Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials
Abstract
:1. Introduction
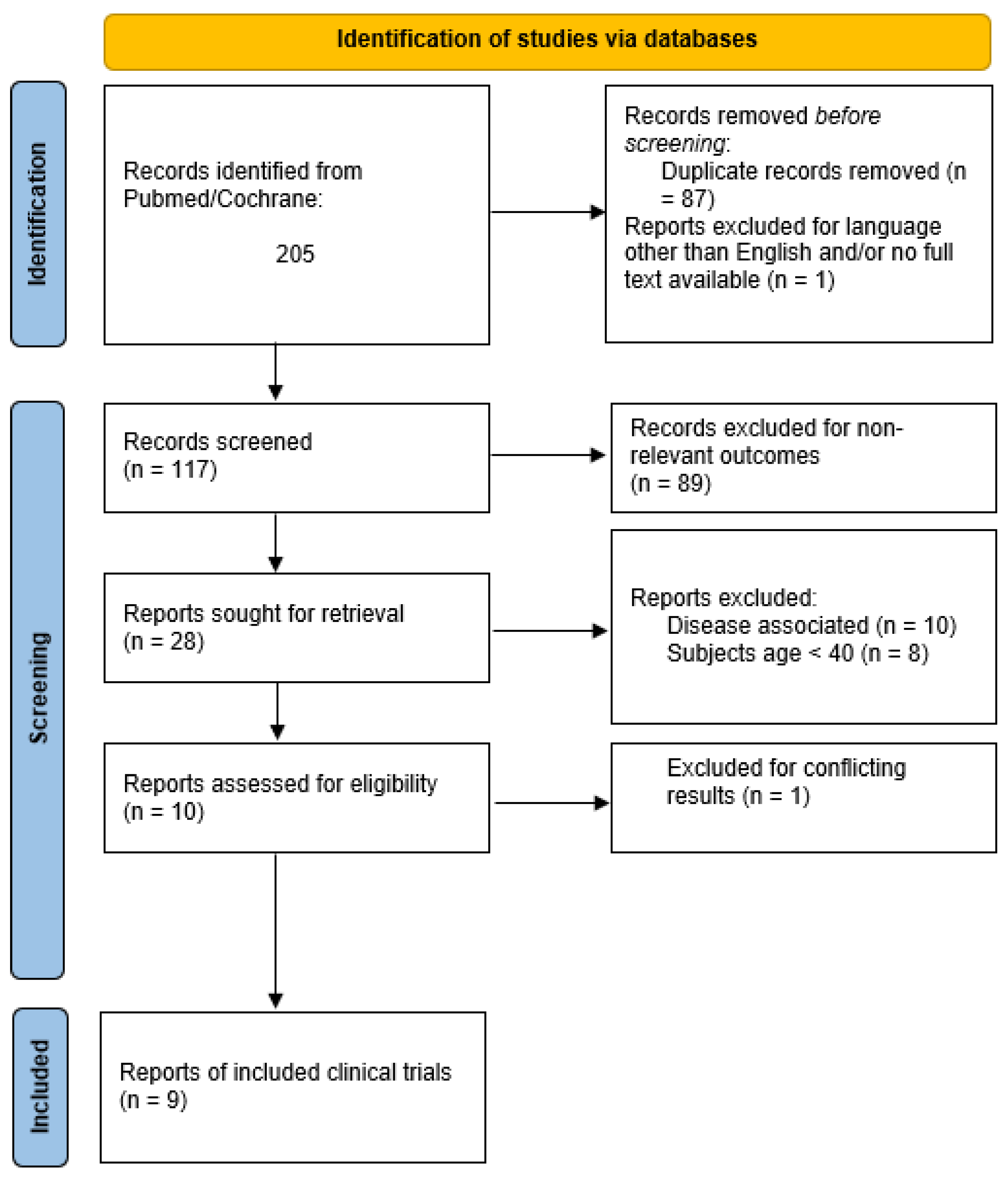
2. Methodology
3. Potential of BDNF, Aβ Peptides, NGF, and Tau Proteins as Biomarkers of Brain Aging
3.1. BDNF
3.2. Aβ Peptides
3.3. NGF and Tau Proteins
4. Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Middle-Aged and Elderly People: Studies’ Overview and Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Boström, K.; Jungbjer, B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. 1997, 94, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Scahill, R.; Frost, C.; Jenkins, R.; Whitwell, J.; Rossor, M.; Fox, N. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003, 60, 989–994. [Google Scholar] [CrossRef]
- Lacreuse, A.; Raz, N.; Schmidtke, D.; Hopkins, W.D.; Herndon, J.G. Age-related decline in executive function as a hallmark of cognitive ageing in primates: An overview of cognitive and neurobiological studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190618. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Vernot, J.P. Senescence-Associated Pro-Inflammatory Cytokines and Tumor Cell Plasticity. Front. Mol. Biosci. 2020, 7, 63. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between Systemic Inflammation and Neuroinflammation Underlies Neuroprotective Mechanism of Several Phytochemicals in Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018, 18, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Britschgi, M.; Herbert, C.; Takeda-Uchimura, Y.; Boxer, A.; Blennow, K.; Friedman, L.F.; Galasko, D.R.; Jutel, M.; Karydas, A.; et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat. Med. 2007, 13, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Chen-Plotkin, A.; Arnold, S.E.; Grossman, M.; Clark, C.M.; Shaw, L.M.; Pickering, E.; Kuhn, M.; Chen, Y.; McCluskey, L.; et al. Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 2010, 119, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Craig-Schapiro, R.; Perrin, R.J.; Roe, C.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF Cytokines in Aging, Multiple Sclerosis, and Dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurología 2021, in press. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1. [Google Scholar]
- Moller, P.; Wallin, H.K.; Knudsen, L.E. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem. Biol. Interact. 1996, 102, 17–36. [Google Scholar] [CrossRef]
- Sies, H. Biological redox systems and oxidative stress. Cell Mol. Life Sci. 2007, 64, 2181–2188. [Google Scholar] [CrossRef]
- Szarc vel Szic, K.; Declerck, K.; Vidakovic, M.; Vanden Berghe, W. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition? Clin. Epigenetics 2015, 7, 33. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, T.; Mursu, J.; Heinonen, M.; Nurmi, A.; Hiltunen, R.; Voutilainen, S. Metabolism of berry anthocyanins to phenolic acids in humans. J. Agric. Food Chem. 2009, 57, 2274–2281. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic Polyphenol pi-pi Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Cheng, N.; Bell, L.; Lamport, D.J.; Williams, C.M. Dietary Flavonoids and Human Cognition: A Meta-Analysis. Mol. Nutr. Food Res. 2022, 66, e2100976. [Google Scholar] [CrossRef]
