Maternal Obesity and Kawasaki Disease-like Vasculitis: A New Perspective on Cardiovascular Injury and Inflammatory Response in Offspring Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
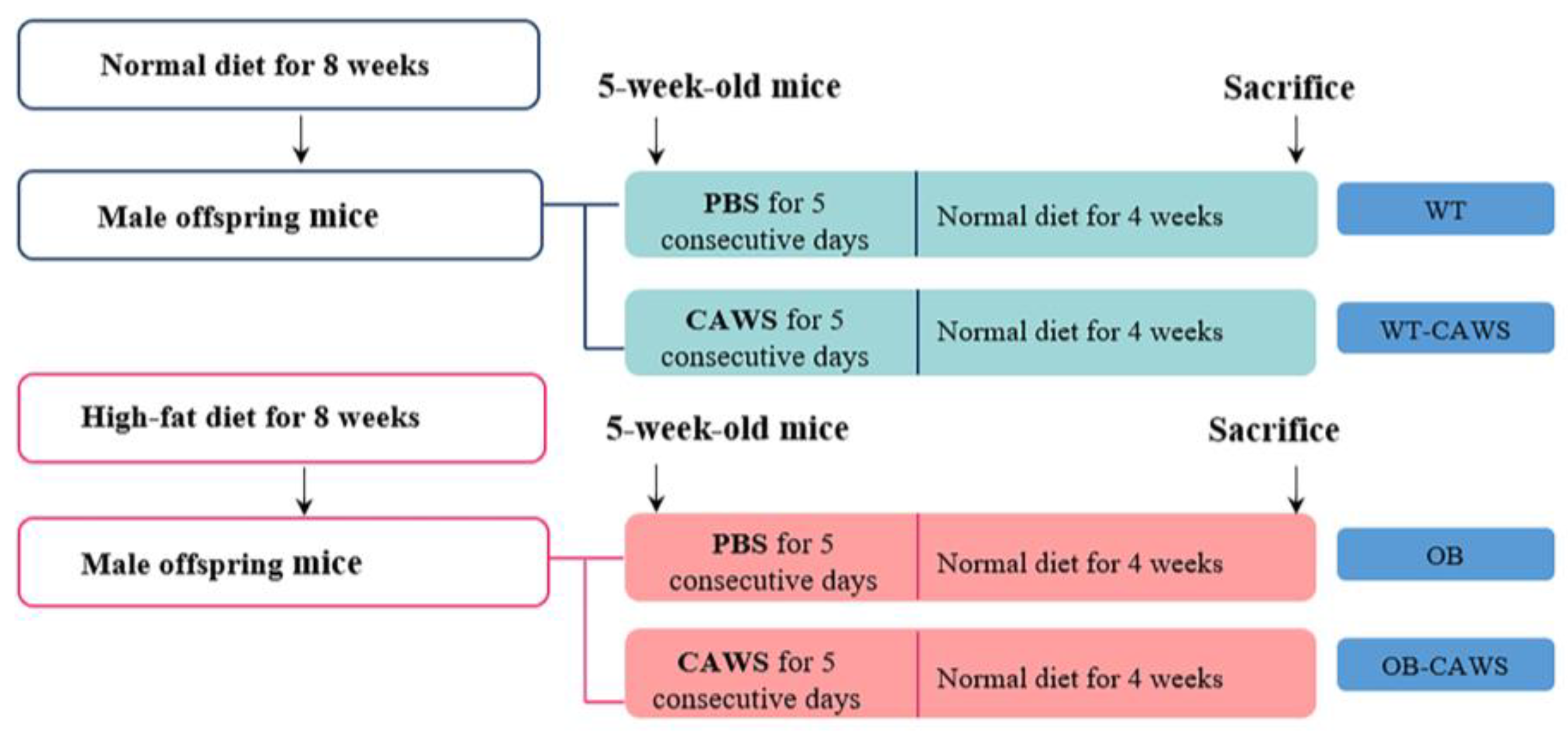
2.2. Obese Mouse Model and Animal Grouping
2.3. KD Mouse Model and Animal Grouping
2.4. Histological Analysis
2.5. Western Blot
2.6. RNA Extraction and Quantitative Real Time PCR (RT-qPCR)
2.7. Measurement of Cytokines and Chemokines
2.8. Echocardiography
2.9. Statistical Analysis
3. Results
3.1. Maternal Obesity Aggravates CAWS-Induced Vasculitis in Offspring Mice
3.2. Maternal Obesity Induces Altered Cardiac Structure in Offspring Mice
3.3. Maternal Obesity Exacerbates Offspring Vasculitis through NF-κB Signaling Pathways
3.4. Transgenerational Effects of Maternal Obesity on Cytokines and Chemokines of Offspring
4. Discussion
5. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalan, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Razaz, N.; Villamor, E.; Muraca, G.M.; Bonamy, A.E.; Cnattingius, S. Maternal obesity and risk of cardiovascular diseases in offspring: A population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol. 2020, 8, 572–581. [Google Scholar] [CrossRef]
- Kislal, S.; Shook, L.L.; Edlow, A.G. Perinatal exposure to maternal obesity: Lasting cardiometabolic impact on offspring. Prenat. Diagn. 2020, 40, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, O.R.; Rosario, F.J.; Chan, J.; Cox, L.A.; Ferchaud-Roucher, V.; Zemski-Berry, K.A.; Reusch, J.E.B.; Keller, A.C.; Powell, T.L.; Jansson, T. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J. Physiol. 2022, 600, 3169–3191. [Google Scholar] [CrossRef]
- Priviero, F. Epigenetic modifications and fetal programming: Molecular mechanisms to control hypertension inheritance. Biochem. Pharmacol. 2023, 208, 115412. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.R.; Glastras, S.J. Maternal Weight Management to Prevent the Developmental Programming of MAFLD in Offspring of Obese Mothers. Nutrients 2023, 15, 2155. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Kadam, I.; Reaz, A.; Bretter, R.; Saxena, A.; Johnson, C.H.; Caviglia, J.M.; Jiang, X. Prenatal Choline Supplement in a Maternal Obesity Model Modulates Offspring Hepatic Lipidomes. Nutrients 2023, 15, 965. [Google Scholar] [CrossRef]
