Adults. J. Am. Coll. Nutr. 2020, 39, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeyan, A.; Lotfi, K.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Saneei, P. The association between egg consumption and metabolic health status in overweight and obese adolescents. Sci. Rep. 2023, 13, 2778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef]
- Smolders, L.; de Wit, N.J.W.; Balvers, M.G.J.; Obeid, R.; Vissers, M.M.M.; Esser, D. Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of A Randomized Trial in Healthy Adults. Nutrients 2019, 11, 2758. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L. Usual Choline Intakes Are Associated with Egg and Protein Food Consumption in the United States. Nutrients 2017, 9, 839. [Google Scholar] [CrossRef]
- Institute of Medicine; Food and Nutrition Board; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline; Subcommittee on Upper Reference Levels of Nutrients. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998.
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 37, 716–723. [Google Scholar] [CrossRef]
- Missimer, A.; Fernandez, M.L.; DiMarco, D.M.; Norris, G.H.; Blesso, C.N.; Murillo, A.G.; Vergara-Jimenez, M.; Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; et al. Compared to an Oatmeal Breakfast, Two Eggs/Day Increased Plasma Carotenoids and Choline without Increasing Trimethyl Amine N-Oxide Concentrations. J. Am. Coll. Nutr. 2018, 37, 140–148. [Google Scholar] [CrossRef]
- Thomas, M.S.; DiBella, M.; Blesso, C.N.; Malysheva, O.; Caudill, M.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients 2022, 14, 1179. [Google Scholar] [CrossRef] [PubMed]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef]
- Finelli, C.; Crispino, P.; Gioia, S.; La Sala, N.; D’Amico, L.; La Grotta, M.; Miro, O.; Colarusso, D. The improvement of large High-Density Lipoprotein (HDL) particle levels, and presumably HDL metabolism, depend on effects of low-carbohydrate diet and weight loss. EXCLI J. 2016, 15, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.S.; Panzoldo, N.B.; Zago, V.H.; Scherrer, D.Z.; Alexandre, F.; Bakkarat, J.; Nunes, V.S.; Nakandakare, E.R.; Quintao, E.C.; Nadruz, W., Jr.; et al. HDL size is more accurate than HDL cholesterol to predict carotid subclinical atherosclerosis in individuals classified as low cardiovascular risk. PLoS ONE 2014, 9, e114212. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Waters, D.; Clark, R.M.; Contois, J.H.; Fernandez, M.L. Plasma LDL and HDL characteristics and carotenoid content are positively influenced by egg consumption in an elderly population. Nutr. Metab. 2006, 3, 6. [Google Scholar] [CrossRef]
- Mutungi, G.; Ratliff, J.; Puglisi, M.; Torres-Gonzalez, M.; Vaishnav, U.; Leite, J.O.; Quann, E.; Volek, J.S.; Fernandez, M.L. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J. Nutr. 2008, 138, 272–276. [Google Scholar] [CrossRef]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef]