- Yang, W.; Cui, K.; Li, X.; Zhao, J.; Zeng, Z.; Song, R.; Qi, X.; Xu, W. Effect of Polyphenols on Cognitive Function: Evidence from Population-Based Studies and Clinical Trials. J. Nutr. Health Aging 2021, 25, 1190–1204. [Google Scholar] [CrossRef]
- Pisani, A.; Paciello, F.; Del Vecchio, V.; Malesci, R.; De Corso, E.; Cantone, E.; Fetoni, A.R. The Role of BDNF as a Biomarker in Cognitive and Sensory Neurodegeneration. J. Pers. Med. 2023, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Naegelin, Y.; Dingsdale, H.; Sauberli, K.; Schadelin, S.; Kappos, L.; Barde, Y.A. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, K.M.; Kennedy, K.M.; Park, D.C. Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 2009, 19, 436–450. [Google Scholar] [CrossRef]
- Li, Y.; Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Weiner, M.W.; Shaw, L.M.; Masters, C.L.; Fowler, C.J.; Trojanowski, J.Q.; et al. Validation of Plasma Amyloid-beta 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology 2022, 98, e688–e699. [Google Scholar] [CrossRef]
- Parikh, V.; Howe, W.M.; Welchko, R.M.; Naughton, S.X.; D’Amore, D.E.; Han, D.H.; Deo, M.; Turner, D.L.; Sarter, M. Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur. J. Neurosci. 2013, 37, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Pîrşcoveanu, D.F.V.; Pirici, I.; Tudorică, V.; Bălşeanu, T.A.; Albu, V.C.; Bondari, S.; Bumbea, A.M.; Pîrşcoveanu, M. Tau protein in neurodegenerative diseases—A review. Rom. J. Morphol. Embryol. 2017, 58, 1141–1150. [Google Scholar]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001, 276, 12660–12666. [Google Scholar] [CrossRef]
- Hempstead, B. Brain-Derived Neurotrophic Factor: Three Ligands, Many Actions. Tansaction Am. Clin. Climatol. Assoc. 2015, 126, 9–19. [Google Scholar]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef]
- Binder, D.; Scharfmann, H. Brain-derived Neurotrophic Factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Acheson, A.; Conover, J.; Fandl, J.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squintot, S.; Yancopoulos, G.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Le Beau, M.M.; Espinosa, R., 3rd; Ip, N.Y.; Belluscio, L.; de la Monte, S.M.; Squinto, S.; Furth, M.E.; Yancopoulos, G.D. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics 1991, 10, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef]
- Diniz, B.S.; Reynolds, C.F., 3rd; Begley, A.; Dew, M.A.; Anderson, S.J.; Lotrich, F.; Erickson, K.I.; Lopez, O.; Aizenstein, H.; Sibille, E.L.; et al. Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: A longitudinal study. J. Psychiatr. Res. 2014, 49, 96–101. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef]
- Siuda, J.; Patalong-Ogiewa, M.; Żmuda, W.; Targosz-Gajniak, M.; Niewiadomska, E.; Matuszek, I.; Jędrzejowska-Szypułka, H.; Lewin-Kowalik, J.; Rudzińska-Bar, M. Cognitive impairment and BDNF serum levels. Neurol. Neurochir. Pol. 2017, 51, 24–32. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Borovecki, F.; Filipcic, I.; Vuic, B.; Milos, T.; Nedic Erjavec, G.; Konjevod, M.; Tudor, L.; Mimica, N.; Uzun, S.; et al. Relationship between Brain-Derived Neurotrophic Factor and Cognitive Decline in Patients with Mild Cognitive Impairment and Dementia. Biomolecules 2023, 13, 570. [Google Scholar] [CrossRef]
