Liver Disease Undernutrition Screening Tool Questionnaire Predicts Decompensation and Mortality in Cirrhotic Outpatients with Portal Hypertension
Abstract
:1. Introduction
2. Materials and Methods
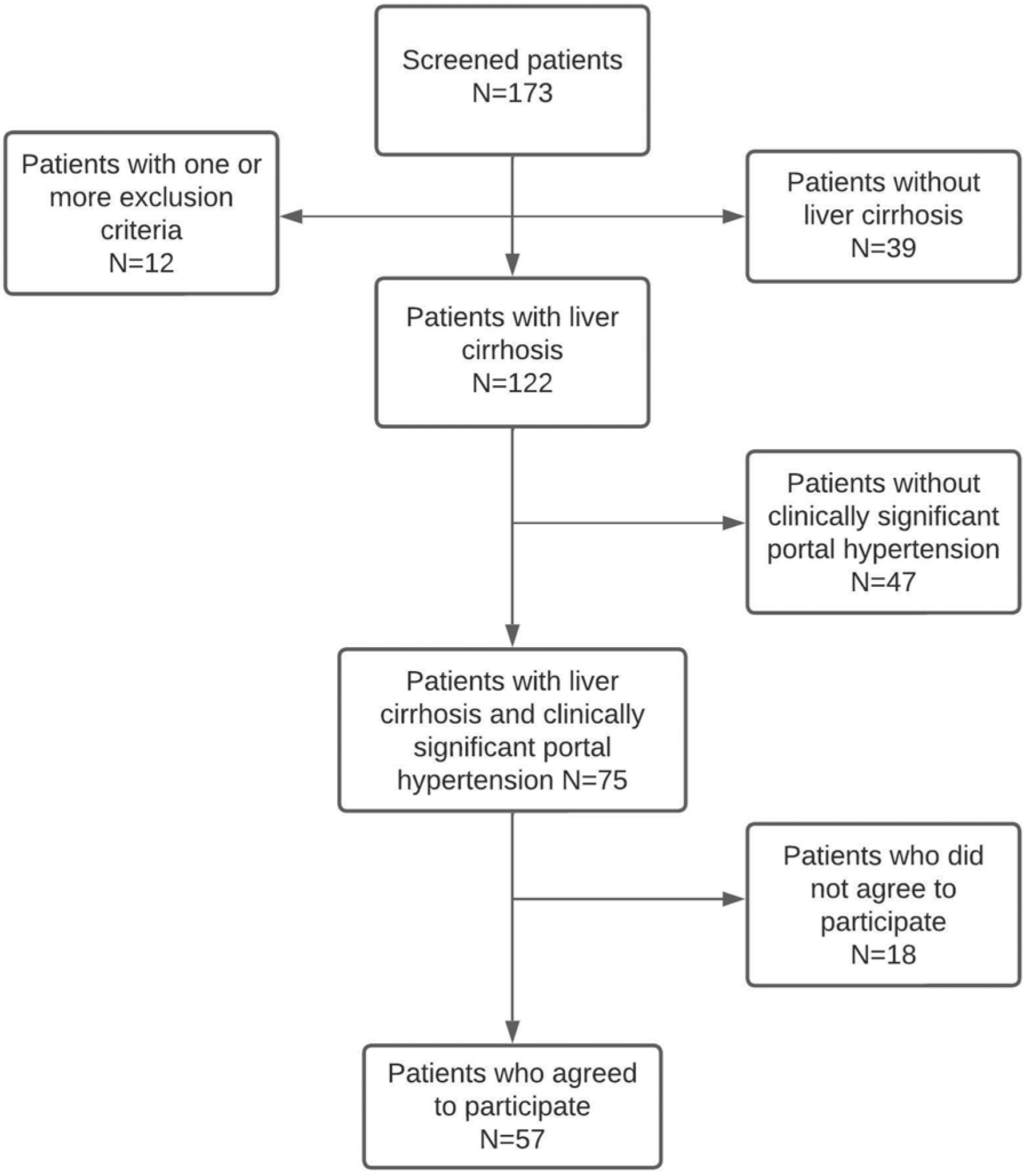
2.1. Study Design and Population
2.2. Baseline Assessment of Patients
2.3. Follow-Up
2.4. Ethical Statement
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Results during Follow-Up
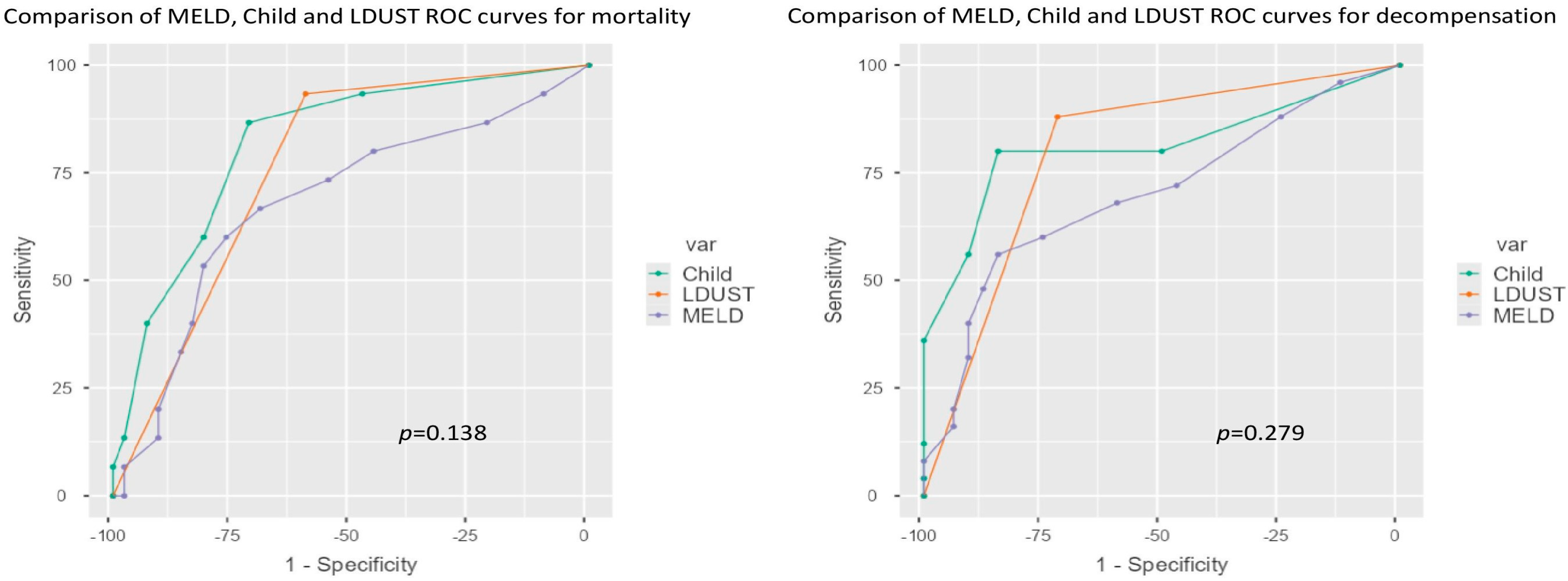
3.3. Comparison of Predictive Ability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Basile-Filho, A.; Nicolini, E.; Auxiliadora-Martins, M.; Alkmim-Teixeira, G.; Martinez, E.; Martins-Filho, O.; Silva, O.d.C.e. Comparison of Acute Physiology and Chronic Health Evaluation II Death Risk, Child-Pugh, Charlson, and Model for End-stage Liver Disease Indexes to Predict Early Mortality After Liver Transplantation. Transplant. Proc. 2011, 43, 1660–1664. [Google Scholar] [CrossRef]
- Boin, I.; Leonardi, M.; Pinto, A.; Leme, R.; Udo, E.; Stucchi, R.; Soares, E.; Leonardi, L. Liver transplant recipients mortality on the waiting list: Long-term comparison to child-pugh classification and meld. Transplant. Proc. 2004, 36, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Cesbron, E.; Tropet, A.-L.; Pilette, C. Comparison and Improvement of MELD and Child-Pugh Score Accuracies for the Prediction of 6-month Mortality in Cirrhotic Patients. J. Clin. Gastroenterol. 2009, 43, 580–585. [Google Scholar] [CrossRef]
- Hong, S.J.; Feuerstadt, P.; Brandt, L.J. MELD is the only predictor of short-term mortality in cirrhotic patients with C. difficile infection. Dig. Liver Dis. 2019, 51, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Rashed, E.; Soldera, J. CLIF-SOFA and CLIF-C scores for the prognostication of acute-on-chronic liver failure and acute decompensation of cirrhosis: A systematic review. World J. Hepatol. 2022, 14, 2025–2043. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, Y.; Liu, L.; Zhu, X. Assessing the prognostic scores for the prediction of the mortality of patients with acute-on-chronic liver failure: A retrospective study. PeerJ 2020, 8, e9857. [Google Scholar] [CrossRef]
- Gnanadeepam, S.; Janeela, A.M.; Zachariah, U.; Eapen, C.E.; Goel, A. Sarcopenia is Closely Associated with Frailty in Decompensated Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 237–238. [Google Scholar] [CrossRef]