- Sundholm, J.K.M.; Litwin, L.; Rono, K.; Koivusalo, S.B.; Eriksson, J.G.; Sarkola, T. Maternal obesity and gestational diabetes: Impact on arterial wall layer thickness and stiffness in early childhood—RADIEL study six-year follow-up. Atherosclerosis 2019, 284, 237–244. [Google Scholar] [CrossRef]
- Menichini, D.; Longo, M.; Facchinetti, F. Maternal interventions to improve offspring outcomes in rodent models of diet-induced obesity: A review. J. Matern-Fetal Neonatal Med. 2019, 32, 2943–2949. [Google Scholar] [CrossRef]
- Imai, A.; Fujimoto, E.; Tamura, K.; Utsuyama, M.; Sato, K. A maternal high-fat diet may accelerate adipo-immunologic aging in offspring. Life Sci. 2019, 219, 100–108. [Google Scholar] [CrossRef]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 2013, 191, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Ghosh, S.; Dai, C.; Barnett, J.A.; Garner, A.M.; Yoo, R.K.H.; Zandberg, W.F.; Botta, A.; Gorzelak, M.A.; Gibson, D.L. Maternal Intake of Dietary Fat Pre-Programs Offspring’s Gut Ecosystem Altering Colonization Resistance and Immunity to Infectious Colitis in Mice. Mol. Nutr. Food Res. 2021, 65, e2000635. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Netea, S.A.; Biesbroek, G.; van Stijn, D.; Ijspeert, H.; van der Made, C.I.; Jansen, M.H.; Geissler, J.; van den Berg, J.M.M.; van der Kuip, M.; Gruppen, M.P.; et al. Transient anti-cytokine autoantibodies superimpose the hyperinflammatory response in Kawasaki disease and multisystem inflammatory syndrome in children: A comparative cohort study on correlates of disease. EBioMedicine 2023, 95, 104736. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Ae, R.; Makino, N.; Kosami, K.; Kuwabara, M.; Matsubara, Y.; Nakamura, Y. Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017–2018. J. Pediatr. 2020, 225, 23–29.e2. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Eun, L.Y.; Han, J.W.; Kim, S.H.; Yoon, K.L.; Han, M.Y.; Yu, J.J.; Choi, J.W.; Rhim, J.W. Epidemiology of Kawasaki Disease in South Korea: A Nationwide Survey 2015–2017. Pediatr. Infect. Dis. J. 2020, 39, 1012–1016. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef]
- Shahi, A.; Afzali, S.; Firoozi, Z.; Mohaghegh, P.; Moravej, A.; Hosseinipour, A.; Bahmanyar, M.; Mansoori, Y. Potential roles of NLRP3 inflammasome in the pathogenesis of Kawasaki disease. J. Cell. Physiol. 2023, 238, 513–532. [Google Scholar] [CrossRef]
- Giryes, S.; McGonagle, D. Immune and non-immune mechanisms that determine vasculitis and coronary artery aneurysm topography in Kawasaki disease and MIS-C. Autoimmun. Rev. 2023, 22, 103240. [Google Scholar] [CrossRef]
