Maternal Obesity and Patterns in Postnatal Diet, Physical Activity and Weight among a Highly Deprived Population in the UK: The GLOWING Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Socio-Demographic Data Collection
2.2. Diet, Physical Activity and Weight Data Collection
2.3. Data Analysis
3. Results
3.1. Participants Characteristics
3.2. Dietary Behaviours
3.3. Physical Activity Behaviours
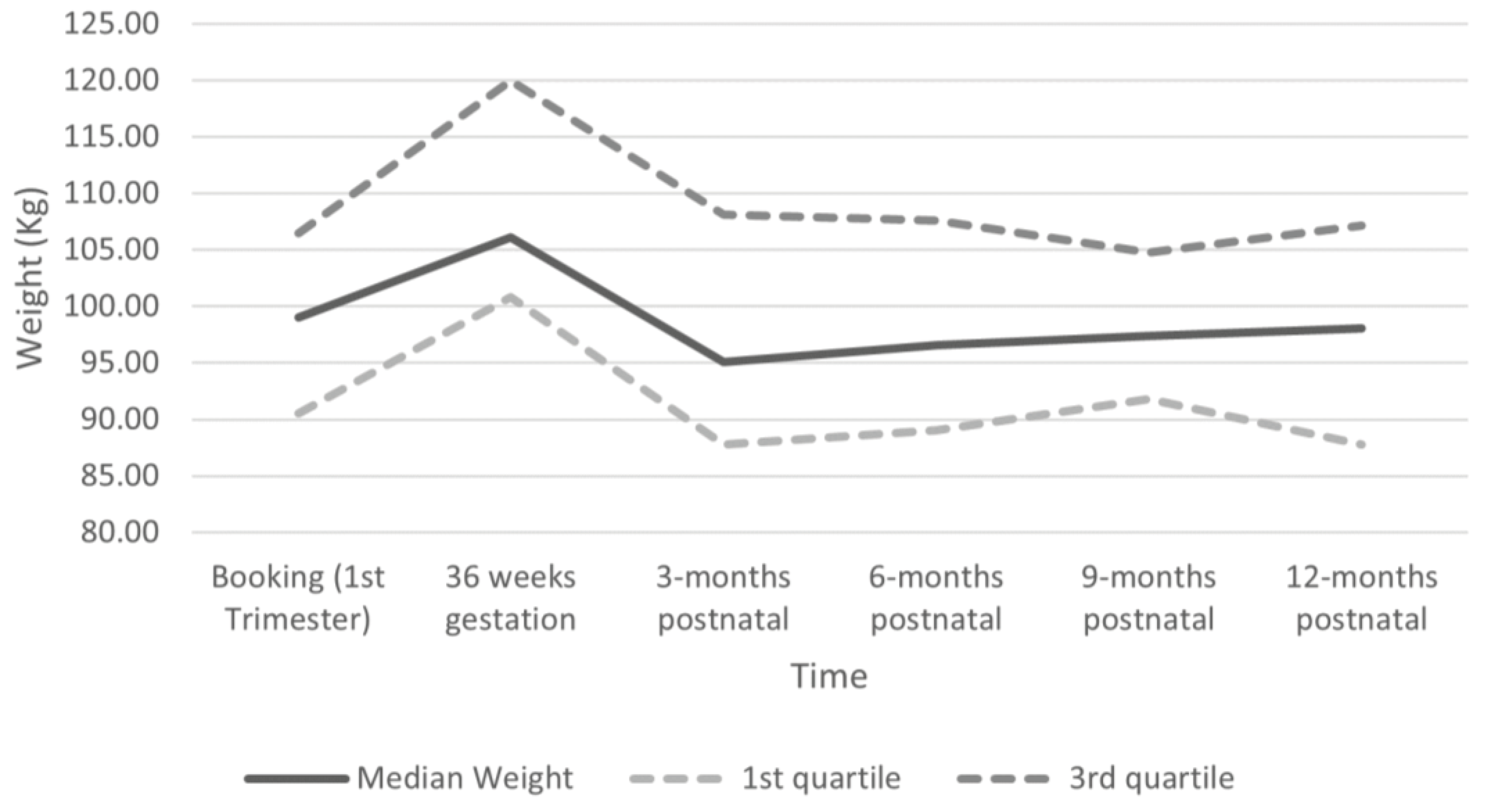
3.4. Postnatal Weight Change
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. Br. Med. J. 2017, 356, j1. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef]
- Relph, S.; NMPA Project Team. NHS Maternity Care for Women with a Body Mass Index of 30 kg/m2 or Above: Births between 1 April 2015 and 31 March 2017 in England, Wales and Scotland; RCOG: London, UK, 2021. [Google Scholar]
- Nguyen, G.; Boath, A.; Heslehurst, N. Addressing inequalities and improving maternal and infant outcomes: The potential power of nutritional interventions across the reproductive cycle. Proc. Nutr. Soc. 2023, 1–12. [Google Scholar] [CrossRef]
- Institute for Medicine and National Research Council. Weight Gain during Pregnancy: Examining the Guidelines; The National Academy Press: Washington, DC, USA, 2009.