- Mehta, M.; Louissaint, J.; Parikh, N.S.; Long, M.T.; Tapper, E.B. Cognitive Function, Sarcopenia, and Inflammation Are Strongly Associated with Frailty: A Framingham Cohort Study. Am. J. Med. 2022, 134, 1530–1538. [Google Scholar] [CrossRef]
- Cullaro, G.; Verna, E.C.; Duarte-Rojo, A.; Kappus, M.R.; Ganger, D.R.; Rahimi, R.S.; Boyarsky, B.; Segev, D.L.; McAdams-DeMarco, M.; Ladner, D.P.; et al. Frailty and the Risk of Acute Kidney Injury Among Patients with Cirrhosis. Hepatol. Commun. 2021, 6, 910–919. [Google Scholar] [CrossRef]
- Lai, J.C.; Shui, A.M.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; Huang, C.; Yao, F.; Kappus, M.; Boyarsky, B.; McAdams-Demarco, M.; et al. Frailty, mortality, and health care utilization after liver transplantation: From the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology 2022, 75, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.; Papatheodoridis, G.V.; Alexopoulou, A.; Deutsch, M.; Vlachogiannakos, I.; Ioannidou, P.; Papageorgiou, M.-V.; Papadopoulos, N.; Tsibouris, P.; Prapa, A.; et al. Evaluation of the effectiveness of eight screening tools in detecting risk of malnutrition in cirrhotic patients: The KIRRHOS study. Br. J. Nutr. 2019, 122, 1368–1376. [Google Scholar] [CrossRef]
- McFarlane, M.; Hammond, C.; Roper, T.; Mukarati, J.; Ford, R.; Burrell, J.; Gordon, V.; Burch, N. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin. Nutr. ESPEN 2018, 23, 156–161. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Booi, A.N.; Menendez, J.; Norton, H.J.; Anderson, W.E.; Ellis, A.C. Validation of a Screening Tool to Identify Undernutrition in Ambulatory Patients with Liver Cirrhosis. Nutr. Clin. Pract. 2015, 30, 683–689. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Peolsson, R.H.A. Intra- and inter-tester reliability and reference values for hand strength. J. Rehabil. Med. 2001, 33, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip Strength across the Life Course: Normative Data from Twelve British Studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and Appendicular Lean Mass Reference Ranges for Australian Men and Women: The Geelong Osteoporosis Study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Krishnan, M.; Temel, J.; Wright, A.; Bernacki, R.; Selvaggi, K.; Balboni, T. Predicting life expectancy in patients with advanced incurable cancer: A review. J. Support. Oncol. 2013, 11, 68–74. [Google Scholar] [CrossRef]
