Post-Diagnosis Dietary Patterns among Cancer Survivors in Relation to All-Cause Mortality and Cancer-Specific Mortality: A Systematic Review and Meta-Analysis of Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
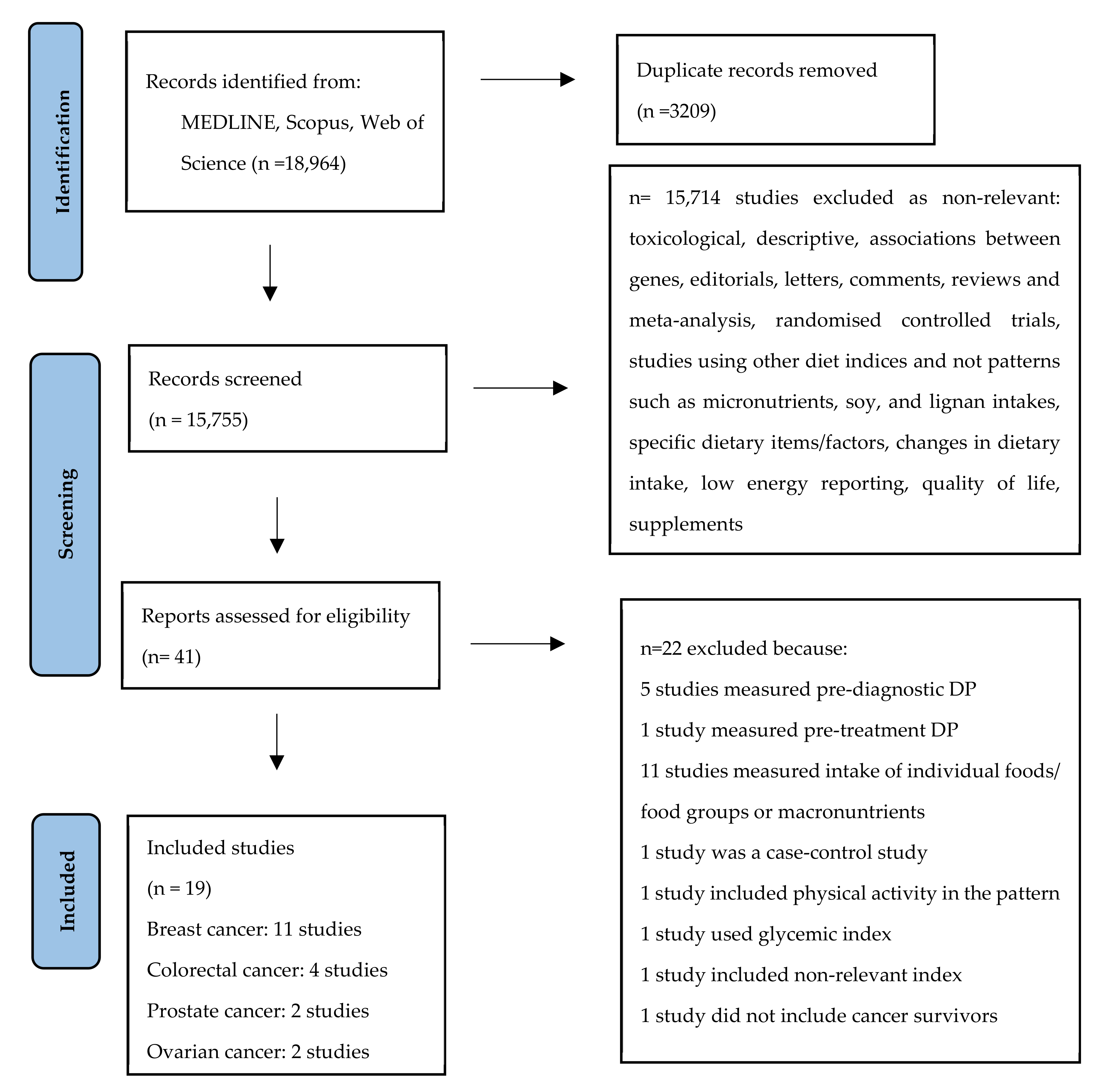
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias Assessment of Included Studies
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies and Risk of Bias
3.2. All-Cause Mortality
3.2.1. A Priori DPs and All-Cause Mortality
3.2.2. A Posteriori DPs and All-Cause Mortality
3.3. Cancer-Specific Mortality
3.3.1. A Priori DPs and Cancer-Specific Mortality
3.3.2. A Posteriori DPs and Cancer-Specific Mortality
3.4. Sensitivity Analysis
3.5. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A. Cancer Survivorship. In The Cancer Atlas, 2nd ed.; American Cancer Society: Atlanta, GA, USA, 2015; p. 136. [Google Scholar]
- Leach, C.R.; Weaver, K.E.; Aziz, N.M.; Alfano, C.M.; Bellizzi, K.M.; Kent, E.E.; Forsythe, L.P.; Rowland, J.H. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J. Cancer Surviv. 2015, 9, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.K.; Nasso, S.F.; Earp, J.A. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017, 18, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Basen-Engquist, K.; Alfano, C.M.; Maitin-Shepard, M.; Thomson, C.A.; Stein, K.; Syrjala, K.L.; Fallon, E.; Pinto, B.M.; Schmitz, K.H.; Zucker, D.S.; et al. Moving Research into Practice: Physical Activity, Nutrition, and Weight Management for Cancer Patients and Survivors. NAM Perspect. 2018. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.; Douglas, P.; Keaver, L. Nutrition Practices among Adult Cancer Survivors Living on the Island of Ireland: A Cross-Sectional Study. Nutrients 2022, 14, 767. [Google Scholar] [CrossRef]
- Lee, E.; Zhu, J.; Velazquez, J.; Bernardo, R.; Garcia, J.; Rovito, M.; Hines, R.B. Evaluation of Diet Quality Among American Adult Cancer Survivors: Results From 2005–2016 National Health and Nutrition Examination Survey. J. Acad. Nutr. Diet. 2021, 121, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Liu, S.; John, E.M.; Must, A.; Demark-Wahnefried, W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer 2015, 121, 4212–4221. [Google Scholar] [CrossRef]
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef]
- Kanellopoulou, A.; Riza, E.; Samoli, E.; Benetou, V. Dietary Supplement Use after Cancer Diagnosis in Relation to Total Mortality, Cancer Mortality and Recurrence: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 16–30. [Google Scholar] [CrossRef]
- Benetou, V. Nutrition for Cancer Survivors. Nutrients 2022, 14, 4093. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Project Expert Report 2018. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 2 July 2022).
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Benetou, V. Impact of Mediterranean Diet on Longevity. In Centenarians: An Example of Positive Biology; Caruso, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 161–168. [Google Scholar]
- Bamia, C. Dietary patterns in association to cancer incidence and survival: Concept, current evidence, and suggestions for future research. Eur. J. Clin. Nutr. 2018, 72, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Salamanca-Fernandez, E.; Garcia-Villanova, B.; Sanchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef]
