Evaluation of the Content of Micro- and Macroelements in Raspberries Depending on the Species, Cultivar Variety, and Geographical Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Samples
2.3. Method
2.4. Validation of Method
2.5. Statistical Analysis
3. Results and Discussion
3.1. Contents of Macro- and Micro-Elements in the Analysed Raspberry Fruits
3.2. Correlations
3.3. Kruskal–Wallis Test
3.4. Post Hoc Dunn’s Test
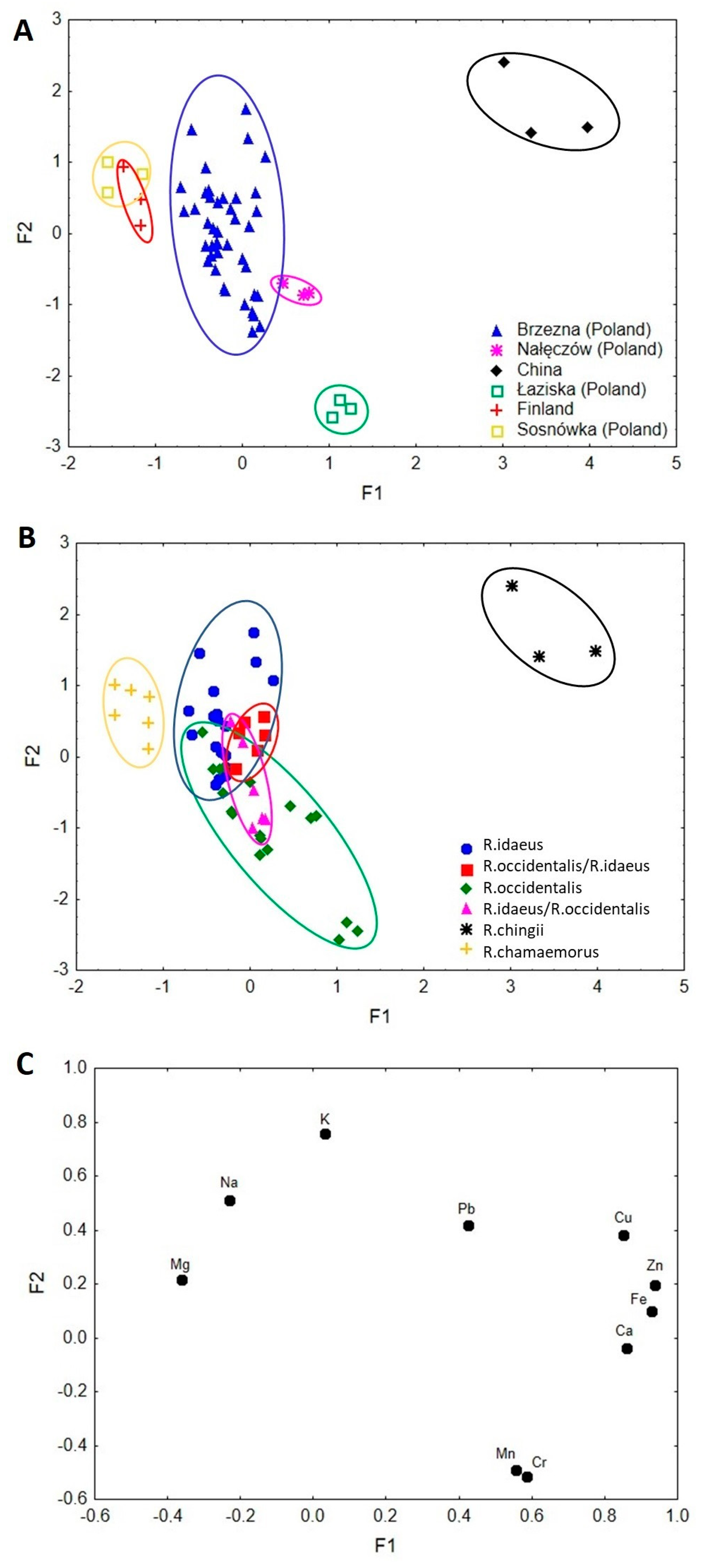
3.5. Factor Analysis
3.6. Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Ahuja, J.K.; Burton-Freeman, B.M. Characterization of the nutrient profile of processed red raspberries for use in nutrition labeling and promoting healthy food choices. Nutr. Healthy Aging 2019, 5, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Bojkovska, K.; Joshevskaand, F.; Tosheva, E.; Jovica, M. Global Raspberries Market Trends and Their Impact on the Macedonian Raspberries Market. Int. J. Res. Rev. 2021, 8, 362–369. [Google Scholar]
- Food and Agriculture Organization of the United Nations. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 23 August 2023).