- Kominiarek, M.A.; Peaceman, A.M. Gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 642–651. [Google Scholar] [CrossRef]
- Davis, E.M.; Zyzanski, S.J.; Olson, C.M.; Stange, K.C.; Horwitz, R.I. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. Am. J. Public Health 2009, 99, 294–299. [Google Scholar] [CrossRef]
- Nurul-Farehah, S.; Rohana, A.J. Maternal obesity and its determinants: A neglected issue? Malays. Fam. Physician 2020, 15, 34–42. [Google Scholar]
- Endres, L.K.; Straub, H.; McKinney, C.; Plunkett, B.; Minkovitz, C.S.; Schetter, C.D.; Ramey, S.; Wang, C.; Hobel, C.; Raju, T.; et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obs. Gynecol 2015, 125, 144–152. [Google Scholar] [CrossRef]
- Nagpal, T.S.; Souza, S.C.S.; Moffat, M.; Hayes, L.; Nuyts, T.; Liu, R.H.; Bogaerts, A.; Dervis, S.; Piccinini-Vallis, H.; Adamo, K.B.; et al. Does prepregnancy weight change have an effect on subsequent pregnancy health outcomes? A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13324. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion number 315: Obesity in Pregnancy. Obstet. Gynecol. 2005, 106, 671–675. [Google Scholar] [CrossRef]
- Hanson, M.A.; Bardsley, A.; De-Redil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrcs (FIGO) recommendations on adolescent, preconception and maternal nutrition: “think nutrition first”. Int. J. Gynecol. Obstet. 2015, 131, S213–S253. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Weight Management before, during and after Pregnancy. Available online: https://www.nice.org.uk/guidance/ph27/resources/weight-management-before-during-and-after-pregnancy-pdf-1996242046405 (accessed on 10 November 2021).
- UK Chief Medical Officers. Physical Activity Guidelines. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf. (accessed on 17 February 2022).
- National Health Service (NHS) England. The Eatwell Guide: Eat Well. Available online: https://www.nhs.uk/live-well/eat-well/the-eatwell-guide/ (accessed on 21 February 2022).
- Public Health England (PHE). The Eatwell Guide; Public Health England (PHE): London, UK, 2016.