- Hou, R.; Wei, J.; Hu, Y.; Zhang, X.; Sun, X.; Chandrasekar, E.K.; Voruganti, V.S. Healthy dietary patterns and risk and survival of breast cancer: A meta-analysis of cohort studies. Cancer Causes Control 2019, 30, 835–846. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet 2018, 118, 74–100.e111. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybylowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, J.Y.; Meyerhardt, J.A. Diet and lifestyle in survivors of colorectal cancer. Hematol. Oncol. Clin. North Am. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Meyerhardt, J.A. Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol. 2015, 33, 1825–1834. [Google Scholar] [CrossRef]
- van Zutphen, M.; Kampman, E.; Giovannucci, E.L.; van Duijnhoven, F.J.B. Lifestyle after Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr. Colorectal. Cancer Rep. 2017, 13, 370–401. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Mele, M.C.; Cintoni, M.; Raoul, P.; Ianiro, G.; Salerno, L.; Pozzo, C.; Bria, E.; Muscaritoli, M.; Molfino, A.; et al. The Facts about Food after Cancer Diagnosis: A Systematic Review of Prospective Cohort Studies. Nutrients 2020, 12, 2345. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, R.F. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat. Med. 1988, 7, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Viechtbauer, W. Publication bias in meta-analysis: Prevention, assessment and adjustments. Psychometrika 2007, 72, 269–271. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Fung, T.T.; Hu, F.B.; Holmes, M.D. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 9295–9303. [Google Scholar] [CrossRef]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011, 22, 589–598. [Google Scholar] [CrossRef]
- Izano, M.A.; Fung, T.T.; Chiuve, S.S.; Hu, F.B.; Holmes, M.D. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr. Cancer 2013, 65, 820–826. [Google Scholar] [CrossRef]
- George, S.M.; Ballard-Barbash, R.; Shikany, J.M.; Caan, B.J.; Freudenheim, J.L.; Kroenke, C.H.; Vitolins, M.Z.; Beresford, S.A.; Neuhouser, M.L. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 2014, 23, 575–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Campbell, P.T.; Wang, Y.; Doyle, C.; Gaudet, M.M. Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II Nutrition Cohort. Cancer Causes Control 2016, 27, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bao, W.; Liu, B.; Caan, B.J.; Lane, D.S.; Millen, A.E.; Simon, M.S.; Thomson, C.A.; Tinker, L.F.; Van Horn, L.V.; et al. Changes in Overall Diet Quality in Relation to Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. J. Acad. Nutr. Diet 2018, 118, 1855–1863.e1856. [Google Scholar] [CrossRef] [PubMed]
- Karavasiloglou, N.; Pestoni, G.; Faeh, D.; Rohrmann, S. Post-Diagnostic Diet Quality and Mortality in Females with Self-Reported History of Breast or Gynecological Cancers: Results from the Third National Health and Nutrition Examination Survey (NHANES III). Nutrients 2019, 11, 2558. [Google Scholar] [CrossRef]
- Wang, F.; Cai, H.; Gu, K.; Shi, L.; Yu, D.; Zhang, M.; Zheng, W.; Zheng, Y.; Bao, P.; Shu, X.O. Adherence to Dietary Recommendations among Long-Term Breast Cancer Survivors and Cancer Outcome Associations. Cancer Epidemiol. Biomark. Prev. 2020, 29, 386–395. [Google Scholar] [CrossRef]