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2009, 23, 554–560. [Google Scholar] [CrossRef]
- Moreno-Medina, B.L.; Casierra-Posada, F.; Cutler, J. Phytochemical Composition and Potential Use of Rubus Species. Gesunde Pflanz. 2018, 70, 65–74. [Google Scholar] [CrossRef]
- Persson, I.L.; Wikan, S.; Swenson, J.E.; Mysterud, I. The diet of the brown bear Ursus arctos in the Pasvik Valley, northeastern Norway. Wildl. Biol. 2001, 7, 27–37. [Google Scholar] [CrossRef]
- Thiem, B. Rubus chamaemorus L.—A boreal plant rich in biologically active metabolites: A review. Biol. Lett. 2003, 40, 3–13. [Google Scholar]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef]
- Seeram, N.P. Berry Fruits: Compositional Elements, Biochemical Activities, and the Impact of Their Intake on Human Health, Performance, and Disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef]
- Dossett, M.; Bassil, N.V.; Lewers, K.S.; Finn, C.E. Genetic diversity in wild and cultivated black raspberry (Rubus occidentalis L.) evaluated by simple sequence repeat markers. Genet. Resour. Crop. Evol. 2012, 59, 1849–1865. [Google Scholar] [CrossRef]
- Orzeł, A.; Król-Dyrek, K.; Kostecka-Gugała, A.; Bieniasz, M.; Augustynowicz, J.; Wyżgolik, G. Evaluation of vegetative growth and fruit chemistry of some raspberry and blackberry cultivars grown in southern Poland. In XI International Rubus and Ribes Symposium; ISHS: Leuven, Belgium, 2020; pp. 371–378. [Google Scholar] [CrossRef]
- Jia-Yun, S.; Si-Qi, W.; Kao-Hua, L.; Bo, Z.; Qiao-Yan, Z.; Lu-Ping, Q.; Jian-Jun, W. Rubus chingii Hu: An overview of botany, traditional uses, phytochemistry, and pharmacology. Chin. J. Nat. Med. 2020, 18, 401–416. [Google Scholar]
- Kim, L.S.; Youn, S.H.; Kim, J.Y. Comparative Study on Antioxidant Effects of Extracts from Rubus coreanus and Rubus occidentalis. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1357–1362. [Google Scholar] [CrossRef]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and Anti-Inflammatory Activities of Tannin Fraction of the Extract from Black Raspberry Seeds Compared to Grape Seeds. J. Food Biochem. 2014, 38, 259–270. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Chen, T.; Kresty, L.A.; Aziz, R.M.; Reinemann, T.; Nines, R. Protection Against Esophageal Cancer in Rodents With Lyophilized Berries: Potential Mechanisms. Nutr. Cancer 2006, 54, 33–46. [Google Scholar] [CrossRef]
- Montrose, D.C.; Horelik, N.A.; Madigan, J.P.; Stoner, G.D.; Wang, L.-S.; Bruno, R.S.; Park, H.J.; Giardina, C.; Rosenberg, D.W. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis 2011, 32, 343–350. [Google Scholar] [CrossRef]
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2016, 68, 18–28. [Google Scholar] [CrossRef]
- Yang, H.M.; Lim, S.S.; Lee, Y.S.; Shin, H.K.; Oh, Y.S. Comparison of the anti-inflammatory effects of the extracts from Rubus coreanus and Rubus occidentalis. Korean J. Food Sci. Technol. 2007, 39, 342–347. [Google Scholar]
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunol. Immunother. 2014, 63, 889–900. [Google Scholar] [CrossRef]