- Abbasi, M.; van den Akker, O. A systematic review of changes in women’s physical activity before and during pregnancy and the postnatal period. J. Reprod. Infant Psychol. 2015, 33, 325–358. [Google Scholar] [CrossRef]
- Moran, L.J.; Sui, Z.; Cramp, C.S.; Dodd, J.M. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post-partum. Int. J. Obes. 2013, 37, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Moran, L.J.; Dodd, J.M. Physical activity levels during pregnancy and gestational weight gain among women who are overweight or obese. Health Promot. J. Aust. 2013, 24, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Freeland-Graves, J.H.; Cahill, J.M.; Lu, H.; Graves, G.R. Diet quality as measured by the healthy eating index and the association with lipid profile in low-income women in early postpartum. J. Am. Diet. Assoc. 2010, 110, 274–279. [Google Scholar] [CrossRef]
- Wiltheiss, G.A.; Lovelady, C.A.; West, D.G.; Brouwer, R.J.; Krause, K.M.; Østbye, T. Diet quality and weight change among overweight and obese postpartum women enrolled in a behavioral intervention program. J. Acad. Nutr. Diet. 2013, 113, 54–62. [Google Scholar] [CrossRef]
- Soltani, H.; Fraser, R.B. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br. J. Nutr. 2000, 84, 95–101. [Google Scholar] [CrossRef]
- Heslehurst, N.; Rankin, J.; McParlin, C.; Sniehotta, F.F.; Howel, D.; Rice, S.; McColl, E. GestationaL Obesity Weight management: Implementation of National Guidelines (GLOWING): A pilot cluster randomised controlled trial of a guideline implementation intervention for the management of maternal obesity by midwives. Pilot Feasibility Stud. 2018, 4, 47. [Google Scholar] [CrossRef]
- Heslehurst, N.; Rankin, J.; Wilkinson, J.R.; Summerbell, C.D. A nationally representative study of maternal obesity in England, UK: Trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int. J. Obes. 2010, 34, 420–428. [Google Scholar] [CrossRef]
- Bradshaw, J. Child Poverty in the North East Region; Child Poverty Action Group: London, UK, 2020. [Google Scholar]
- Public Health England. Public Health Profiles: Overweight in North East England. Available online: https://fingertips.phe.org.uk/search/overweight (accessed on 9 December 2021).
- Heslehurst, N.; Flynn, A.C.; Ngongalah, L.; McParlin, C.; Dalrymple, K.V.; Best, K.E.; Rankin, J.; McColl, E. Diet, Physical Activity and Gestational Weight Gain Patterns among Pregnant Women Living with Obesity in the North East of England: The GLOWING Pilot Trial. Nutrients 2021, 13, 1981. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consultation on Obesity (1999: Geneva, Switzerland). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. 2000. Available online: https://apps.who.int/iris/handle/10665/42330 (accessed on 17 February 2022).
- Ministry of Housing, C.L.G. English Indices of Deprivation 2019: Postcode Lookup. Available online: https://imd-by-postcode.opendatacommunities.org/imd/2019 (accessed on 18 January 2022).
- Flynn, A.C.; Seed, P.T.; Patel, N.; Barr, S.; Bell, R.; Briley, A.L.; Godfrey, K.M.; Nelson, S.M.; Oteng-Ntim, E.; Robinson, S.M. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Bingham, S.A.; Welch, A.A.; McTaggart, A.; Mulligan, A.A.; Runswick, S.A.; Luben, R.; Oakes, S.; Khaw, K.T.; Wareham, N.; Day, N.E. Nutritional methods in the European prospective investigation of cancer in Norfolk. Public Health Nutr. 2001, 4, 847–858. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004, 3, 1750–1760. [Google Scholar] [CrossRef]
- Roberts, D.E.; Fragala, M.S.; Pober, D.; Chasan-Taber, L.; Freedson, P.S. Energy Cost of Physical Activities During Pregnancy. Med. Sci. Sports Exerc. 2002, 34. [Google Scholar] [CrossRef]
- NMPA Project Team. National Maternity and Perinatal Audit: Clinical Report 2021. Based on Births in NHS Maternity Services in England, Scotland and Wales between 1 April 2017 and 31 March 2018; RCOG: London, UK, 2021. [Google Scholar]
- Office for National Statistics (ONS). Female Employment Rate (Aged 6 to 64, Seasonally Adjusted). Available online: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/timeseries/lf25/lms (accessed on 17 February 2022).
- Office for National Statistics (ONS). Conceptions in England and Wales: 2014. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/conceptionandfertilityrates/bulletins/conceptionstatistics/2014#marital-status-at-conception (accessed on 17 February 2022).
- Office for National Statistics (ONS), Released 6 December 2022, ONS Website, Statistical Bulletin, Adult Smoking Habits in the UK: 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2021. (accessed on 17 February 2022).