- Ergas, I.J.; Cespedes Feliciano, E.M.; Bradshaw, P.T.; Roh, J.M.; Kwan, M.L.; Cadenhead, J.; Santiago-Torres, M.; Troeschel, A.N.; Laraia, B.; Madsen, K.; et al. Diet Quality and Breast Cancer Recurrence and Survival: The Pathways Study. JNCI Cancer Spectr. 2021, 5. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Hu, F.B.; Mayer, R.J.; Nelson, H.; Whittom, R.; Hantel, A.; Thomas, J.; et al. Association of Dietary Patterns With Cancer Recurrence and Survival in Patients With Stage III Colon Cancer. JAMA 2007, 298, 754–764. [Google Scholar] [CrossRef]
- Fung, T.T.; Kashambwa, R.; Sato, K.; Chiuve, S.E.; Fuchs, C.S.; Wu, K.; Giovannucci, E.; Ogino, S.; Hu, F.B.; Meyerhardt, J.A. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE 2014, 9, e115377. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; di Giuseppe, R.; Waniek, S.; Plachta-Danielzik, S.; Koch, M.; Nothlings, U.; Hampe, J.; Schlesinger, S.; Lieb, W. Postdiagnostic Mediterranean and Healthy Nordic Dietary Patterns Are Inversely Associated with All-Cause Mortality in Long-Term Colorectal Cancer Survivors. J. Nutr. 2017, 147, 636–644. [Google Scholar] [CrossRef]
- Guinter, M.A.; McCullough, M.L.; Gapstur, S.M.; Campbell, P.T. Associations of Pre- and Postdiagnosis Diet Quality With Risk of Mortality Among Men and Women With Colorectal Cancer. J. Clin. Oncol. 2018, 36, 3404–3410. [Google Scholar] [CrossRef]
- Kenfield, S.A.; DuPre, N.; Richman, E.L.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E.L. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur. Urol. 2014, 65, 887–894. [Google Scholar] [CrossRef]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev. Res. 2015, 8, 545–551. [Google Scholar] [CrossRef]
- Al Ramadhani, R.M.; Nagle, C.M.; Ibiebele, T.I.; Grant, P.; Friedlander, M.; DeFazio, A.; Webb, P.M.; Ovarian Cancer, P.; Lifestyle Study, G. Pre- and Post-Diagnosis Diet Quality and Ovarian Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, N.; Wang, T.; Townsend, M.K.; Eliassen, A.H.; Tabung, F.K.; Giovannucci, E.L.; Matulonis, U.A.; Terry, K.L.; Tworoger, S.S.; Harris, H.R. Pre-diagnosis and post-diagnosis dietary patterns and survival in women with ovarian cancer. Br. J. Cancer 2022, 127, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the Healthy Eating Index: HEI-2010. J. Acad. Nutr. Diet 2013, 113, 569–580. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Wilson, M.M.; Lerman, J.L.; Tooze, J.A. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet 2018, 118, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index revised: A measurement instrument for populations. J. Am. Diet Assoc. 1999, 99, 697–704. [Google Scholar] [CrossRef]
- Mai, V.; Kant, A.K.; Flood, A.; Lacey, J.V., Jr.; Schairer, C.; Schatzkin, A. Diet quality and subsequent cancer incidence and mortality in a prospective cohort of women. Int. J. Epidemiol. 2005, 34, 54–60. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. Bmj 2005, 330, 991. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Ball, K.; Crawford, D.; Mishra, G.D. An index of diet and eating patterns is a valid measure of diet quality in an Australian population. J. Nutr. 2008, 138, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Lay, S.; Yu, H.N.; Shen, S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 2016, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Q.; Li, F.; Wu, H.; Wang, Y.C.; Chen, J.S.; He, G.S.; Li, S.G.; Chen, B. Evaluation of the Validity and Reliability of the Chinese Healthy Eating Index. Nutrients 2018, 10, 114. [Google Scholar] [CrossRef]