- Tsoukas, P.; Yeung, R.S.M. Kawasaki disease and MIS-C share a host immune response. Nat. Rev. Rheumatol. 2022, 18, 555–556. [Google Scholar] [CrossRef]
- Fukazawa, R.; Kobayashi, J.; Ayusawa, M.; Hamada, H.; Miura, M.; Mitani, Y.; Tsuda, E.; Nakajima, H.; Matsuura, H.; Ikeda, K.; et al. JCS/JSCS 2020 Guideline on Diagnosis and Management of Cardiovascular Sequelae in Kawasaki Disease. Circ. J. 2020, 84, 1348–1407. [Google Scholar] [CrossRef]
- Manlhiot, C.; Niedra, E.; McCrindle, B.W. Long-term management of Kawasaki disease: Implications for the adult patient. Pediatr. Neonatol. 2013, 54, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Yoshitomi, T.; Nishikawa, M.; Fujiwara, M.; Matsubara, T.; Hayashi, T.; Furukawa, S. NF-kappaB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin. Immunol. 2001, 99, 373–377. [Google Scholar] [CrossRef]
- Shi, H.; Weng, F.; Li, C.; Jin, Z.; Hu, J.; Chu, M.; Qiu, H. Overweight, obesity and coronary artery lesions among Kawasaki disease patients. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1604–1612. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Huo, Y.; Zheng, Y.; Gui, Y. Effects of the Leptin-Mediated MAPK/ERK Signaling Pathway on Collagen II Expression in Knee Cartilage of Newborn Male Mice from Obese Maternal Offspring. Biomolecules 2022, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, S.; Zhang, J.; Hou, J.; Wu, F.; Wang, W.; Han, X.; Gui, Y. Melatonin alleviates vascular endothelial cell damage by regulating an autophagy-apoptosis axis in Kawasaki disease. Cell Prolif. 2022, 55, e13251. [Google Scholar] [CrossRef]
- Huang, C.; Tan, H.; Song, M.; Liu, K.; Liu, H.; Wang, J.; Shi, Y.; Hou, F.; Zhou, Q.; Huang, R.; et al. Maternal Western diet mediates susceptibility of offspring to Crohn’s-like colitis by deoxycholate generation. Microbiome 2023, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Leon-Aguilar, L.F.; Croyal, M.; Ferchaud-Roucher, V.; Huang, F.; Marchat, L.A.; Barraza-Villarreal, A.; Romieu, I.; Ramakrishnan, U.; Krempf, M.; Ouguerram, K.; et al. Maternal obesity leads to long-term altered levels of plasma ceramides in the offspring as revealed by a longitudinal lipidomic study in children. Int. J. Obes. 2019, 43, 1231–1243. [Google Scholar] [CrossRef]
- Gilley, S.P.; Harrall, K.K.; Friedman, C.; Glueck, D.H.; Cohen, C.C.; Perng, W.; Sauder, K.A.; Krebs, N.F.; Shankar, K.; Dabelea, D. Association of Maternal BMI and Rapid Infant Weight Gain with Childhood Body Size and Composition. Pediatrics 2023, 151, e2022059244. [Google Scholar] [CrossRef]