- Public Health England (PHE). NDNS: Results from Years 9 to 11 (Combined)—Statistical Summary; Public Health England (PHE): London, UK, 2020.
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Ashman, A.M.; Collins, C.E.; Hure, A.J.; Jensen, M.; Oldmeadow, C. Maternal diet during early childhood, but not pregnancy, predicts diet quality and fruit and vegetable acceptance in offspring. Matern. Child Nutr. 2016, 12, 579–590. [Google Scholar] [CrossRef]
- Makama, M.; Brown, W.J.; Lim, S.; Skouteris, H.; Harrison, C.L.; Joham, A.E.; Mishra, G.D.; Teede, H.; Moran, L.J. Levels of physical activity and sitting time in women with infants, toddlers and preschoolers: A population-based cross-sectional study. Public Health 2023, 214, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; Uwhubetine, O.; Flynn, A.C.; Pasupathy, D.; Briley, A.L.; Relph, S.A.; Seed, P.T.; O’Keeffe, M.; Poston, L. Modifiable Determinants of Postpartum Weight Loss in Women with Obesity: A Secondary Analysis of the UPBEAT Trial. Nutrients 2021, 13, 1979. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Doi, S.A.; Mamun, A.A. Association between weight gain during pregnancy and postpartum weight retention and obesity: A bias-adjusted meta-analysis. Nutr. Rev. 2013, 71, 343–352. [Google Scholar] [CrossRef]
- McAndrew, F.; Thompson, J.; Fellows, L.; Large, A.; Speed, M.; Renfrew, M.J. Infant Feeding Survey 2010; Health and Social Care Information Centre: Leeds, UK, 2012.
- Marshall, N.E.; Lau, B.; Purnell, J.Q.; Thornburg, K.L. Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern. Child Nutr. 2019, 15, e12732. [Google Scholar] [CrossRef] [PubMed]
- Oakley, L.L.; Renfrew, M.J.; Kurinczuk, J.J.; Quigley, M.A. Factors associated with breastfeeding in England: An analysis by primary care trust. BMJ Open 2013, 3, e002765. [Google Scholar] [CrossRef] [PubMed]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunešová, M.; Hejgaard, T.; García Solano, M.; Fijałkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.A.; McAuliffe, F.M. Maternal nutrient intakes and levels of energy underreporting during early pregnancy. Eur. J. Clin. Nutr. 2012, 66, 906–913. [Google Scholar] [CrossRef]
- McNitt, K.M.; Hohman, E.E.; Rivera, D.E.; Guo, P.; Pauley, A.M.; Gernand, A.D.; Symons Downs, D.; Savage, J.S. Underreporting of Energy Intake Increases over Pregnancy: An Intensive Longitudinal Study of Women with Overweight and Obesity. Nutrients 2022, 14, 2326. [Google Scholar] [CrossRef]
| 3-Months | 6-Months | 9-Months | 12-Months | |
|---|---|---|---|---|
| Milk mL/d | 142 (142, 285) | 142 (142, 285) | 142 (142, 285) | 142 (36, 285) |
| Reduced fat (n, %) | 19 (79.2) | 18 (81.8) | 8 (66.7) | 11 (55) |
| Full fat (n, %) | 3 (12.5) | 3 (13.6) | 3 (25) | 5 (25) |