- Cechova, K.; Angelucci, F.; Markova, H.; Nikolai, T.; Matuskova, V.; Laczó, J.; Nedelska, Z.; Vyhnalek, M.; Hort, J. Ratio of serum proBDNF to BDNF and its association with cognitive performance and brain morphometry in mild cognitive impairment. Alzheimer’s Dement. 2020, 16, e046340. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.G.; Gobbi, S.; Andreatto, C.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduroz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Castano, L.A.A.; Castillo de Lima, V.; Barbieri, J.F.; de Lucena, E.G.P.; Gaspari, A.F.; Arai, H.; Teixeira, C.V.L.; Coelho-Junior, H.J.; Uchida, M.C. Resistance Training Combined with Cognitive Training Increases Brain Derived Neurotrophic Factor and Improves Cognitive Function in Healthy Older Adults. Front. Psychol. 2022, 13, 870561. [Google Scholar] [CrossRef]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women with MCI: A Small-Scale Study. Am. J. Alzheimers Dis. Other Demen 2018, 33, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.V.; Bystryak, S.; Galzitskaya, O.V. The role of beta-amyloid peptide in neurodegenerative diseases. Ageing Res. Rev. 2011, 10, 440–452. [Google Scholar] [CrossRef]
- Coulson, E.J.; Paliga, K.; Beyreuther, K.; Masters, C.L. What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem. Int. 2000, 36, 175–184. [Google Scholar] [CrossRef]
- Priller, C.; Bauer, T.; Mitteregger, G.; Krebs, B.; Kretzschmar, H.A.; Herms, J. Synapse formation and function is modulated by the amyloid precursor protein. J. Neurosci. 2006, 26, 7212–7221. [Google Scholar] [CrossRef]
- Turner, P.R.; O’Connor, K.; Tate, W.P.; Abraham, W.C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003, 70, 1–32. [Google Scholar] [CrossRef]
- Duce, J.A.; Tsatsanis, A.; Cater, M.A.; James, S.A.; Robb, E.; Wikhe, K.; Leong, S.L.; Perez, K.; Johanssen, T.; Greenough, M.A.; et al. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 2010, 142, 857–867. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Nunan, J.; Small, D.H. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000, 483, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Olsson, F.; Schmidt, S.; Althoff, V.; Munter, L.M.; Jin, S.; Rosqvist, S.; Lendahl, U.; Multhaup, G.; Lundkvist, J. Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions. J. Biol. Chem. 2014, 289, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Nagashima, Y.; Sano, Y.; Ishihara, S.; Morishima-Kawashima, M.; Funamoto, S.; Ihara, Y. gamma-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J. Neurosci. 2009, 29, 13042–13052. [Google Scholar] [CrossRef]
- Gu, L.; Guo, Z. Alzheimer’s Abeta42 and Abeta40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Dupuis, N.F.; Lazo, N.D.; Wyttenbach, T.; Condron, M.M.; Bitan, G.; Teplow, D.B.; Shea, J.E.; Ruotolo, B.T.; Robinson, C.V.; et al. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 2009, 1, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Kuperstein, I.; Broersen, K.; Benilova, I.; Rozenski, J.; Jonckheere, W.; Debulpaep, M.; Vandersteen, A.; Segers-Nolten, I.; Van Der Werf, K.; Subramaniam, V.; et al. Neurotoxicity of Alzheimer’s disease Abeta peptides is induced by small changes in the Abeta42 to Abeta40 ratio. EMBO J. 2010, 29, 3408–3420. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Li, X.; Ma, L.; Hou, M.; Zhou, H.; Zhou, R. Based on molecular structures: Amyloid-beta generation, clearance, toxicity and therapeutic strategies. Front. Mol. Neurosci. 2022, 15, 927530. [Google Scholar] [CrossRef]