- Blesso, C.N. Egg phospholipids and cardiovascular health. Nutrients 2015, 7, 2731–2747. [Google Scholar] [CrossRef]
- Didichenko, S.A.; Navdaev, A.V.; Cukier, A.M.; Gille, A.; Schuetz, P.; Spycher, M.O.; Therond, P.; Chapman, M.J.; Kontush, A.; Wright, S.D. Enhanced HDL Functionality in Small HDL Species Produced Upon Remodeling of HDL by Reconstituted HDL, CSL112: Effects on Cholesterol Efflux, Anti-Inflammatory and Antioxidative Activity. Circ. Res. 2016, 119, 751–763. [Google Scholar] [CrossRef]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, Dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Mutharasan, R.K.; Thaxton, C.S.; Berry, J.; Daviglus, M.L.; Yuan, C.; Sun, J.; Ayers, C.; Lloyd-Jones, D.M.; Wilkins, J.T. HDL efflux capacity, HDL particle size, and high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: The Chicago Healthy Aging Study. J. Lipid Res. 2017, 58, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Momeni, Z.; Dehghani, A.; Fallahzadeh, H.; Koohgardi, M.; Dafei, M.; Hekmatimoghaddam, S.H.; Mohammadi, M. The impacts of pill contraceptive low-dose on plasma levels of nitric oxide, homocysteine, and lipid profiles in the exposed vs. non exposed women: As the risk factor for cardiovascular diseases. Contracept. Reprod. Med. 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Amigo, N.; Tellez-Plaza, M.; Pardo-Cea, M.A.; Dominguez-Lucas, A.; Marrachelli, V.G.; Monleon, D.; Martin-Escudero, J.C.; Ascaso, J.F.; Chaves, F.J.; et al. LDL particle size and composition and incident cardiovascular disease in a South-European population: The Hortega-Liposcale Follow-up Study. Int. J. Cardiol. 2018, 264, 172–178. [Google Scholar] [CrossRef]
- Daniels, K.; Abma, J.C. Current Contraceptive Status among Women Aged 15–49: United States, 2017–2019; NCHS Data Brief, No 388; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Guo, J.; Hobbs, D.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Lovegrove, J.A.; Givens, D.I. Association between egg consumption and cardiovascular disease events, diabetes and all-cause mortality. Eur. J. Nutr. 2018, 57, 2943–2952. [Google Scholar] [CrossRef]
- Hernandez-Alvarez, M.I.; Diaz-Ramos, A.; Berdasco, M.; Cobb, J.; Planet, E.; Cooper, D.; Pazderska, A.; Wanic, K.; O’Hanlon, D.; Gomez, A.; et al. Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Sci. Rep. 2017, 7, 13850. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef]
- Glynn, E.L.; Piner, L.W.; Huffman, K.M.; Slentz, C.A.; Elliot-Penry, L.; AbouAssi, H.; White, P.J.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 2015, 58, 2324–2335. [Google Scholar] [CrossRef]
- Sajadi Hezaveh, Z.; Sikaroudi, M.K.; Vafa, M.; Clayton, Z.S.; Soltani, S. Effect of egg consumption on inflammatory markers: A systematic review and meta-analysis of randomized controlled clinical trials. J. Sci. Food Agric. 2019, 99, 6663–6670. [Google Scholar] [CrossRef]