- Arjal, R.; Trotter, J.F. International Normalized Ratio of Prothrombin Time in the Model for End-stage Liver Disease Score: An Unreliable Measure. Clin. Liver Dis. 2009, 13, 67–71. [Google Scholar] [CrossRef]
- Trotter, J.F.; Olson, J.; Lefkowitz, J.; Smith, A.D.; Arjal, R.; Kenison, J. Changes in International Normalized Ratio (INR) and Model for Endstage Liver Disease (MELD) Based on Selection of Clinical Laboratory. Am. J. Transplant. 2007, 7, 1624–1628. [Google Scholar] [CrossRef]
- Zheng, J.; Xing, R.-C.; Zheng, W.-H.; Liu, W.; Yao, R.-C.; Li, X.-S.; Du, J.-P.; Li, L. A comparative study on postoperative mortality prediction of SFLI scoring system and Child-Pugh classification in patients with hepatocellular carcinoma. J. BUON 2017, 22, 709–713. [Google Scholar] [PubMed]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Morabito, A.; Pagliaro, L.; Marubini, E.; The Liver Study Group of “V. Cervello” Hospital. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig. Dis. Sci. 1986, 31, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Groszmann, R.; Garcia–Tsao, G.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Garcia–Pagan, J.C.; Makuch, R.; Patch, D.; et al. Hepatic Venous Pressure Gradient Predicts Clinical Decompensation in Patients with Compensated Cirrhosis. Gastroenterology 2007, 133, 481–488. [Google Scholar] [CrossRef]
- Tejedor, M.; Selzner, N.; Berenguer, M. Are MELD and MELDNa Still Reliable Tools to Predict Mortality on the Liver Transplant Waiting List? Transplantation 2022, 106, 2122–2136. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, D.; Shi, X.; Liu, S.; Hu, X.; Zhou, C.; Tian, X.; Liu, H.; Long, H.; Li, Z.; et al. High Child-Pugh and CRUB65 scores predict mortality of decompensated cirrhosis patients with COVID-19: A 23-center, retrospective study. Virulence 2021, 12, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Krige, J.; Spence, R.T.; Jonas, E.; Hoogerboord, M.; Ellsmere, J. A New Recalibrated Four-Category Child–Pugh Score Performs Better than the Original Child–Pugh and MELD Scores in Predicting In-Hospital Mortality in Decompensated Alcoholic Cirrhotic Patients with Acute Variceal Bleeding: A Real-World Cohort Analysis. World J. Surg. 2020, 44, 241–246. [Google Scholar] [CrossRef]