- Ibanez, C.A.; Lira-Leon, G.; Reyes-Castro, L.A.; Rodriguez-Gonzalez, G.L.; Lomas-Soria, C.; Hernandez-Rojas, A.; Bravo-Flores, E.; Solis-Paredes, J.M.; Estrada-Gutierrez, G.; Zambrano, E. Programming Mechanism of Adipose Tissue Expansion in the Rat Offspring of Obese Mothers Occurs in a Sex-Specific Manner. Nutrients 2023, 15, 2245. [Google Scholar] [CrossRef]
- Wang, L.; O’Kane, A.M.; Zhang, Y.; Ren, J. Maternal obesity and offspring health: Adapting metabolic changes through autophagy and mitophagy. Obes. Rev. 2023, 24, e13567. [Google Scholar] [CrossRef]
- Corken, A.; Thakali, K.M. Maternal Obesity Programming of Perivascular Adipose Tissue and Associated Immune Cells: An Understudied Area with Few Answers and Many Questions. Front. Physiol. 2021, 12, 798987. [Google Scholar] [CrossRef]
- Chang, L.; Garcia-Barrio, M.T.; Chen, Y.E. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1094–1109. [Google Scholar] [CrossRef]
- Barreca, M.M.; Alessandro, R.; Corrado, C. Effects of Flavonoids on Cancer, Cardiovascular and Neurodegenerative Diseases: Role of NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 9236. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shu, L.; Zhang, J.; Yang, X.; Cheng, X.; Zhao, X.; Qu, W.; Zhu, Q.; Shou, Y.; Peng, G.; et al. Ogt-mediated O-GlcNAcylation inhibits astrocytes activation through modulating NF-kappaB signaling pathway. J. Neuroinflamm. 2023, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Marwick, J.; Kirkham, P. Redox modulation of chromatin remodeling: Impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem. Pharmacol. 2004, 68, 1255–1267. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Almalki, W.H.; Kazmi, I.; Javed Shaikh, M.A.; Thangavelu, L.; Gulati, M.; Singh, S.K.; Jha, N.K.; Gupta, P.K.; et al. Nuclear factor-kappa B (NF-kappaB) inhibition as a therapeutic target for plant nutraceuticals in mitigating inflammatory lung diseases. Chem. Biol. Interact. 2022, 354, 109842. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Padin-Iruegas, M.E.; Caponio, V.C.A.; Lorenzo-Pouso, A.I.; Saavedra-Nieves, P.; Chamorro-Petronacci, C.M.; Suarez-Penaranda, J.; Perez-Sayans, M. Caspase 3 and Cleaved Caspase 3 Expression in Tumorogenesis and Its Correlations with Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 1937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.M.; Hayes, D.A.; Singh-Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem inflammatory syndrome in children and Kawasaki disease: A critical comparison. Nat. Rev. Rheumatol. 