- Petersen, C.A.H.; Alikhani, N.; Behbahani, H.; Wiehager, B.; Pavlov, P.; Alafuzoff, I.; AkiraIto, V.L.; Winblad, B.; Glaser, E.; Ankarcrona, M. The amyloid-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2008, 105, 13145–13150. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-beta and tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-Beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Ellis, K.A.; Lim, Y.Y.; Harrington, K.; Ames, D.; Bush, A.I.; Darby, D.; Martins, R.N.; Masters, C.L.; Rowe, C.C.; Savage, G.; et al. Decline in cognitive function over 18 months in healthy older adults with high amyloid-β. J. Alzheimers Dis. 2013, 34, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Giudici, K.V.; de Souto Barreto, P.; Guyonnet, S.; Li, Y.; Bateman, R.J.; Vellas, B. Assessment of Plasma Amyloid-β42/40 and Cognitive Decline Among Community-Dwelling Older Adults. JAMA Netw. Open 2020, 3, e2028634. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Razlighi, Q.R.; Zahodne, L.B.; Janicki, S.C.; Ichise, M.; Manly, J.J.; Devanand, D.P.; Brickman, A.M.; Schupf, N.; Mayeux, R.; et al. Brain Amyloid Deposition and Longitudinal Cognitive Decline in Nondemented Older Subjects: Results from a Multi-Ethnic Population. PLoS ONE 2015, 10, e0123743. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Villemagne, V.; Ong, K.; Chetélat, G.; Lamb, F.; Reininger, C.B.; Woodward, M.; Rowe, C.C. Prediction of amyloid-β pathology in amnestic mild cognitive impairment with neuropsychological tests. J. Alzheimers Dis. 2013, 33, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef]
- Pérez-Grijalba, V.; Romero, J.; Pesini, P.; Sarasa, L.; Monleón, I.; San-José, I.; Arbizu, J.; Martínez-Lage, P.; Munuera, J.; Ruiz, A.; et al. Plasma Aβ42/40 Ratio Detects Early Stages of Alzheimer’s Disease and Correlates with CSF and Neuroimaging Biomarkers in the AB255 Study. J. Prev. Alzheimers Dis. 2019, 6, 34–41. [Google Scholar] [CrossRef]
- Jagust, W. Is amyloid-beta harmful to the brain? Insights from human imaging studies. Brain 2016, 139, 23–30. [Google Scholar] [CrossRef]
- Hempstead, B.; Martin-Zanca, D.; Kaplan, D.; Parada, L.F.; Chao, M.V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature 1991, 350, 678–683. [Google Scholar] [CrossRef]
- Niewiadomska, G.; Mietelska-Porowska, A.; Mazurkiewicz, M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav. Brain Res. 2011, 221, 515–526. [Google Scholar] [CrossRef]
- Eu, W.Z.; Chen, Y.J.; Chen, W.T.; Wu, K.Y.; Tsai, C.Y.; Cheng, S.J.; Carter, R.N.; Huang, G.J. The effect of nerve growth factor on supporting spatial memory depends upon hippocampal cholinergic innervation. Transl. Psychiatry 2021, 11, 162. [Google Scholar] [CrossRef]