- Madhusoodhanan, R.; Natarajan, M.; Singh, J.V.N.; Jamgade, A.; Awasthi, V. Effect of black raspberry extract in inhibiting NF B dependent radioprotection in human breast cancer cells. Nutr. Cancer 2010, 62, 93–104. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry Extracts Inhibit Growth and Stimulate Apoptosis of Human Cancer Cells In Vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice—In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.J.; Martin, J.R.; Wulff, B.C.; Stoner, G.D.; Tober, K.L.; Oberyszyn, T.M.; Kusewitt, D.F.; Van Buskirk, A.M. Topical Treatment with Black Raspberry Extract Reduces Cutaneous UVB-Induced Carcinogenesis and Inflammation. Cancer Prev. Res. 2009, 2, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Dombkowski, A.A.; Seguin, C.; Rocha, C.; Cukovic, D.; Mukundan, A.; Henry, C.; Stoner, G.D. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carcinogenesis. Mol. Carcinog. 2011, 50, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Hecht, S.; Carmella, S.; Seguin, C.; Rocha, C.; Yu, N.; Stoner, K.; Chiu, S.; Stoner, G. Berry Ellagitannins May Not Be Sufficient for Prevention of Tumors in the Rodent Esophagus. J. Agric. Food Chem. 2010, 58, 3992–3995. [Google Scholar] [CrossRef]
- Bi, X.; Fang, W.; Wang, L.S.; Stoner, G.D.; Yang, J. Black raspberries inhibit intestinal tumorigenesis in mouse models of colorectal cancer. Cancer Prev. Res. 2010, 3, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Ma, H.; Yuan, T.; Chichester, C.O.; Seeram, N.P. Anti-inflammatory Effects of Polyphenolic-Enriched Red Raspberry Extract in an Antigen-Induced Arthritis Rat Model. J. Agric. Food Chem. 2012, 60, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.E.; Câmara, M.B.; Direito, R.; Rocha, J.; Serra, A.T.; Duarte, C.M.M.; Fernandes, A.; Freitas, M.; Marques, M.C.; Bronze, M.R.; et al. Chemical characterization of a red raspberry fruit extract and evaluation of its pharmacological effects in experimental models of acute inflammation and collagen-induced arthritis. Food Funct. 2014, 5, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus Berries for the Control of Gastric Inflammation: In Vitro and In Vivo Studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Gu, J.; Ahn-Jarvis, J.H.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Vodovotz, Y. Characterization of Black Raspberry Functional Food Products for Cancer Prevention Human Clinical Trials. J. Agric. Food Chem. 2014, 62, 3997–4006. [Google Scholar] [CrossRef]
- Roberts, K.M.; Grainger, E.M.; Thomas-Ahner, J.M.; Hinton, A.; Gu, J.; Riedl, K.; Vodovotz, Y.; Abaza, R.; Schwartz, S.J.; Clinton, S.K. Dose-Dependent Increases in Ellagitannin Metabolites as Biomarkers of Intake in Humans Consuming Standardized Black Raspberry Food Products Designed for Clinical Trials. Mol. Nutr. Food Res. 2020, 64, e1900800. [Google Scholar] [CrossRef]
- An, J.H.; Kim, D.L.; Lee, T.B.; Kim, K.J.; Kim, S.H.; Kim, N.H.; Kim, H.Y.; Choi, D.S.; Kim, S.G. Effect of Rubus occidentalis Extract on Metabolic Parameters in Subjects with Prediabetes: A Proof-of-concept, Randomized, Double-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2016, 30, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Cho, J.Y.; Lee, T.-B.; Kwon, J.-W.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.-S. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Renai, L.; Scordo, C.V.A.; Chiuminatto, U.; Ulaszewska, M.; Giordani, E.; Petrucci, W.A.; Tozzi, F.; Nin, S.; Del Bubba, M. Liquid Chromatographic Quadrupole Time-of-Flight Mass Spectrometric Untargeted Profiling of (Poly)phenolic Compounds in Rubus idaeus L. and Rubus occidentalis L. Fruits and Their Comparative Evaluation. Antioxidants 2021, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Johnson, J.L.; Bomser, J.A.; Scheerens, J.C.; Giusti, M.M. Effect of Black Raspberry (Rubus occidentalis L.) Extract Variation Conditioned by Cultivar, Production Site, and Fruit Maturity Stage on Colon Cancer Cell Proliferation. J. Agric. Food Chem. 2011, 59, 1638–1645. [Google Scholar] [CrossRef]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of Lyophilized Black Raspberries on Azoxymethane-Induced Colon Cancer and 8-Hydroxy-2′-Deoxyguanosine Levels in the Fischer 344 Rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef]