2021, 17, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Doratt, B.M.; Mendza, N.; Varlamov, O.; Rincon, M.; Marshall, N.E.; Messaoudi, I. Maternal obesity blunts antimicrobial responses in fetal monocytes. Elife 2023, 12, e81320. [Google Scholar] [CrossRef]
- Sureshchandra, S.; Wilson, R.M.; Rais, M.; Marshall, N.E.; Purnell, J.Q.; Thornburg, K.L.; Messaoudi, I. Maternal Pregravid Obesity Remodels the DNA Methylation Landscape of Cord Blood Monocytes Disrupting Their Inflammatory Program. J. Immunol. 2017, 199, 2729–2744. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.M.; Corey, S.J. G-CSF, the guardian of granulopoiesis. Semin. Immunol. 2021, 54, 101515. [Google Scholar] [CrossRef]
- Semerad, C.L.; Liu, F.; Gregory, A.D.; Stumpf, K.; Link, D.C. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 2002, 17, 413–423. [Google Scholar] [CrossRef]
- Martin, K.R.; Wong, H.L.; Witko-Sarsat, V.; Wicks, I.P. G-CSF—A double edge sword in neutrophil mediated immunity. Semin. Immunol. 2021, 54, 101516. [Google Scholar] [CrossRef]
- e-Lacerda, R.R.; Teixeira, C.J.; Bordin, S.; Antunes, E.; Anhe, G.F. Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring. Nutrients 2019, 11, 2902. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Hassanshahi, G.; Khorramdelazad, H.; Zainodini, N.; Koochakzadeh, L. An Overlook to the Characteristics and Roles Played by Eotaxin Network in the Pathophysiology of Food Allergies: Allergic Asthma and Atopic Dermatitis. Inflammation 2016, 39, 1253–1267. [Google Scholar] [CrossRef]
- Nazarinia, D.; Behzadifard, M.; Gholampour, J.; Karimi, R.; Gholampour, M. Eotaxin-1 (CCL11) in neuroinflammatory disorders and possible role in COVID-19 neurologic complications. Acta Neurol. Belg. 2022, 122, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Mohite, S.; Cordeiro, T.; Tannous, J.; Mwangi, B.; Selvaraj, S.; Soares, J.C.; Sanches, M.; Teixeira, A.L. Eotaxin-1/CCL11 correlates with left superior temporal gyrus in bipolar disorder: A preliminary report suggesting accelerated brain aging. J. Affect. Disord. 2020, 273, 592–596. [Google Scholar] [CrossRef]
- Mayer, M.R.; Stone, M.J. Identification of receptor binding and activation determinants in the N-terminal and N-loop regions of the CC chemokine eotaxin. J. Biol. Chem. 2001, 276, 13911–13916. [Google Scholar] [CrossRef] [PubMed]