- Pham, T.M.; Söderström, S.; Winblad, B.; Mohammed, A.H. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav. Brain Res. 1999, 103, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Kutiyanawalla, A.; Pillai, A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiol. Behav. 2011, 102, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Binder, L.; Counts, S.E.; DeKosky, S.T.; de Toledo-Morrell, L.; Ginsberg, S.D.; Ikonomovic, M.D.; Perez, S.E.; Scheff, S.W. Mild cognitive impairment: Pathology and mechanisms. Acta Neuropathol. 2012, 123, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Bhaskar, C.D. The Role of Tau Protein in Diseases. Ann. Adv. Chem. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Dage, J.L.; Wennberg, A.M.V.; Airey, D.C.; Hagen, C.E.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Jack, C.R., Jr.; Petersen, R.C.; Mielke, M.M. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016, 12, 1226–1234. [Google Scholar] [CrossRef]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.; Pino, A.; Cuevas, C.; Puertas-Martin, V.; San Roman, R.; de Pascual-Teresa, S. Neurocognitive Effects of Cocoa and Red-Berries Consumption in Healthy Adults. Nutrients 2021, 14, 1. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Maruyama, K.; Hossain, S.; Sumiyoshi, E.; Wakatsuki, H.; Kato, S.; Ohno, M.; Tanabe, Y.; Kuroda, Y.; et al. Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: A possible supplement for brain health in the elderly. Food Funct. 2022, 13, 2768–2781. [Google Scholar] [CrossRef]
- Igwe, E.O.; Roodenrys, S.; Probst, Y.C.; do Rosario, V.; Netzel, M.E.; Hong, H.T.; Netzel, G.; Phan, A.D.T.; Charlton, K.E. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: A randomized crossover trial. Nutr. Res. 2020, 82, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef]
- Baba, Y.; Inagaki, S.; Nakagawa, S.; Kaneko, T.; Kobayashi, M.; Takihara, T. Effect of Daily Intake of Green Tea Catechins on Cognitive Function in Middle-Aged and Older Subjects: A Randomized, Placebo-Controlled Study. Molecules 2020, 25, 4265. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, H.; Tanaka, A.; Nishihira, J.; Murayama, N. Effect of quercetin glycosides on cognitive functions and cerebral blood flow: A randomized, double-blind, and placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8700–8712. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef]
- Janelidze, S.; Teunissen, C.E.; Zetterberg, H.; Allue, J.A.; Sarasa, L.; Eichenlaub, U.; Bittner, T.; Ovod, V.; Verberk, I.M.W.; Toba, K.; et al. Head-to-Head Comparison of 8 Plasma Amyloid-Beta 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021, 78, 1375–1382. [Google Scholar] [CrossRef]
- Cheng, L.; Li, W.; Chen, Y.; Lin, Y.; Wang, B.; Guo, Q.; Miao, Y. Plasma Abeta as a biomarker for predicting Abeta-PET status in Alzheimer’s disease: A systematic review with meta-analysis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 513–520. [Google Scholar] [CrossRef]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; Cózar-Santiago, M.D.P.; et al. Genistein effect on cognition in prodromal Alzheimer’s disease patients. The GENIAL clinical trial. Alzheimers Res. Ther. 2022, 14, 164. [Google Scholar] [CrossRef]
| Reference | Subjects | Study Design | Intervention | Main Results |
|---|---|---|---|---|
| Ahles 2020 [89] | Healthy adults, age 40–60 | Randomized, placebo-controlled, double-blinded, parallel-grouped study | Aronia extract in capsules providing 16–27 mg of anthocyanins/day for 24 weeks or placebo | No effects on serum BDNF |
| Garcia-Cordero 2021 [90] | Healthy adults, age 50–75 | Randomized, double-blinded, parallel-grouped study | Red berry powder (100 mg anthocyanins/day), cocoa powder (200 mg flavanols/day) or both combined and dissolved in water for 12 weeks | No effects on serum BDNF and NGF-R, but positive correlation between total polyphenol intake and BDNF levels |