- Wang, L.-S.; Kuo, C.-T.; Cho, S.-J.; Seguin, C.; Siddiqui, J.; Stoner, K.; Weng, Y.-I.; Huang, T.H.-M.; Tichelaar, J.; Yearsley, M.; et al. Black Raspberry-Derived Anthocyanins Demethylate Tumor Suppressor Genes Through the Inhibition of DNMT1 and DNMT3B in Colon Cancer Cells. Nutr. Cancer 2013, 65, 118–125. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. Int. Rev. J. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Abo-Elmatty, D.M.; Mesbah, N.M.; Saleh, S.M.; Ali, A.-M.A.; Sakr, A.T. Raspberry ketone preserved cholinergic activity and antioxidant defense in obesity induced Alzheimer disease in rats. BioMedicine 2018, 107, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Kankainen, M.; Maukonen, J.; Törrönen, R.; Kolehmainen, M.; Leppänen, T.; Moilanen, E.; Nohynek, L.; Aura, A.-M.; et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries Decrease Cardiovascular Risk Factors in Obese Men and Women with Metabolic Syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Leal, M.F.C.; Catarino, R.I.L.; Pimenta, A.M.; Souto, M.R.S. Roles of Metal Microelements in Neurodegenerative Diseases. Neurophysiology 2020, 52, 80–88. [Google Scholar] [CrossRef]
- Gutowska, I.; Żwierełło, W.; Piorun, K.; Skórka-Majewicz, M.; Maciejewska-Markiewicz, D.; Kupnicka, P.; Baranowska-Bosiacka, I.; Dalewski, B.; Chlubek, D. The Extent of Burn Injury Significantly Affects Serum Micro- and Macroelement Concentrations in Patients on the First Day of Hospitalisation. Nutrients 2022, 14, 4248. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.Y.; Koltsov, V.A.; Zhbanova, E.V.; Akimova, O.M. Nutritional value of promising raspberry varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022078. [Google Scholar] [CrossRef]
- Tesovic, Z.; Dulic, I. Microelement level in the fruits of red raspberry (Rubus idaeus L.) cultivars and selections. Acta Hotic. 1989, 262, 327–332. [Google Scholar]
- Kresty, L.A.; Morse, M.A.; Morgan, C.; Carlton, P.S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, G.D. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119. [Google Scholar]
- Kresty, L.A.; Frankel, W.L.; Hammond, C.D.; Baird, M.E.; Mele, J.M.; Stoner, G.D.; Fromkes, J.J. Transitioning From Preclinical to Clinical Chemopreventive Assessments of Lyophilized Black Raspberries: Interim Results Show Berries Modulate Markers of Oxidative Stress in Barrett’s Esophagus Patients. Nutr. Cancer 2006, 54, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Jorhem, L.; Sundström, B. Levels of Lead, Cadmium, Zinc, Copper, Nickel, Chromium, Manganese, and Cobalt in Foods on the Swedish Market, 1983–1990. J. Food Compos. Anal. 1993, 6, 223–241. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Milinković, M.; Vranić, D.; Đurić, M.; Paunović, M. Chemical composition of organically and conventionally grown fruits of raspberry (Rubus idaeus L.) cv. Willamette. Acta Agric. Serb. 2021, 26, 83–88. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ. Monit. Assess. 2016, 188, 183. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International. Official method 999.11 determination of lead, cadmium, copper, iron, and zinc in foods atomic absorption spectrophotometry after dry ashing. J. AOAC Int. 2000, 83, 1204. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory: A Practical Approach; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Szefer, P. Chemometric techniques in analytical evaluation of food quality. In Mineral Components in Foods; Szefer, P., Nriagu, J., Eds.; CRC Press—Taylor & Francis: Boca Raton, FL, USA, 2019; pp. 163–229. [Google Scholar]