- Tannock, L.R.; O’Brien, K.D.; Knopp, R.H.; Retzlaff, B.; Fish, B.; Wener, M.H.; Kahn, S.E.; Chait, A. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation 2005, 111, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, J.C.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr. Metab. 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Kolobaric, N.; Drenjancevic, I.; Matic, A.; Susnjara, P.; Mihaljevic, Z.; Mihalj, M. Dietary Intake of n-3 PUFA-Enriched Hen Eggs Changes Inflammatory Markers’ Concentration and Treg/Th17 Cells Distribution in Blood of Young Healthy Adults—A Randomised Study. Nutrients 2021, 13, 1851. [Google Scholar] [CrossRef]
- Andersen, C.J.; Vance, T.M. Gender Dictates the Relationship between Serum Lipids and Leukocyte Counts in the National Health and Nutrition Examination Survey 1999–2004. J. Clin. Med. 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Vance, T.M. Sex-Specific Associations Between Serum Lipids, Antinuclear Antibodies, and Statin Use in National Health and Nutrition Examination Surveys 1999–2004. Front. Med. 2022, 9, 887741. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Gong, S.; Gao, X.; Xu, F.; Shang, Z.; Li, S.; Chen, W.; Yang, J.; Li, J. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine (Baltim.) 2018, 97, e12813. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Cauci, S.; Di Santolo, M.; Culhane, J.F.; Stel, G.; Gonano, F.; Guaschino, S. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obs. Gynecol. 2008, 111, 857–864. [Google Scholar] [CrossRef]
- Divani, A.A.; Luo, X.; Datta, Y.H.; Flaherty, J.D.; Panoskaltsis-Mortari, A. Effect of oral and vaginal hormonal contraceptives on inflammatory blood biomarkers. Mediat. Inflamm. 2015, 2015, 379501. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.J.; Park, B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine (Baltim.) 2019, 98, e15340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Yuan, S.G.; Peng, D.Q.; Zhao, S.P. High-density lipoprotein affects antigen presentation by interfering with lipid raft: A promising anti-atherogenic strategy. Clin. Exp. Immunol. 2010, 160, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Vyletelova, V.; Novakova, M.; Paskova, L. Alterations of HDL’s to piHDL’s Proteome in Patients with Chronic Inflammatory Diseases, and HDL-Targeted Therapies. Pharmaceuticals 2022, 15, 1278. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Fernandez, M.L. DIetary approaches to improving atheroprotective HDL functions. Food Funct. 2013, 4, 1304–1313. [Google Scholar] [CrossRef]
- Rueda, C.M.; Rodriguez-Perea, A.L.; Moreno-Fernandez, M.; Jackson, C.M.; Melchior, J.T.; Davidson, W.S.; Chougnet, C.A. High density lipoproteins selectively promote the survival of human regulatory T cells. J. Lipid Res. 2017, 58, 1514–1523. [Google Scholar] [CrossRef]