- Terai, M.; Yasukawa, K.; Honda, T.; Jibiki, T.; Hirano, K.; Sato, J.; Ishiwada, N.; Seguchi, M.; Ueda, S.; Kohno, Y. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr. Infect. Dis. J. 2002, 21, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Toor, I.S.; Ruckerl, D.; Mair, I.; Ainsworth, R.; Meloni, M.; Spiroski, A.M.; Benezech, C.; Felton, J.M.; Thomson, A.; Caporali, A.; et al. Eosinophil Deficiency Promotes Aberrant Repair and Adverse Remodeling Following Acute Myocardial Infarction. JACC Basic Transl. Sci. 2020, 5, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Xiong, Y.Y.; Tang, R.J.; Jiang, W.Y.; Ning, Y.; Gong, Z.T.; Huang, P.S.; Chen, G.H.; Xu, J.; Wu, C.X.; et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc. Res. 2022, 118, 2165–2178. [Google Scholar] [CrossRef] [PubMed]





| Group (Mean ± SD) | p-Value (Two-Way ANOVA) | ||||||
|---|---|---|---|---|---|---|---|
| Variables | WT | WT-CAWS | OB | OB-CAWS | KD | OB | Interaction |
| Eotaxin (pg/mL) | 1840 ± 473.0 | 983.1 ± 313.5 | 699.5 ± 104.7 | 1141 ± 346.9 | 0.2410 | 0.0129 * | 0.0023 ** |
| G-CSF (pg/mL) | 118.5 ± 32.21 | 324.9 ± 127.4 | 164.6 ± 29.88 | 1114 ± 568.0 | 0.0019 ** | 0.0143 * | 0.0256 * |
| GM-CSF (pg/mL) | 105.9 ± 16.22 | 101.9 ± 5.014 | 95.47 ± 7.042 | 102.9 ± 18.57 | 0.7971 | 0.4861 | 0.3996 |
| IFN-g (pg/mL) | 31.49 ± 7.096 | 27.02 ± 5.440 | 28.04 ± 1.386 | 28.36 ± 5.864 | 0.4568 | 0.7024 | 0.3912 |
| IL-1a (pg/mL) | 23.95 ± 8.032 | 17.81 ± 1.085 | 28.19 ± 3.511 | 21.81 ± 2.555 | 0.0186 * | 0.0984 | 0.9588 |
| IL-1b (pg/mL) | 8.693 ± 1.382 | 10.90 ± 2.122 | 7.065 ± 0.785 | 12.18 ± 4.718 | 0.0191 * | 0.8992 | 0.3024 |
| IL-2 (pg/mL) | 18.95 ± 3.053 | 18.86 ± 6.524 | 14.95 ± 1.473 | 21.66 ± 3.405 | 0.1284 | 0.7740 | 0.1193 |
| IL-3 (pg/mL) | 10.05 ± 1.499 | 9.215 ± 1.140 | 11.84 ± 1.777 | 9.743 ± 1.578 | 0.0773 | 0.1511 | 0.4186 |
| IL-4 (pg/mL) | 13.62 ± 1.179 | 11.18 ± 0.675 | 17.15 ± 5.582 | 13.43 ± 2.432 | 0.0718 | 0.0884 | 0.6888 |
| IL-5 (pg/mL) | 23.19 ± 3.992 | 23.19 ± 3.798 | 19.13 ± 0.790 | 22.15 ± 6.978 | 0.5108 | 0.2745 | 0.5114 |
| IL-6 (pg/mL) | 17.40 ± 2.250 | 40.11 ± 19.04 | 15.93 ± 0.472 | 61.67 ± 35.08 | 0.0050 ** | 0.3348 | 0.2718 |
| IL-9 (pg/mL) | 90.26 ± 11.13 | 76.99 ± 5.113 | 85.88 ± 7.074 | 73.01 ± 11.09 | 0.0131 * | 0.3704 | 0.9652 |
| IL-10 (pg/mL) | 163.8 ± 51.58 | 144.4 ± 12.49 | 149.9 ± 7.942 | 126.7 ± 7.674 | 0.4120 | 0.2667 | 0.8992 |