- Boliņa, L.; Osvalde, A.; Karlsons, A. Habitat Characteristics and Mineral Nutrition Status of Rubus chamaemorus L. in Latvia. Plants 2023, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- The World’s First Cloudberry Cultivation in an Agroforestry System. Available online: https://sir.cdr.gov.pl/2021/09/20/uprawa-maliny-moroszki-w-systemie-agrolesnym/ (accessed on 23 August 2023).
- European Network for Rural Development. Available online: https://ec.europa.eu/enrd/projects-practice/innovative-model-production-processing-and-distribution-herbs-zielawa-valley_de.html (accessed on 23 August 2023).
- Ryzhakova, N.K.; Babeshina, L.G.; Kondratyeva, A.G.; Sechnaya, D.Y. Contents of macro-, microelements and long-lived radionuclides in the medicinal plants belonging to the wetland community of Siberian region, Russia. Phytochem. Lett. 2017, 22, 280–286. [Google Scholar] [CrossRef]
- Dresler, S.; Bednarek, W.; Tkaczyk, P.; Hawrylak-Nowak, B. Estimation of the macro- and micronutrient status of raspberries grown in the Lublin region. Folia Hortic. 2015, 27, 53–62. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Interactions of Zinc with other nutrients in soils and plants—A review. Indian J. Fertil. 2016, 12, 16–26. [Google Scholar]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium Intake and Risk of Type 2 Diabetes in Men and Women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef]
- Scheelbeek, P.F.D.; Khan, A.E.; Mojumder, S.; Elliott, P.; Vineis, P. Drinking Water Sodium and Elevated Blood Pressure of Healthy Pregnant Women in Salinity—Affected Coastal Areas. Hypertension 2016, 68, 467–470. [Google Scholar] [CrossRef]
- Horuz, A.; Korkmaz, A.; Rüştü-Karaman, M. The evaluation of leaf nutrient contents and element ratios of different raspberry varieties. J. Food Agric. Environ. 2013, 11, 588–593. [Google Scholar]
- Preedy, V.R. Tea in Health and Disease Prevention; Elsevier: Gainesville, FL, USA, 2013. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Chrzanowska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020. [Google Scholar]
- Anderson, R.A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000, 26, 22–27. [Google Scholar] [PubMed]
- Martin, J.; Wang, Z.Q.; Zhang, X.H.; Wachtel, D.; Volaufova, J.; Matthews, D.E.; Cefalu, W.T. Chromium Picolinate Supplementation Attenuates Body Weight Gain and Increases Insulin Sensitivity in Subjects With Type 2 Diabetes. Diabetes Care 2006, 29, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R. Nature—The underestimated source of asorbic acid. Post Fitot. 2004, 1, 13–18. [Google Scholar]
- Jomova, K.; Baros, S.; Valko, M. Redox active metal-induced oxidative stress in biological systems. Transit. Met. Chem. 2012, 37, 127–134. [Google Scholar] [CrossRef]
- Rivera-Mancía, S.; Pérez-Neri, I.; Ríos, C.; Tristán-López, L.; Rivera-Espinosa, L.; Montes, S. The transition metals copper and iron in neurodegenerative diseases. Chem. Interact. 2010, 186, 184–199. [Google Scholar] [CrossRef]


| Cultivar or Hybrids/Colour of Fruit | Origin of the Cultivar | Pedigree * Op–Open-Pollination |
|---|---|---|
| R. occidentalis ‘Jewel’/black | Niwa Berry Breeding Ltd. (Brzezna, Poland) | ‘Dundee’ x (‘Bristol’ x ‘Dundee’) |
| R. occidentalis ‘Niwot’/black | Niwa Berry Breeding Ltd. (Brzezna, Poland) | A complex cross between two breeding clones from a natural environment of origin in the USA |
| R. occidentalis ‘MacBlack’/black | Niwa Berry Breeding Ltd. (Brzezna, Poland) | - |