- Souza Junior, D.R.; Silva, A.R.M.; Rosa-Fernandes, L.; Reis, L.R.; Alexandria, G.; Bhosale, S.D.; Ghilardi, F.R.; Dalcoquio, T.F.; Bertolin, A.J.; Nicolau, J.C.; et al. HDL proteome remodeling associates with COVID-19 severity. J. Clin. Lipidol. 2021, 15, 796–804. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, H.; Zhao, Y.; Luo, X.; Gao, W. Role of platelet biomarkers in inflammatory response. Biomark. Res. 2020, 8, 28. [Google Scholar] [CrossRef]
- Vinholt, P.J.; Hvas, A.M.; Frederiksen, H.; Bathum, L.; Jorgensen, M.K.; Nybo, M. Platelet count is associated with cardiovascular disease, cancer and mortality: A population-based cohort study. Thromb. Res. 2016, 148, 136–142. [Google Scholar] [CrossRef]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Nogueira-Pedro, A.; Santos, E.W.; Hastreiter, A.; Silva, G.B.; Borelli, P.; Fock, R.A. A review of select minerals influencing the haematopoietic process. Nutr. Res. Rev. 2018, 31, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Lynch, S.R.; Trinidad, T.P.; Dassenko, S.A.; Cook, J.D. Iron absorption in humans: Bovine serum albumin compared with beef muscle and egg white. Am. J. Clin. Nutr. 1988, 47, 102–107. [Google Scholar] [CrossRef]
- Hallberg, L.; Hulthen, L. Prediction of dietary iron absorption: An algorithm for calculating absorption and bioavailability of dietary iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef]
- Makrides, M.; Hawkes, J.S.; Neumann, M.A.; Gibson, R.A. Nutritional effect of including egg yolk in the weaning diet of breast-fed and formula-fed infants: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 75, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, H.; Zhang, W.G.; Lee, E.J.; Ahn, D.U. Egg yolk phosvitin and functional phosphopeptides—Review. J. Food Sci. 2011, 76, R143–R150. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Rupa, P.; Kovacs-Nolan, J.; Turner, P.V.; Matsui, T.; Mine, Y. Oral administration of hen egg white ovotransferrin attenuates the development of colitis induced by dextran sodium sulfate in mice. J. Agric. Food Chem. 2015, 63, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.J.; Chalmers, K.A.; Collins, C.E.; Patterson, A.J. The effect of nutrition knowledge and dietary iron intake on iron status in young women. Appetite 2014, 81, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rojo, R.; Baeza-Richer, C.; Lopez-Parra, A.M.; Perez-Granados, A.M.; Brichs, A.; Bertoncini, S.; Buil, A.; Arroyo-Pardo, E.; Soria, J.M.; Vaquero, M.P. Four variants in transferrin and HFE genes as potential markers of iron deficiency anaemia risk: An association study in menstruating women. Nutr. Metab. 2011, 8, 69. [Google Scholar] [CrossRef]
- Rigas, A.S.; Sorensen, C.J.; Pedersen, O.B.; Petersen, M.S.; Thorner, L.W.; Kotze, S.; Sorensen, E.; Magnussen, K.; Rostgaard, K.; Erikstrup, C.; et al. Predictors of iron levels in 14,737 Danish blood donors: Results from the Danish Blood Donor Study. Transfusion 2014, 54, 789–796. [Google Scholar] [CrossRef]