- Borhofen, S.M.; Gerner, C.; Lehmann, J.; Fimmers, R.; Görtzen, J.; Hey, B.; Geiser, F.; Strassburg, C.P.; Trebicka, J. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig. Dis. Sci. 2016, 61, 1735–1743. [Google Scholar] [CrossRef]
- Tapper, E.B.; Finkelstein, D.; Mittleman, M.A.; Piatkowski, G.; Lai, M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology 2015, 62, 584–590. [Google Scholar] [CrossRef]
- Wang, S.; Whitlock, R.; Xu, C.; Taneja, S.; Singh, S.; Abraldes, J.G.; Burak, K.W.; Bailey, R.J.; Lai, J.C.; Tandon, P. Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology 2022, 75, 600–609. [Google Scholar] [CrossRef]
- Demirel, B.; Atasoy, B.M. Comparison of Three Nutritional Screening Tools to Predict Malnutrition Risk and Detect Distinctions Between Tools in Cancer Patients Receiving Radiochemotherapy. Nutr. Cancer 2018, 70, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Deza, D.C.; Glaria, M.E.B.; Sanz-París, A.; Blasco, M.L.; Bonilla, E.M.F.; Monterde, V.B.; Mainar, J.M.A.; Olmo, J.F. Mini Nutritional Assessment - Short Form Is a Useful Malnutrition Screening Tool in Patients with Liver Cirrhosis, Using the Global Leadership Initiative for Malnutrition Criteria as the Gold Standard. Nutr. Clin. Pract. 2021, 36, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Stites, S.; Navarro, V. Bringing Assessment of Patient-Reported Outcomes to Hepatology Practice. Clin. Gastroenterol. Hepatol. 2018, 16, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Orman, E.S.; Ghabril, M.S.; Desai, A.P.; Nephew, L.; Patidar, K.R.; Gao, S.; Xu, C.; Chalasani, N. Patient-Reported Outcome Measures Modestly Enhance Prediction of Readmission in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2022, 20, e1426–e1437. [Google Scholar] [CrossRef]
- Burns, D.J.; Arora, J.; Okunade, O.; Beltrame, J.F.; Bernardez-Pereira, S.; Crespo-Leiro, M.G.; Filippatos, G.S.; Hardman, S.; Hoes, A.W.; Hutchison, S.; et al. International Consortium for Health Outcomes Measurement (ICHOM): Standardized Patient-Centered Outcomes Measurement Set for Heart Failure Patients. JACC Heart Fail. 2020, 8, 212–222. [Google Scholar] [CrossRef]
- Wong, D.; Matini, L.; Kormilitzin, A.; Kantschuster, R.; Simadibrata, D.M.; Lyden, S.; Wilson, J.; A Brain, O.; Palmer, R.; Ambrose, T.; et al. Patient-reported Outcomes: The ICHOM Standard Set for Inflammatory Bowel Disease in Real-life Practice Helps Quantify Deficits in Current Care. J. Crohn’s Colitis 2022, 16, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Schouten, E.; Haupt, J.; Ramirez, J.; Sillett, N.; Nielsen, C.; Clarke, A.; Matkin, L.; Joseph, A.; Been, J.; González, I.B.; et al. Standardized Outcome Measures for Preterm and Hospitalized Neonates: An ICHOM Standard Set. Neonatology 2022, 119, 443–454. [Google Scholar] [CrossRef]