| None (n, %) | 2 (8.3) | 1 (4.5) | 1 (8.3) | 4 (20) |
| Spread g/d | 6 (0, 11) | 9 (4, 23) | 10 (5, 14) | 5 (1, 9) |
| Reduced fat (n, %) | 11 (45.8) | 13 (59.1) | 6 (50) | 12 (60) |
| Full fat (n, %) | 7 (29.2) | 8 (36.4) | 5 (41.7) | 4 (20) |
| None (n, %) | 6 (25) | 1 (4.5) | 1 (8.3) | 4 (20) |
| Cheese g/d | 4 (0, 30) | 9 (0, 36) | 4 (0, 22) | 12 (0, 25) |
| Reduced fat (n, %) | 3 (12.5) | 3 (13.6) | 2 (16.7) | 2 (10.0) |
| Full fat (n, %) | 12 (50.0) | 13 (59.1) | 6 (50.0) | 12 (60.0) |
| None (n, %) | 9 (37.5) | 6 (27.3) | 4 (33.3) | 6 (30.0) |
| Sugary Drinks mL/d | 158 (40, 500) | 80 (3, 279) | 14 (0, 211) | 57 (14, 518) |
| Fruit juice | 0 (0, 27) | 0 (0, 28) | 7 (0, 24) | 14 (0, 24) |
| Sugar-sweetened beverages | 151 (0, 490) | 76 (0, 226) | 0 (0, 178) | 10 (0, 500) |
| Starchy carbohydrate foods g/d | 103 (62, 140) | 93 (71, 158) | 109 (88, 160) | 85 (29, 149) |
| Rice, pasta, noodles, potatoes | 14 (12, 17) | 16 (13, 18) | 16 (11, 20) | 15 (13, 19) |
| Takeaway and oven chips | 4 (3, 6) | 5 (4, 6) | 5 (3, 6) | 4 (4, 6) |
| Bread g/d | 51 (26, 76) | 54 (31, 90) | 72 (57, 126) | 36 (12, 72) |
| Wholemeal bread (n, %) | 12 (50.0) | 11 (50.0) | 5 (41.7) | 10 (50.0) |
| White bread (n, %) | 9 (37.5) | 10 (45.5) | 7 (58.3) | 7 (35.0) |
| No bread (n, %) | 3 (12.5) | 1 (4.5) | 0 | 3 (15.0) |
| Breakfast cereal g/d | 10 (0, 30) | 0 (0, 38) | 0 (0, 20) | 0 (0, 29) |
| Refined breakfast cereal (n, %) | 7 (29.2) | 3 (13.6) | 3 (25) | 2 (10.0) |
| Non-refined breakfast cereal (n, %) | 7 (29.2) | 6 (27.3) | 1 (8.3) | 5 (25.0) |
| No breakfast cereal (n, %) | 10 (41.7) | 13 (59.1) | 8 (66.7) | 13 (65.0) |
| Fruits and Vegetables g/d | 161 (80, 355) | 147 (58, 256) | 131 (41, 306) | 136 (47, 286) |
| Vegetables | 89 (33, 178) | 78 (42, 132) | 75 (41, 306) | 68 (22, 115) |
| Fruits | 50 (20, 200) | 81 (17, 152) | 67 (35, 156) | 45 (16, 137) |
| Servings of fruit and vegetables per day a | 2.0 | 1.8 | 1.6 | 1.7 |
| Snacks g/d | 43 (26, 84) | 51 (24, 93) | 61 (27, 145) | 31 (14, 70) |
| Crisps and fried snacks | 3 (2, 4) | 5 (4, 5) | 4 (2, 5) | 2 (2, 5) |
| Sweet snacks | 33 (22, 75) | 45 (18, 84) | 52 (18, 140) | 26 (9, 64) |
| Yoghurt | 3 (1, 5) | 3 (1, 4) | 3 (1, 6) | 2 (1, 4) |
| Meat and fish g/d | 151 (93, 222) | 169 (128, 195) | 147 (95, 197) | 124 (85, 169) |
| Red meat | 21 (11, 68) | 68 (22, 96) | 68 (3, 68) | 22 (14, 68) |
| Processed meat and fish | 19 (19, 54) | 28 (0, 55) | 19 (19, 38) | 19 (10, 35) |
| Fish (including processed) | 16 (7, 32) | 17 (0, 35) | 37 (15, 53) | 23 (0, 35) |
| Oily fish | 0 (0, 7) | 0 (0, 13) | 4 (0, 17) | 0 (0, 9) |
| MET-h/Week Median (IQR) | ||||
|---|---|---|---|---|
| 3-Months | 6-Months | 9-Months | 12-Months | |
| Total (EE) | 213.4 (137.9–295.5) | 224.3 (163.8–292.1) | 239.3 (163.2–313.2) | 300.7 (198.2–415.5) |