- Werner, E.R.; Arnold, C.D.; Caswell, B.L.; Iannotti, L.L.; Lutter, C.K.; Maleta, K.M.; Stewart, C.P. The Effects of 1 Egg per Day on Iron and Anemia Status among Young Malawian Children: A Secondary Analysis of a Randomized Controlled Trial. Curr. Dev. Nutr. 2022, 6, nzac094. [Google Scholar] [CrossRef]
- Engel, R.W. Anemia and edema of chronic choline deficiency in the rat. J. Nutr. 1948, 36, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Mesquita, R.; Martins, E.S.J.; Saldanha, C. Effects of choline on hemorheological properties and NO metabolism of human erythrocytes. Clin. Hemorheol. Microcirc. 2003, 29, 41–51. [Google Scholar] [PubMed]
- McEwen, B.J. The influence of diet and nutrients on platelet function. Semin. Thromb. Hemost. 2014, 40, 214–226. [Google Scholar] [CrossRef] [PubMed]





| Nutrient | Egg White Diet | Whole Egg Diet |
|---|---|---|
| Total energy, kcal | 75 1 | 215.7 |
| Total carbohydrates, g | 3 1 | 1.4 |
| Total protein, g | 15 1 | 18.7 |
| Total fat, g | 0 1 | 15 |
| Cholesterol, mg | 0 1 | 621 |
| Vitamin A, μg | 0 1 | 271.5 |
| Vitamin C, mg | 0 1 | 0 |
| Vitamin D, μg | 0 | 3.7 |
| Vitamin B2, mg | 0.4 | 0.6 |
| Choline, mg | 0 | 507 |
| Lutein + Zeaxanthin, μg | 0 | 693 |
| Selenium, μg | 18.2 | 46.8 |
| Sodium, mg | 225 1 | 194.7 |
| Zinc, mg | 0 | 1.9 |
| Iron, mg | 0 1 | 2.5 |
| Calcium, mg | 0 1 | 72.3 |
| Daily Intake Totals | Egg-Free Diet | Egg White Diet | Whole Egg Diet | p-Value |
|---|---|---|---|---|
| Total energy, kcal | 1948.4 ± 817.7 | 1837.0 ± 633.2 | 2069.3 ± 586.7 | 0.240 |
| Carbohydrates, % of kcal | 49.5 ± 7.0 a | 45.6 ± 6.7 b | 41.8 ± 7.7 c | <0.001 |
| Protein, % of kcal | 16.0 ± 4.4 | 18.3 ± 4.6 | 17.8 ± 3.9 | 0.062 |
| Fat, % of kcal | 32.2 ± 4.3 a | 34.8 ± 5.2 b | 39.4 ± 6.0 c | <0.001 |
| Total carbohydrates, g | 244.1 ± 109.6 | 209.9 ± 75.9 | 221.6 ± 80.8 | 0.201 |
| Total protein, g | 77.4 ± 36.7 | 79.2 ± 25.1 | 88.0 ± 32.6 | 0.215 |
| Animal protein, g | 45.6 ± 26.4 a | 52.4 ± 19.2 ab | 58.4 ± 23.3 b | 0.023 |
| Vegetable protein, g | 31.8 ± 15.3 | 26.7 ± 10.2 | 29.5 ± 19.6 | 0.333 |
| Alanine, g | 3.45 ± 1.64 | 3.80 ± 1.26 | 4.12 ±1.54 | 0.080 |
| Glycine, g | 3.06 ± 1.38 | 3.21 ± 1.22 | 3.42 ± 1.36 | 0.390 |
| Isoleucine, g | 3.44 ± 1.71 | 3.71 ± 1.14 | 4.08 ± 1.50 | 0.101 |
| Leucine, g | 5.96 ± 2.88 | 6.23 ± 1.90 | 6.90 ± 2.50 | 0.145 |
| Valine, g | 3.88 ± 1.86 | 4.22 ± 1.21 | 4.56 ± 1.59 | 0.150 |
| Total fat, g | 71.6 ± 31.5 a | 75.8 ± 33.5 a | 92.1 ± 25.9 b | 0.003 |
| Saturated fat, g | 24.1 ± 11.3 | 25.1 ± 13.1 | 29.5 ± 8.3 | 0.058 |
| Monounsaturated fat, g | 24.7 ± 10.9 a | 25.9 ± 12.7 a | 32.6 ± 9.8 b | 0.001 |
| Polyunsaturated fat, g | 16.7 ± 9.2 a | 18.8 ± 9.5 ab | 21.4 ± 9.6 b | 0.035 |
| Arachidonic acid, g | 0.12 ± 0.13 a | 0.11 ± 0.06 a | 0.29 ± 0.08 b | <0.001 |
| EPA, g | 0.03 ± 0.06 | 0.03 ± 0.05 | 0.04 ± 0.07 | 0.598 |
| DHA, g | 0.07 ± 0.13 | 0.06 ± 0.11 | 0.12 ± 0.21 | 0.245 |