| No Undernutrition (n = 26) | Undernutrition (n = 31) | All (N = 57) | p-Value | |
|---|---|---|---|---|
| Sex | 0.182 | |||
| Female | 12 (46.2%) | 9 (29.0%) | 21 (36.8%) | |
| Male | 14 (53.8%) | 22 (71.0%) | 36 (63.2%) | |
| Age (years) | 0.103 | |||
| Mean (SD) | 61.2 (10.3) | 65.5 (9.2) | 63.5 (9.9) | |
| Alcohol | 0.221 | |||
| No | 23 (88.5%) | 30 (96.8%) | 53 (93.0%) | |
| Yes | 3 (11.5%) | 1 (3.2%) | 4 (7.0%) | |
| Etiology | 0.497 | |||
| HCV | 6 (23.1%) | 7 (22.6%) | 13 (22.8%) | |
| HBV | 3 (11.5%) | 1 (3.2%) | 4 (7.0%) | |
| Alcohol | 14 (53.8%) | 15 (48.4%) | 29 (50.9%) | |
| Autoimmune | 0 (0.0%) | 3 (9.7%) | 3 (5.3%) | |
| MAFLD | 1 (3.8%) | 1 (3.2%) | 2 (3.5%) | |
| Idiopathic | 2 (7.7%) | 4 (12.9%) | 6 (10.5%) | |
| Child–Pugh | 0.090 | |||
| A | 19 (73.1%) | 13 (41.9%) | 32 (56.1%) | |
| B/C | 7 (26.9%) | 18 (58.1%) | 25 (43.9%) | |
| MELD | 9.7 (3.0) | 11.4 (4.1) | 10.6 (3.7) | 0.096 |
| Charlson Index | 0.895 | |||
| Mean (SD) | 4.2 (1.4) | 4.3 (1.9) | 4.3 (1.7) | |
| Previous decompensation | 0.508 | |||
| No | 6 (23.1%) | 5 (16.1%) | 11 (19.3%) | |
| Yes | 20 (76.9%) | 26 (83.9%) | 46 (80.7%) | |
| Ascitis | 0.571 | |||
| No | 12 (46.2%) | 12 (38.7%) | 24 (42.1%) | |
| Yes | 14 (53.8%) | 19 (61.3%) | 33 (57.9%) | |
| Esophageal varices | 0.176 | |||
| No | 5 (19.2%) | 5 (16.1%) | 10 (17.5%) | |
| Small | 18 (69.2%) | 16 (51.6%) | 34 (59.6%) | |
| Large | 3 (11.5%) | 10 (32.3%) | 13 (22.8%) |
| No Undernutrition (n = 26) | Undernutrition (n = 31) | All (N = 57) | p-Value | |
|---|---|---|---|---|
| GLIM | 0.001 | |||
| Normal | 22 (84.6%) | 12 (40%) | 34 (60.7%) | |
| Mild malnutrition | 3 (11.5%) | 6 (20%) | 9 (16.1%) | |
| Severe malnutrition | 1 (3.8%) | 12 (40%) | 13 (23.2%) | |
| Sarcopenia | 0.038 | |||
| Normal | 25 (96.2%) | 23 (76.7%) | 48 (85.7%) | |
| Sarcopenia | 1 (3.8%) | 7 (23.3%) | 8 (14.3%) | |
| Abdominal perimeter | 0.642 | |||
| Normal | 8 (30.8%) | 11 (36.7%) | 19 (33.9%) | |
| Metabolic syndrome criteria * | 18 (69.2%) | 19 (63.3%) | 37 (66.1%) | |
| Calf circumference | 0.038 | |||