| R. occidentalis ‘Heban’ (R139501)/black | Niwa Berry Breeding Ltd. (Brzezna, Poland) | (purple raspberry x ‘Polka’) x op |
| R. occidentalis ‘Bristol’ A/black | “Czarna malina” Barbara Rusiecka-Górniak (Nałęczów, Poland) | - |
| R. occidentalis ‘Bristol’ B/black | “BiGrim” Grzegorz Maryniowski (Łaziska, Poland) | - |
| R. idaeus ‘Husaria’/red | Niwa Berry Breeding Ltd. (Brzezna, Poland) | R120701 x ‘Sokolica’ |
| R. idaeus ‘Delniwa’/red | Niwa Berry Breeding Ltd. (Brzezna, Poland) | ‘Polka’ x R1211101 |
| R. idaeus ‘Poranna rosa’/yellow | Niwa Berry Breeding Ltd. (Brzezna, Poland) | 89112(83291 x ‘ORUS’ 10 98-1) |
| R. idaeus ‘Jantar’/yellow | Niwa Berry Breeding Ltd. (Brzezna, Poland) | R126107 (‘Heritage’ x ‘Polesie’) |
| R. idaeus ‘Promyk’/yellow | Niwa Berry Breeding Ltd. (Brzezna, Poland) | ‘Poemat’ x R127302 (‘Pingvin’ x op) |
| R. occidentalis/R. idaeus R1613411/Purple | Niwa Berry Breeding Ltd. (Brzezna, Poland). | ‘Jewel’ x R121304 (‘Litacz’ (‘Bristol’ x op) x purple raspberry x op) |
| R. idaeus/R. occidentalis R1613409/dark purple | Niwa Berry Breeding Ltd. (Brzezna, Poland) | ‘Jewel’ x R121304 (purple raspberry x op) |
| R. occidentalis/R. idaeus R1613412/purple | Niwa Berry Breeding Ltd. (Brzezna, Poland) | ‘Jewel’ x R121304 (‘Litacz’ x purple raspberry) x op |
| R. idaeus/ R. occidentalis R1314701/purple | Niwa Berry Breeding Ltd. (Brzezna, Poland) | ‘Litacz’ x ‘Sokolica’ |
| R. idaeus R1616002/red | Niwa Berry Breeding Ltd. (Brzezna, Poland) | R1634401 x ‘Polana’ |
| R. chamaemorus | Commercial source, Finland | - |
| R. chamaemorus | Lubelskie Zioła, Poland (Sosnówka, Poland). | - |
| R. chingii | Commercial source, China | - |
| Element | LOD (µg/mL) | LOQ (µg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Ca | 0.0001 | 0.0003 | 92 | 4.15 |
| Cu | 0.0002 | 0.0006 | 97 | 1.42 |
| Cd | 0.0002 | 0.0006 | 101 | 3.42 |
| Cr | 0.0002 | 0.0006 | 95 | 0.02 |
| Mg | 0.0005 | 0.0015 | 99 | 0.59 |
| Mn | 0.0001 | 0.0003 | 108 | 1.57 |
| Zn | 0.0001 | 0.0003 | 104 | 3.31 |
| K | 0.0005 | 0.0015 | 102 | 2.06 |
| Na | 0.0002 | 0.0006 | 98 | 10.1 |
| Pb | 0.0002 | 0.0006 | 92 | 4.50 |
| Fe | 0.0002 | 0.0006 | 101 | 0.62 |
| Species/Variety/Hybrid | Mg | Ca | Na | K | Fe | Zn | Cr | Cu | Mn | Pb | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rubus idaeus/ ‘Poranna Rosa’ | 126.22 ± 5.44 | 9.33 ± 0.84 | 6.11 ± 0.84 | 1374.74 ± 14.3 | 4.27 ± 0.33 | 2.68 ± 0.06 | 0.025 ± 0.004 | 0.85 ± 0.02 | 0.66 ± 0.01 | 0.0299 ± 0.0015 | ND |
| ‘Promyk’ | 138.34 ± 3.12 | 3.96 ± 0.06 | 7.96 ± 0.4 | 1373.69 ± 3.77 | 2.84 ± 0.16 | 1.82 ± 0.04 | 0.051 ± 0.004 | 0.84 ± 0.02 | 0.4 ± 0.03 | 0.012 ± 0.0008 | ND |
| ‘Jantar’ | 168.64 ± 1.28 | 7.79 ± 0.35 | 3.3 ± 0.21 | 1266.25 ± 5.97 | 2.31 ± 0.21 | 2.29 ± 0.11 | 0.027 ± 0.007 | 1.01 ± 0.04 | 0.13 ± 0.02 | 0.0163 ± 0.001 | ND |
| ‘Delniwa’ | 113.63 ± 8.12 | 7.47 ± 0.9 | 3.99 ± 0.41 | 935.06 ± 8.42 | 2.76 ± 0.02 | 1.58 ± 0.13 | 0.032 ± 0.006 | 0.77 ± 0.18 | 0.16 ± 0.02 | 0.0176 ± 0.0004 | ND |
| ‘Husaria’ | 161.35 ± 8.75 | 5.03 ± 0.46 | 3.03 ± 0.27 | 1109.32 ± 15.14 | 2.88 ± 0.07 | 2.26 ± 0.12 | 0.042 ± 0.007 | 0.72 ± 0.21 | 0.17 ± 0.01 | 0.0124 ± 0.0006 | 0.009 ± 0.001 |
| Rubus occidentalis/ ‘Jewel’ | 110.11 ± 6.13 | 19.02 ± 0.85 | 1.37 ± 0.23 | 1003.98 ± 7.92 | 2.43 ± 0.32 | 2.03 ± 0.22 | 0.019 ± 0.004 | 0.95 ± 0.09 | 0.84 ± 0.01 | 0.0123 ± 0.0009 | 0.086 ± 0.002 |
| ‘MacBlack’ | 134.05 ± 4.97 | 29.51 ± 2.27 | 0.32 ± 0.05 | 933.14 ± 1.78 | 1.6 ± 0.33 | 1.81 ± 0.19 | 0.046 ± 0.005 | 0.94 ± 0.11 | 0.73 ± 0.01 | 0.0124 ± 0.0004 | ND |