| Sedentary PA | 17.9 (14.9–29.4) | 17.9 (7.4–18.1) | 17.9 (17.9–29.4) | 17.9 (7.4–29.3) |
| Light PA | 102.5 (76.4–150.9) | 111.3 (75.3–155.4) | 111.7 (93.4–146.0) | 146.0 (105.4–204.0) |
| Moderate PA | 89.6 (51.1–115.1) | 89.2 (53.8–138.4) | 76.6 (40.2–127.3) | 109.6 (66.5–210.4) |
| Vigorous PA | 0.8 (0.0–0.8) | 0.0 (0.0–0.8) | 0.0 (0.0–0.0) | 0.0 (0.0–1.4) |
| Household/ care PA | 166.6 (102.84–212.2) | 180.1 (107.4–234.2) | 131.9 (101.2–180.1) | 163.5 (107.4–238.0) |
| Occupational PA | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–88.9) | 0.0 (0.0–123.5) |
| Sport PA | 5.3 (1.7–13.6) | 4.0 (1.3–5.5) | 0.5 (0.0–5.3) | 1.6 (0.1–6.4) |
| Transport PA | 28.0 (10.2–43.3) | 23.3 (10.5–31.5) | 13.4 (10.7–22.6) | 17.4 (10.7–40.7) |
| Inactive PA | 21.5 (16.7–32.8) | 17.9 (7.4–22.0) | 29.4 (17.9–30.5) | 17.9 (7.4–34.5) |
| Median (IQR) Postnatal Weight Change | |||||
|---|---|---|---|---|---|
| Population | Weight Change Period | 3 Months | 6 Months | 9 Months | 12 Months |
| All women | From booking | −2.3 (−9.7, 1.6) | −1.3 (−6.9, 4.8) | −0.8 (−3.5, 3.2) | −1.6 (−8.8, 3.3) |
| From 3rd trimester | −11.6 (−15.6, −5.2) | −10.2 (−12.7, −5.0) | −9.0 (−11.2, −6.0) | −9.0 (−10.8, −4.1) | |
| GWG “not excessive” | From booking | −9.7 (−13.5, −2.2) | −8.0 (−10.8, −2.5) | −2.7 | −4.4 (−9.4, 3.6) |
| From 3rd trimester | −11.5 (−13.7, −4.2) | −10.2 (−12.7, −5.4) | −6.1 | −7.2 (−12.0, −0.6) | |
| GWG “excessive” | From booking | −1.8 (−2.5, 4.2) | 2.3 (−3.4 6.0) | −0.8 (−3.0, 0.8) | −0.3 (−7.1, 2.2) |
| From 3rd trimester | −11.9 (−16.1, −9.4) | −10.1 (−13.3, −4.2) | −9.8 (−11.1, −6.8) | −9.0 (−18.9, −5.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heslehurst, N.; Cullen, E.; Flynn, A.C.; Briggs, C.; Smart, L.; Rankin, J.; McColl, E.; Sniehotta, F.F.; McParlin, C. Maternal Obesity and Patterns in Postnatal Diet, Physical Activity and Weight among a Highly Deprived Population in the UK: The GLOWING Pilot Trial. Nutrients 2023, 15, 3805. https://doi.org/10.3390/nu15173805
Heslehurst N, Cullen E, Flynn AC, Briggs C, Smart L, Rankin J, McColl E, Sniehotta FF, McParlin C. Maternal Obesity and Patterns in Postnatal Diet, Physical Activity and Weight among a Highly Deprived Population in the UK: The GLOWING Pilot Trial. Nutrients. 2023; 15(17):3805. https://doi.org/10.3390/nu15173805
Chicago/Turabian StyleHeslehurst, Nicola, Emer Cullen, Angela C. Flynn, Chloe Briggs, Lewis Smart, Judith Rankin, Elaine McColl, Falko F. Sniehotta, and Catherine McParlin. 2023. "Maternal Obesity and Patterns in Postnatal Diet, Physical Activity and Weight among a Highly Deprived Population in the UK: The GLOWING Pilot Trial" Nutrients 15, no. 17: 3805. https://doi.org/10.3390/nu15173805