| Undernourished | 1 (3.8%) | 7 (23.3%) | 8 (14.3%) | |
| Normal | 25 (96.2%) | 23 (76.7%) | 48 (85.7%) | |
| Arm circumference | 0.029 | |||
| Undernourished | 0 (0%) | 6 (20%) | 6 (10.7%) | |
| Regular | 1 (3.8%) | 3 (10%) | 4 (7.1%) | |
| Normal | 25 (96.2%) | 21 (70%) | 46 (82.1%) | |
| Strength (hand-grip) | 0.122 | |||
| Normal | 22 (84.6%) | 20 (66.7%) | 42 (75%) | |
| Low | 4 (15.4%) | 10 (33.3%) | 14 (25%) | |
| BMI (kg/m2) | 0.346 | |||
| <19 | 0 (0%) | 2 (6.7%) | 2 (3.6%) | |
| 19–21 | 2 (7.7%) | 3 (10%) | 5 (8.9%) | |
| 21–23 | 2 (7.7%) | 5 (16.7%) | 7 (12.5%) | |
| >23 | 22 (84.6%) | 20 (66.7%) | 42 (75%) | |
| FFMI | 0.097 | |||
| Normal | 26 (100%) | 27 (90%) | 53 (94.6%) | |
| Low | 0 (%) | 3 (10%) | 3 (5.4%) |
| No Undernutrition (n = 26) | Undernutrition (n = 31) | All (N = 57) | p-Value | |
|---|---|---|---|---|
| Exitus | <0.001 | |||
| No | 25 (96.2%) | 17 (54.8%) | 42 (73.7%) | |
| Yes | 1 (3.8%) | 14 (45.2%) | 15 (26.3%) | |
| Emergency care | <0.001 | |||
| No | 23 (88.5%) | 10 (32.3%) | 33 (57.9%) | |
| Yes | 3 (11.5%) | 21 (67.7%) | 24 (42.1%) | |
| Hospital admission | <0.001 | |||
| No | 23 (88.5%) | 11 (35.5%) | 34 (59.6%) | |
| Yes | 3 (11.5%) | 20 (64.5%) | 23 (40.4%) | |
| Number of admissions | <0.001 | |||
| Mean (SD) | 0.2 (0.8) | 1.9 (2.0) | 1.1 (1.8) | |
| ICU admission | 0.103 | |||
| No | 26 (100.0%) | 28 (90.3%) | 54 (94.7%) | |
| Yes | 0 (0.0%) | 3 (9.7%) | 3 (5.3%) | |
| New or further decompensation | <0.001 | |||
| No | 23 (88.5%) | 9 (29.0%) | 32 (56.1%) | |
| Yes | 3 (11.5%) | 22 (71.0%) | 25 (43.9%) | |
| Ascitis (new or worsen) | <0.001 | |||
| No | 26 (100.0%) | 15 (48.4%) | 41 (71.9%) | |
| Yes | 0 (0.0%) | 16 (51.6%) | 16 (28.1%) | |
| Increased doses of diuretics | <0.001 | |||
| No | 26 (100.0%) | 16 (51.6%) | 42 (73.7%) | |
| Yes | 0 (0.0%) | 15 (48.4%) | 15 (26.3%) | |
| Evacuative paracentesis | <0.001 | |||
| No | 26 (100.0%) | 17 (54.8%) | 43 (75.4%) | |
| Yes | 0 (0.0%) | 14 (45.2%) | 14 (24.6%) | |
| Variceal bleeding | 0.076 | |||
| No | 25 (96.2%) | 25 (80.6%) | 50 (87.7%) | |
| Yes | 1 (3.8%) | 6 (19.4%) | 7 (12.3%) | |
| Encephalopathy | 0.042 | |||
| No | 24 (92.3%) | 22 (71.0%) | 46 (80.7%) | |
| Yes | 2 (7.7%) | 9 (29.0%) | 11 (19.3%) | |
| Severe infection | 0.042 | |||
| No | 24 (92.3%) | 22 (71.0%) | 46 (80.7%) | |
| Yes | 2 (7.7%) | 9 (29.0%) | 11 (19.3%) |
| Coefficients of the Child-LDUST Model—Decompensation | ||||