| IL-12 (P40) (pg/mL) | 1876 ± 759.4 | 1559 ± 347.1 | 1125 ± 110.6 | 2189 ± 545.9 | 0.1627 | 0.8147 | 0.0176 * |
| IL-12 (P70) (pg/mL) | 467.8 ± 110.8 | 393.0 ± 51.12 | 510.7 ± 30.79 | 331.4 ± 25.70 | 0.0028 * | 0.7866 | 0.1502 |
| IL-13 (pg/mL) | 157.2 ± 24.59 | 151.1 ± 25.99 | 125.1 ± 7.921 | 213.7 ± 85.61 | 0.1081 | 0.5230 | 0.0648 |
| IL-17A (pg/mL) | 121.6 ± 57.88 | 101.4 ± 23.91 | 133.1 ± 16.24 | 95.19 ± 30.26 | 0.1294 | 0.8844 | 0.6304 |
| KC (pg/mL) | 140.2 ± 12.22 | 124.1 ± 28.39 | 77.39 ± 6.945 | 80.95 ± 21.25 | 0.5228 | 0.0001 ** | 0.3226 |
| MCP-1 (pg/mL) | 614.2 ± 80.10 | 543.9 ± 36.54 | 518.8 ± 27.17 | 544.4 ± 81.61 | 0.4820 | 0.1494 | 0.1452 |
| MIP-1a (pg/mL) | 6.918 ± 1.239 | 5.950 ± 0.642 | 6.350 ± 0.114 | 7.370 ± 1.344 | 0.9578 | 0.3971 | 0.0632 |
| MIP-1b (pg/mL) | 244.6 ± 46.02 | 230.1 ± 13.72 | 238.6 ± 16.00 | 290.5 ± 57.56 | 0.3496 | 0.1813 | 0.1089 |
| RANTES (pg/mL) | 324.8 ± 71.44 | 342.5 ± 67.87 | 210.6 ± 31.95 | 440.9 ± 133.0 | 0.0123 * | 0.8546 | 0.0268 * |
| TNF-a (pg/mL) | 119.7 ± 40.79 | 105.7 ± 22.20 | 116.8 ± 8.292 | 124.3 ± 37.14 | 0.0149 * | 0.0005 ** | 0.6012 |
| Group (Mean ± SD) | p-Value (Two-Way ANOVA) | ||||||
|---|---|---|---|---|---|---|---|
| Variables | WT | WT-CAWS | OB | OB-CAWS | KD | OB | Interaction |
| Eotaxin (pg/mL) | 6.790 ± 2.093 | 7.328 ± 2.661 | 5.313 ± 1.044 | 8.943 ± 3.311 | 0.1113 | 0.9557 | 0.2263 |
| G-CSF (pg/mL) | 2.635 ± 0.689 | 3.470 ± 0.500 | 3.303 ± 0.688 | 4.548 ± 1.082 | 0.0009 ** | 0.1464 | 0.0115 |
| GM-CSF (pg/mL) | 12.67 ± 1.716 | 13.84 ± 3.106 | 11.31 ± 1.345 | 12.73 ± 4.083 | 0.3720 | 0.3939 | 0.9328 |
| IFN-g (pg/mL) | 7.313 ± 1.299 | 9.198 ± 2.331 | 6.843 ± 1.756 | 7.895 ± 0.800 | 0.3028 | 0.3028 | 0.6223 |
| IL-1a (pg/mL) | 6.943 ± 0.772 | 7.718 ± 2.671 | 8.763 ± 1.345 | 7.830 ± 2.158 | 0.9348 | 0.3257 | 0.3830 |
| IL-1b (pg/mL) | 1.720 ± 0.161 | 1.963 ± 0.305 | 1.510 ± 0.352 | 1.925 ± 0.414 | 0.0640 | 0.4573 | 0.6022 |
| IL-2 (pg/mL) | 13.88 ± 1.652 | 16.45 ± 5.196 | 18.70 ± 2.447 | 17.39 ± 3.326 | 0.7195 | 0.1184 | 0.2790 |
| IL-3 (pg/mL) | 0.885 ± 0.281 | 0.903 ± 0.109 | 6.433 ± 11.71 | 0.790 ± 0.243 | 0.4119 | 0.1502 | 0.4876 |
| IL-4 (pg/mL) | 1.163 ± 0.189 | 1.255 ± 0.359 | 0.972 ± 0.493 | 0.880 ± 0.317 | 0.1395 | 1.0000 | 0.6137 |
| IL-5 (pg/mL) | 1.188 ± 0.331 | 1.128 ± 0.591 | 1.065 ± 0.510 | 1.070 ± 0.300 | 0.7416 | 0.9196 | 0.9050 |
| IL-6 (pg/mL) | 1.815 ± 1.233 | 3.043 ± 0.832 | 2.443 ± 1.314 | 3.325 ± 1.234 | 0.0962 | 0.4513 | 0.7729 |