| Alcohol, g | 6.1 ± 8.6 | 3.7 ± 6.1 | 4.0 ± 6.8 | 0.084 |
| Cholesterol, mg | 224.0 ± 286.6 a | 170.1 ± 89.4 a | 657.8 ± 125.1 b | <0.001 |
| Vitamin A, μg | 1001.1 ± 615.1 | 1036.5 ± 605.8 | 1156.1 ± 569.4 | 0.501 |
| Pantothenic acid, mg | 5.0 ± 2.5 ab | 4.2 ± 1.4 a | 5.9 ± 1.5 b | 0.001 |
| Vitamin B6, mg | 1.9 ± 0.8 | 1.7 ± 0.8 | 2.0 ± 1.3 | 0.377 |
| Folic acid, μg | 475.9 ± 270.7 | 385.4 ± 144.6 | 551.5 ± 667.2 | 0.298 |
| Vitamin B12, μg | 3.72 ± 2.7 ab | 3.0 ± 1.8 a | 4.8 ± 2.7 b | 0.016 |
| Vitamin C, mg | 88.2 ± 63.1 | 75.8 ± 42.0 | 90.0 ± 62.5 | 0.440 |
| Vitamin D, μg | 4.2 ± 4.4 ab | 3.1 ± 2.6 a | 6.1 ± 2.7 b | 0.006 |
| Vitamin E, mg α-tocopherol | 11.9 ± 8.7 | 10.5 ± 6.5 | 13.5 ± 7.0 | 0.239 |
| Vitamin K, μg | 107.8 ± 74.4 | 142.7 ± 81.2 | 141.7 ± 106.9 | 0.113 |
| Calcium, mg | 919.5 ± 471.8 | 795.9 ± 314.1 | 910.7 ± 355.4 | 0.293 |
| Copper, mg | 1.26 ± 0.65 | 1.12 ± 0.49 | 1.14 ± 0.59 | 0.216 |
| Iron, mg | 15.8 ± 8.0 | 12.0 ± 5.0 | 16.1 ± 14.1 | 0.204 |
| Magnesium, mg | 300.4 ± 140.2 | 260.0 ± 106.1 | 264.0 ± 125.0 | 0.075 |
| Phosphorus, mg | 1197.8 ± 510.9 ab | 1042.8 ± 375.1 a | 1304.5 ± 454.0 b | 0.020 |
| Selenium, μg | 111.4 ± 61.3 a | 117.4 ± 36.0 a | 143.1 ± 43.8 b | 0.017 |
| Sodium, mg | 3170.7 ± 1146.4 a | 3387.0 ± 1238.6 ab | 3862.5 ± 1328.8 b | 0.032 |
| Zinc, mg | 10.4 ± 4.4 | 8.8 ± 3.8 | 11.1 ± 6.5 | 0.127 |
| Choline, mg | 289.5 ± 253.1 a | 221.2 ± 78.4 a | 614.1 ± 147.2 b | <0.001 |
| Betaine, mg | 180.4 ± 150.5 | 126.9 ± 60.2 | 143.1 ± 80.3 | 0.155 |
| Lutein + Zeaxanthin, μg | 1425.1 ± 1007.2 a | 2063.0 ± 1901.5 ab | 2471.6 ± 2208.1 b | 0.039 |
| Egg-Free Diet | Egg White Diet | Whole Egg Diet | p-Value | |
|---|---|---|---|---|
| Body weight, kg | 66.7 ± 12.1 a | 67.3 ± 12.7 b | 67.2 ± 12.7 ab | 0.038 |
| BMI, kg/m2 | 22.9 ± 2.8 | 23.0 ± 3.0 | 23.0 ± 3.0 | 0.054 |
| Body fat, % | 24.8 ± 7.1 | 25.5 ± 7.3 | 26.1 ± 7.7 | 0.189 |
| Fat mass, g | 36.9 ± 15.1 a | 37.7 ± 15.9 ab | 38.2 ± 16.2 b | 0.020 |
| Fat free mass, g | 109.8 ± 20.1 | 110.3 ± 21.0 | 109.6 ± 20.3 | 0.241 |
| Muscle mass, g | 104.0 ± 19.5 | 104.5 ± 20.3 | 102.8 ± 20.7 | 0.142 |
| Sodium, mmol/L | 138.8 ± 1.6 | 139.2 ± 1.5 | 139.0 ± 1.8 | 0.623 |
| Potassium, mmol/L | 4.13 ± 0.24 | 4.11 ± 0.23 | 4.11 ± 0.26 | 0.913 |
| Chloride, mmol/L | 104.0 ± 1.8 | 104.7 ± 2.0 | 104.1 ± 1.8 | 0.248 |
| Carbon dioxide, mmol/L | 23.9 ± 3.0 | 23.9 ± 2.4 | 23.8 ± 2.5 | 0.977 |
| Calcium, mg/dL | 9.35 ± 0.29 | 9.32 ± 0.30 | 9.35 ± 0.24 | 0.921 |
| BUN, mg/dL | 12.7 ± 3.2 | 13.0 ± 3.0 | 12.9 ± 3.0 | 0.907 |
| Protein, g/dL | 0.807 ± 0.09 | 0.784 ± 0.13 | 0.78 ± 0.11 | 0.160 |
| Albumin, g/dL | 4.42 ± 0.30 | 4.43 ± 0.26 | 4.40 ± 0.23 | 0.735 |
| Globulin, g/dL | 2.41 ± 0.32 | 2.42 ± 0.33 | 2.52 ± 0.34 | 0.170 |
| Albumin:Globulin | 1.88 ± 0.33 | 1.86 ± 0.30 | 1.78 ± 0.25 | 0.190 |
| Bilirubin, mg/dL | 0.53 ± 0.25 | 0.55 ± 0.33 | 0.50 ± 0.2 | 0.681 |
| ALP, U/L | 55.9 ± 14.1 | 55.9 ± 17.0 | 54.5 ± 11.9 | 0.983 |