| Hashimoto 2022 [91] | Healthy elderlies, age 60–85 | Randomized, double-blinded, parallel-grouped study | Encapsulated perilla seed oil (0.88 g ALA/day) alone or in combination with ponkan powder (2.91 mg nobiletin/day) for 12 months | Serum BDNF levels increased in oil + powder group over time |
| Igwe 2020 [92] | Healthy adults, age > 55 | Randomized, controlled, cross-over trial | Plum nectar providing 7.4–10.6 mg anthocyanins/day for 8 weeks compared to raspberry cordial | No effects on serum levels of BDNF |
| Neshatdoust 2016 [93] | Healthy adults, age 62–75 | Randomized, controlled, double-masked, cross-over trial | High flavanol (494 mg flavanols/day) or low flavanol (23 mg flavanols/day) cocoa drink for 28 days | High flavanol intervention was associated with increased serum BDNF levels compared to low flavanol intervention |
| Baba 2020 [94] | Healthy adults and elderlies, age 50–69 with mild cognitive decline (MMSE score ≥ 24) | Randomized, placebo-controlled, double-blinded, parallel-grouped study | Capsules providing 336.4 g/day of catechins in capsules for 12 weeks or placebo | No significant differences between placebo and catechin group for serum Aβ1-40, Aβ1-42, Aβ1-40/Aβ1-42 ratio, Aβ precursor protein α (sAPPα), Aβ precursor protein (APP)770, and serum BDNF levels |
| Nakamura 2022 [95] | Healthy elderlies, age 60–80 with mild cognitive decline (MMSE score ≥ 24) | Randomized, placebo-controlled, double-blinded, parallel-grouped study | Barley tea beverage providing 110 mg quercetin/day for 40 weeks or placebo beverage | Plasma levels of Aβ42/Aβ40 decreased in both groups but change was only significant in placebo |
| Small 2018 [96] | Healthy middle-aged and elderlies, age 50–90 | Randomized, placebo-controlled double-blinded, parallel-grouped study | 180 mg of encapsulated curcumin/day for 18 months or placebo | Significant reduction of Aβ and tau plaques in the amygdala (PET measurement) compared to baseline, no change of hypothalamic Aβ and tau plaques in curcumin group but significantly increased in placebo |
| Di Silvestro 2012 [97] | Healthy middle-aged, age 40–60 years | Placebo-controlled, parallel-grouped study | 80 mg of encapsulated curcumin/day for 4 weeks or placebo | Plasma Aβ levels decreased in curcumin group but not in placebo group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegler, T.; Tsiountsioura, M.; Meixner-Goetz, L.; Cvirn, G.; Lamprecht, M. Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials. Nutrients 2023, 15, 3770. https://doi.org/10.3390/nu15173770
Ziegler T, Tsiountsioura M, Meixner-Goetz L, Cvirn G, Lamprecht M. Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials. Nutrients. 2023; 15(17):3770. https://doi.org/10.3390/nu15173770
Chicago/Turabian StyleZiegler, Tobias, Melina Tsiountsioura, Lisa Meixner-Goetz, Gerhard Cvirn, and Manfred Lamprecht. 2023. "Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials" Nutrients 15, no. 17: 3770. https://doi.org/10.3390/nu15173770
APA StyleZiegler, T., Tsiountsioura, M., Meixner-Goetz, L., Cvirn, G., & Lamprecht, M. (2023). Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials. Nutrients, 15(17), 3770. https://doi.org/10.3390/nu15173770