| Predictor | Exp (B) | EE | Z | p |
| Constant | −11.18 | 3.001 | −3.72 | <0.001 |
| LDUST | ||||
| Risky–normal | 4.10 | 1.207 | 3.40 | <0.001 |
| Child (points) | 1.29 | 0.379 | 3.41 | <0.001 |
| Coefficients of the MELD-LDUST model—Decompensation | ||||
| Predictor | Exp (B) | EE | Z | p |
| Constant | −4.475 | 1.298 | −3.45 | <0.001 |
| LDUST | ||||
| Risky–normal | 2.841 | 0.750 | 3.79 | 3.79 |
| Child (points) | 0.227 | 0.101 | 2.24 | 2.24 |
| Decompensation | Mortality | |||
|---|---|---|---|---|
| AUC Value | Difference | AUC Value | Difference | |
| Child | 0.811 | 0.125 | 0.700 | 0.166 |
| Child-LDUST | 0.936 | 0.866 | ||
| MELD | 0.819 | 0.085 | 0.683 | 0.141 |
| MELD-LDUST | 0.904 | 0.824 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Deza, D.; Bernal-Monterde, V.; Betoré-Glaria, E.; Julián-Gomara, A.B.; Yagüe-Caballero, C.; Sanz-París, A.; Fernández-Bonilla, E.M.; Fuentes-Olmo, J.; Arbones-Mainar, J.M. Liver Disease Undernutrition Screening Tool Questionnaire Predicts Decompensation and Mortality in Cirrhotic Outpatients with Portal Hypertension. Nutrients 2023, 15, 3780. https://doi.org/10.3390/nu15173780
Casas-Deza D, Bernal-Monterde V, Betoré-Glaria E, Julián-Gomara AB, Yagüe-Caballero C, Sanz-París A, Fernández-Bonilla EM, Fuentes-Olmo J, Arbones-Mainar JM. Liver Disease Undernutrition Screening Tool Questionnaire Predicts Decompensation and Mortality in Cirrhotic Outpatients with Portal Hypertension. Nutrients. 2023; 15(17):3780. https://doi.org/10.3390/nu15173780
Chicago/Turabian StyleCasas-Deza, Diego, Vanesa Bernal-Monterde, Elena Betoré-Glaria, Ana Belén Julián-Gomara, Carmen Yagüe-Caballero, Alejandro Sanz-París, Eva María Fernández-Bonilla, Javier Fuentes-Olmo, and Jose M. Arbones-Mainar. 2023. "Liver Disease Undernutrition Screening Tool Questionnaire Predicts Decompensation and Mortality in Cirrhotic Outpatients with Portal Hypertension" Nutrients 15, no. 17: 3780. https://doi.org/10.3390/nu15173780
APA StyleCasas-Deza, D., Bernal-Monterde, V., Betoré-Glaria, E., Julián-Gomara, A. B., Yagüe-Caballero, C., Sanz-París, A., Fernández-Bonilla, E. M., Fuentes-Olmo, J., & Arbones-Mainar, J. M. (2023). Liver Disease Undernutrition Screening Tool Questionnaire Predicts Decompensation and Mortality in Cirrhotic Outpatients with Portal Hypertension. Nutrients, 15(17), 3780. https://doi.org/10.3390/nu15173780