| ‘Niwot’ | 174.68 ± 10.6 | 30.97 ± 2.24 | 2.85 ± 0.22 | 969.65 ± 7.74 | 2.24 ± 0.06 | 1.86 ± 0.14 | 0.089 ± 0.009 | 0.86 ± 0.08 | 0.8 ± 0.02 | 0.0095 ± 0.001 | ND |
| ‘Heban’ | 224.08 ± 4.43 | 21.56 ± 1.05 | 1.54 ± 0.59 | 1040.46 ± 18.51 | 1.96 ± 0.05 | 2.12 ± 0.02 | 0.02 ± 0.007 | 0.94 ± 0.07 | 0.68 ± 0.02 | 0.0134 ± 0.0013 | ND |
| ‘Bristol’ A | 148.36 ± 4.28 | 17.62 ± 0.38 | 1.27 ± 0.2 | 1140.27 ± 4.11 | 5.15 ± 0.06 | 3.07 ± 0.1 | 0.038 ± 0.012 | 1.28 ± 0.03 | 6.91 ± 0.01 | 0.0131 ± 0.0006 | 0.0119 ± 0.0015 |
| ‘Bristol’ B | 95.41 ± 4.48 | 24.47 ± 0.61 | 2.83 ± 0.57 | 648.73 ± 0.85 | 6.08 ± 0.36 | 2.86 ± 0.02 | 0.077 ± 0.003 | 1.19 ± 0.05 | 8.45 ± 0.03 | 0.0135 ± 0.0008 | 0.0164 ± 0.0026 |
| R. occidentalis/R. idaeus | |||||||||||
| R1613411 | 190.78 ± 4.21 | 26.91 ± 1.66 | 2.58 ± 0.11 | 1221.16 ± 1.62 | 2.76 ± 0.1 | 2.62 ± 0.29 | 0.025 ± 0.001 | 0.98 ± 0.03 | 0.83 ± 0.06 | 0.0097 ± 0.0015 | ND |
| R1613412 | 116.2 ± 5.45 | 19.31 ± 0.8 | 2.99 ± 0.04 | 986.07 ± 7.27 | 2.25 ± 0.37 | 2.38 ± 0.14 | 0.029 ± 0.004 | 0.9 ± 0.1 | 0.85 ± 0.01 | 0.0333 ± 0.0009 | ND |
| R. idaeus/R. occidentalis R1613409 | 135.05 ± 7.31 | 27.47 ± 0.12 | 2.5 ± 0.12 | 819.93 ± 5.77 | 1.97 ± 0.24 | 2.76 ± 0.29 | 0.051 ± 0.005 | 0.97 ± 0.05 | 1.11 ± 0.04 | 0.0154 ± 0.0012 | ND |
| R1314701 | 137.4 ± 6.61 | 21.39 ± 1.31 | 4.78 ± 0.91 | 1185.37 ± 2.94 | 2.39 ± 0.08 | 2.19 ± 0.12 | 0.04 ± 0.002 | 0.96 ± 0.08 | 0.81 ± 0.05 | 0.0137 ± 0.0008 | 0.0041 ± 0.0007 |
| R. idaeus | |||||||||||
| R1616002 | 163.53 ± 6.88 | 5.03 ± 0.15 | 4.09 ± 0.4 | 1460.19 ± 0.49 | 1.84 ± 0.03 | 1.71 ± 0.01 | 0.025 ± 0.01 | 0.77 ± 0.03 | 0.18 ± 0.01 | 0.0118 ± 0.0004 | ND |
| Rubus chingii | 122.52 ± 2.45 | 72.13 ± 6.84 | 7.29 ± 1.02 | 1273.07 ± 5.28 | 10.22 ± 0.11 | 6.84 ± 0.15 | 0.062 ± 0.019 | 17.42 ± 0.78 | 3.4 ± 0.08 | 0.0273 ± 0.0051 | 0.028 ± 0.009 |
| Rubus chamaemorus Poland | 184.11 ± 2.96 | 1.56 ± 0.17 | 18.72 ± 0.49 | 1046.54 ± 7.78 | 0.28 ± 0.06 | 0.33 ± 0.01 | 0.012 ± 0.001 | 0.05 ± 0.01 | 0.63 ± 0.03 | 0.0183 ± 0.0026 | 0.0233 ± 0.0011 |
| Rubus chamaemorus Finland | 215.77 ± 9.73 | 1.31 ± 0.02 | 8.63 ± 1.32 | 1023.51 ± 2.9 | 0.25 ± 0.1 | 0.35 ± 0.01 | 0.011 ± 0.001 | 0.07 ± 0.01 | 0.59 ± 0.11 | 0.0077 ± 0.0005 | 0.0105 ± 0.0013 |
| Element | Recommended Daily Allowance (RDA) (mg/Day/Person) | Average Content in 100 g Rubus idaeus Lyophilised Fruits | Percentage of RDA | Average Content in 100 g Rubus occidentalis Lyophilised Fruits | Percentage of RDA | Average Content in 100 g Rubus chamaemorus Lyophilised Fruits | Percentage of RDA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (31–50 Years) | Female (31–50 Years) | Male (31–50 Years) | Female (31–50 Years) | Male (31–50 Years) | Female (31–50 Years) | Male (31–50 Years) | Female (31–50 Years) | ||||
| Mg | 420 | 320 | 113–168 | 26.9–40 | 35–52.5 | 95–224 | 22.6–53.3 | 29.7–70 | 184–215 | 43.8–51.2 | 57.5–67.2 |
| K | 4700 | 4700 | 935–1460 | 19.9–31.1 | 19.9–31.1 | 648–1140 | 13.8–24.2 | 13.8–24.2 | 1023–1046 | 21.8–22.2 | 21.8–22.2 |
| Fe | 10 | 18 | 1.84–4.27 | 18.4–42.7 | 10.2–23.7 | 1.6–6.08 | 16–60.8 | 8.8–33.8 | 0.25–0.28 | 2.5–2.8 | 1.4–1.5 |
| Zn | 11 | 8 | 1.58–2.68 | 14.4–24.4 | 19.7–33.5 | 1.81–3.07 | 16.4–27.9 | 22.6–38.4 | 0.33–0.35 | 3–3.2 | 4.1–4.4 |
| Cu | 0.9 | 0.9 | 0.72–1.01 | 80–112.2 | 80–112.2 | 0.86–1.28 | 95.5–142.2 | 95.5–142.2 | 0.05–0.07 | 5.5–7.8 | 5.5–7.8 |
| Mn | 2.3 | 1.8 | 0.13–0.66 | 5.6–28.7 | 7.2–36.7 | 0.68–8.45 | 29.6–367.4 | 37.7–469.4 | 0.59–0.63 | 25.6–27.4 | 32.7–35 |
| Cr a | 0.035 | 0.025 | 0.025–0.051 | 71.4–145.7 | 100–204 | 0.019–0.089 | 54.3–254.3 | 76–356 | 0.011–0.012 | 31.4–34.3 | 44–48 |