| IL-9 (pg/mL) | 14.26 ± 4.443 | 20.08 ± 3.839 | 15.26 ± 2.311 | 17.64 ± 2.606 | 0.0335 * | 0.6795 | 0.3328 |
| IL-10 (pg/mL) | 4.150 ± 4.327 | 7.105 ± 1.084 | 3.738 ± 1.691 | 6.685 ± 2.607 | 0.0507 | 0.7647 | 0.9978 |
| IL-12 (P40) (pg/mL) | 6.820 ± 1.450 | 26.41 ± 18.36 | 6.250 ± 1.062 | 15.95 ± 3.511 | 0.2628 | 0.0089 * | 0.3128 |
| IL-12 (P70) (pg/mL) | 33.58 ± 5.116 | 36.95 ± 8.059 | 26.17 ± 12.44 | 30.64 ± 2.953 | 0.3456 | 0.1110 | 0.8924 |
| IL-13 (pg/mL) | 53.74 ± 25.18 | 74.52 ± 16.67 | 57.30 ± 15.76 | 59.03 ± 7.316 | 0.2209 | 0.5067 | 0.2958 |
| IL-17A (pg/mL) | 1.828 ± 0.495 | 2.000 ± 0.232 | 1.580 ± 0.507 | 1.848 ± 0.259 | 0.2872 | 0.3313 | 0.8140 |
| KC (pg/mL) | 9.075 ± 1.413 | 9.643 ± 0.545 | 8.060 ± 2.345 | 10.69 ± 2.804 | 0.1323 | 0.9882 | 0.3183 |
| MCP-1 (pg/mL) | 32.79 ± 15.21 | 77.80 ± 41.88 | 8.393 ± 14.54 | 75.98 ± 67.69 | 0.0353 * | 0.8295 | 0.6364 |
| MIP-1a (pg/mL) | 0.722 ± 0.121 | 4.223 ± 1.115 | 0.572 ± 0.153 | 2.555 ± 1.029 | 0.0001 ** | 0.0346 * | 0.0693 |
| MIP-1b (pg/mL) | 5.147 ± 4.156 | 24.37 ± 13.09 | 5.890 ± 2.969 | 18.51 ± 10.98 | 0.0120 * | 0.6333 | 0.5398 |
| RANTES (pg/mL) | 41.01 ± 6.148 | 75.05 ± 20.95 | 45.88 ± 8.427 | 128.2 ± 63.89 | 0.0051 ** | 0.1139 | 0.1815 |
| TNF-a (pg/mL) | 6.950 ± 3.140 | 9.090 ± 0.712 | 6.670 ± 1.612 | 10.79 ± 1.412 | 0.0071 ** | 0.4777 | 0.3267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, W.; Huo, Y.; Gui, Y. Maternal Obesity and Kawasaki Disease-like Vasculitis: A New Perspective on Cardiovascular Injury and Inflammatory Response in Offspring Male Mice. Nutrients 2023, 15, 3823. https://doi.org/10.3390/nu15173823
Zheng Y, Wang W, Huo Y, Gui Y. Maternal Obesity and Kawasaki Disease-like Vasculitis: A New Perspective on Cardiovascular Injury and Inflammatory Response in Offspring Male Mice. Nutrients. 2023; 15(17):3823. https://doi.org/10.3390/nu15173823
Chicago/Turabian StyleZheng, Yuanzheng, Wenji Wang, Yu Huo, and Yonghao Gui. 2023. "Maternal Obesity and Kawasaki Disease-like Vasculitis: A New Perspective on Cardiovascular Injury and Inflammatory Response in Offspring Male Mice" Nutrients 15, no. 17: 3823. https://doi.org/10.3390/nu15173823
APA StyleZheng, Y., Wang, W., Huo, Y., & Gui, Y. (2023). Maternal Obesity and Kawasaki Disease-like Vasculitis: A New Perspective on Cardiovascular Injury and Inflammatory Response in Offspring Male Mice. Nutrients, 15(17), 3823. https://doi.org/10.3390/nu15173823