| ALT, U/L | 14.4 ± 6.1 | 13.0 ± 6.8 | 13.2 ± 5.1 | 0.453 |
| AST, U/L | 18.5 ± 5.5 | 17.9 ± 5.2 | 18.0 ± 4.5 | 0.751 |
| hsCRP, mg/L | 1.5 ± 1.9 | 1.7 ± 3.1 | 2.2 ± 3.8 | 0.629 |
| Total ketones, μmol/L | 170.9 ± 105.3 | 167.2 ± 141.4 | 137.4 ± 54.6 | 0.468 |
| BHB, μmol/L | 173.7 ± 106.6 | 170.1 ± 143.7 | 138.6 ± 55.4 | 0.462 |
| Acetoacetate, μmol/L | 39.8 ± 21.7 | 40.4 ± 29.6 | 38.0 ± 16.0 | 0.934 |
| Acetone, μmol/L | 36.3 ± 16.3 | 34.3 ± 15.5 | 31.5 ± 11.8 | 0.482 |
| Choline, μM | 8.8 ± 2.8 a | 9.6 ± 2.3 ab | 10.6 ± 2.6 b | 0.025 |
| Betaine, μM | 32.4 ± 10.4 a | 31.6 ± 10.9 a | 36.6 ± 13.4 b | <0.001 |
| TMAO, μM | 2.1 ± 1.5 | 4.0 ± 6.4 | 1.6 ± 0.9 | 0.092 |
| Egg-Free Diet | Egg White Diet | Whole Egg Diet | p-Value | |
|---|---|---|---|---|
| Glucose, mg/dL | 81.6 ± 9.6 | 82.5 ± 9.4 | 83.5 ± 9.4 | 0.423 |
| GlycA, μmol/L | 380.2 ± 75.1 | 367.1 ± 44.7 | 386.9 ± 79.7 | 0.333 |
| LP–IR risk index, 0–100 | 34.1 ± 16.6 | 33.4 ± 15.9 | 31.4 ± 13.6 | 0.701 |
| DRI, 0–100 | 32.7 ± 11.7 | 32.8 ± 10.3 | 33.4 ± 10.1 | 0.947 |
| Total BCAA, μmol/L | 361.9 ± 44.1 | 376.1 ± 50.8 | 384.1 ± 46.5 | 0.079 |
| Leucine, μmol/L | 123.4 ± 20.0 | 120.9 ± 24.8 | 126.4 ± 19.0 | 0.612 |
| Isoleucine, μmol/L | 50.4 ± 10.6 a | 56.9 ± 9.5 b | 58.4 ± 11.1 b | 0.001 |
| Valine, μmol/L | 188.0 ± 28.5 | 198.3 ± 31.5 | 199.2 ± 30.6 | 0.133 |
| Alanine, μmol/L | 363.4 ± 98.0 | 365.5 ± 83.0 | 378.7 ± 65.9 | 0.608 |
| Glycine, μmol/L | 202.9 ± 53.2 a | 196.0 ± 47.7 a | 219.2 ± 61.2 b | 0.004 |
| Citrate, μmol/L | 86.0 ± 24.4 | 87.6 ± 24.9 | 81.3 ± 19.2 | 0.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, C.J.; Huang, L.; Zhai, F.; Esposito, C.P.; Greco, J.M.; Zhang, R.; Woodruff, R.; Sloan, A.; Van Dyke, A.R. Consumption of Different Egg-Based Diets Alters Clinical Metabolic and Hematological Parameters in Young, Healthy Men and Women. Nutrients 2023, 15, 3747. https://doi.org/10.3390/nu15173747
Andersen CJ, Huang L, Zhai F, Esposito CP, Greco JM, Zhang R, Woodruff R, Sloan A, Van Dyke AR. Consumption of Different Egg-Based Diets Alters Clinical Metabolic and Hematological Parameters in Young, Healthy Men and Women. Nutrients. 2023; 15(17):3747. https://doi.org/10.3390/nu15173747
Chicago/Turabian StyleAndersen, Catherine J., Lindsey Huang, Fangyi Zhai, Christa Palancia Esposito, Julia M. Greco, Ruijie Zhang, Rachael Woodruff, Allison Sloan, and Aaron R. Van Dyke. 2023. "Consumption of Different Egg-Based Diets Alters Clinical Metabolic and Hematological Parameters in Young, Healthy Men and Women" Nutrients 15, no. 17: 3747. https://doi.org/10.3390/nu15173747
APA StyleAndersen, C. J., Huang, L., Zhai, F., Esposito, C. P., Greco, J. M., Zhang, R., Woodruff, R., Sloan, A., & Van Dyke, A. R. (2023). Consumption of Different Egg-Based Diets Alters Clinical Metabolic and Hematological Parameters in Young, Healthy Men and Women. Nutrients, 15(17), 3747. https://doi.org/10.3390/nu15173747