| R. idaeus | R. occidentalis/ R. idaeus | R. occidentalis | R. idaeus/ R. occidenatlis | R. chingii | R. chamaemorus | |
|---|---|---|---|---|---|---|
| R. idaeus | - | Ca a, Mn a | K b, Na c, Ca c, Mnc | Ca a, Mn b | Ca b, Cu a, Mn b | Fe b |
| R. occidentalis/ R. idaeus | Ca a, Mn b | - | Na a, Ca b, Fe a, Zn b | |||
| R. occidentalis | K b, Na c, Ca c, Mn c | - | Na a | Na c, Ca c, Fe a, Zn b, Cu c, Cr a | ||
| R. idaeus/ R. occidenatlis | Ca a, Mn b | - | Ca b, Zn a, Cu a, Cr a | |||
| R. chingii | Ca b,Cu a, Mn b | Na a | - | Ca c, Fe c, Zn c, Cu c, Cr a | ||
| R. chamaemorus | Fe b | Na a, Ca b, Fe a, Zn b | Na c, Ca c, Fe a, Zn b, Cu c, Cr a | Ca b, Zn a, Cu a, Cr a | Ca c, Fe c, Zn c, Cu c, Cr a | - |
| Brzezna/Lesser/ Poland | Nałęczów/Lublin Voivodship/Poland | China | Łaziska/Lublin Voivodship/Poland | Finland | Sosnówka/Lublin Voivodship/Poland | |
|---|---|---|---|---|---|---|
| Brzezna/Lesser Poland | - | Mna | Naa | |||
| Nałęczów/Lublin Voivodship/Poland | - | Na a, Fe b, Zn b, Cu b | Na b, Fe b, Zn b, Cu b | |||
| China | - | K a | Ca b, Fe b, Zn b, Cu b, Cr a | Ca b, Fe b, Zn b, Cu b, Pb b | ||
| Łaziska/Lublin Voivodship/Poland | Mn a | K a | - | Fe b, Zn a, Cu a, Cr b | Fe b, Zn a, Cu b, Cr a | |
| Finland | Na a, Fe b, Zn b, Cu b | Ca b, Fe b, Zn b, Cu b, Cr a | Fe b, Zn a, Cu a, Cr b | - | ||
| Sosnówka/Lublin Voivodship/Poland | Na a | Na b, Fe b, Zn b, Cu b | Ca b, Fe b, Zn b, Cu b, Pb b | Fe a,b, Zn a, Cu b, Cr a | - |
| R. idaeus | R. occidentalis/ R. idaeus | R. occidentalis | R. idaeus/ R. occidenatlis | R. chamaemorus | |
|---|---|---|---|---|---|
| R. idaeus | - | Ca a, Mn b | K b, Na c, Ca c,Mn c, Cu a | Ca b, Mn c | Fe a |
| R. occidentalis/ R. idaeus | Ca a, Mn b | - | Cab, Znb | ||
| R. occidentalis | K b, Na c, Ca c, Mn c, Cu a | - | Na c, Ca b, Zn a, Cu b | ||
| R. idaeus/ R. occidenatlis | Ca b, Mn c | - | Ca b, Cu a, Pb a | ||
| R. chamaemorus | Fe a | Ca b, Zn b | Na c, Ca b, Zn a, Cu b | Ca b, Cu a, Pb a | - |
| Brzezna/Lesser/Poland | Nałęczów/Lublin Voivodship/Poland | Łaziska/Lublin Voivodship/Poland | Sosnówka/Lublin Voivodship/Poland | |
|---|---|---|---|---|
| Brzezna/Lesser Poland | - | Cu a, Mn a | K a, Fe a, Cu a, Mn a | Na a, Ca a |
| Nałęczów/Lublin Voivodship/Poland | Cu a, Mn a | - | Na b, Fe b, Zn c, Cu c | |
| Łaziska/Lublin Voivodship/Poland | K a, Fe a, Cu a, Mn a | - | Mg a, Ca a, Fe c, Zn b, Cu c, Mn a, Cr a | |
| Sosnówka/Lublin Voivodship/Poland | Na a, Ca a | Na a,b, Fe b, Zn c, Cu c | Mg a, Ca a, Fe c, Zn b, Cu c, Mn a, Cr a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczuk, N.; Ośko, J.; Grembecka, M.; Konieczyński, P.; Migas, P.; Orzeł, A.; Baj-Wójtowicz, B.; Krauze-Baranowska, M. Evaluation of the Content of Micro- and Macroelements in Raspberries Depending on the Species, Cultivar Variety, and Geographical Environment. Nutrients 2023, 15, 3782. https://doi.org/10.3390/nu15173782
Adamczuk N, Ośko J, Grembecka M, Konieczyński P, Migas P, Orzeł A, Baj-Wójtowicz B, Krauze-Baranowska M. Evaluation of the Content of Micro- and Macroelements in Raspberries Depending on the Species, Cultivar Variety, and Geographical Environment. Nutrients. 2023; 15(17):3782. https://doi.org/10.3390/nu15173782
Chicago/Turabian StyleAdamczuk, Natalia, Justyna Ośko, Małgorzata Grembecka, Paweł Konieczyński, Piotr Migas, Agnieszka Orzeł, Barbara Baj-Wójtowicz, and Mirosława Krauze-Baranowska. 2023. "Evaluation of the Content of Micro- and Macroelements in Raspberries Depending on the Species, Cultivar Variety, and Geographical Environment" Nutrients 15, no. 17: 3782. https://doi.org/10.3390/nu15173782