- Olsen, A.; Egeberg, R.; Halkjær, J.; Christensen, J.; Overvad, K.; Tjønneland, A. Healthy aspects of the Nordic diet are related to lower total mortality. J. Nutr. 2011, 141, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Satija, A.; Stampfer, M.J.; Rimm, E.B.; Willett, W.; Hu, F.B. Perspective: Are Large, Simple Trials the Solution for Nutrition Research? Adv. Nutr. 2018, 9, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Yilmaz, O.; Wang, M.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Low-Carbohydrate Diet Score and Macronutrient Intake in Relation to Survival After Colorectal Cancer Diagnosis. JNCI Cancer Spectr. 2018, 2, pky077. [Google Scholar] [CrossRef] [PubMed]
- Kunnavuttivanich, V.; Pramyothin, P.; Ithimakin, S. Association between dietary patterns and disease recurrence in Thai colorectal cancer patients. Medicine 2020, 99, e19522. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790, Erratum in JAMA Oncol. 2019, 5, 579. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Sato, K.; Niedzwiecki, D.; Ye, C.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J. Natl. Cancer Inst. 2012, 104, 1702–1711. [Google Scholar] [CrossRef]
- Jacobs, S.; Harmon, B.E.; Ollberding, N.J.; Wilkens, L.R.; Monroe, K.R.; Kolonel, L.N.; Le Marchand, L.; Boushey, C.J.; Maskarinec, G. Among 4 Diet Quality Indexes, Only the Alternate Mediterranean Diet Score Is Associated with Better Colorectal Cancer Survival and Only in African American Women in the Multiethnic Cohort. J. Nutr. 2016, 146, 1746–1755. [Google Scholar] [CrossRef]
- Shen, G.P.; Xu, F.H.; He, F.; Ruan, H.L.; Cui, C.; Chen, L.Z.; Zeng, Y.X.; Jia, W.H. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PLoS ONE 2012, 7, e36515. [Google Scholar] [CrossRef]
- van den Berg, M.G.; Rütten, H.; Rasmussen-Conrad, E.L.; Knuijt, S.; Takes, R.P.; van Herpen, C.M.; Wanten, G.J.; Kaanders, J.H.; Merkx, M.A. Nutritional status, food intake, and dysphagia in long-term survivors with head and neck cancer treated with chemoradiotherapy: A cross-sectional study. Head Neck 2014, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Wilson, K.M.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dairy intake after prostate cancer diagnosis in relation to disease-specific and total mortality. Int. J. Cancer 2015, 137, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Shivappa, N.; Schafmayer, C.; Burmeister, G.; Nöthlings, U.; Hampe, J.; Hébert, J.R.; Lieb, W.; Schlesinger, S. Association between the dietary inflammatory index and all-cause mortality in colorectal cancer long-term survivors. Int. J. Cancer 2019, 144, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.E.; Peterson, K.E.; Rozek, L.S.; Taylor, J.M.; Light, E.; Chepeha, D.B.; Hébert, J.R.; Terrell, J.E.; Wolf, G.T.; Duffy, S.A.; et al. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am. J. Clin. Nutr. 2013, 97, 360–368. [Google Scholar] [CrossRef]
- Vrieling, A.; Buck, K.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br. J. Cancer 2013, 108, 188–192. [Google Scholar] [CrossRef]
- Thomson, C.A.; ECrane, T.; Wertheim, B.C.; Neuhouser, M.L.; Li, W.; Snetselaar, L.G.; Basen-Engquist, K.M.; Zhou, Y.; Irwin, M.L. Diet quality and survival after ovarian cancer: Results from the Women’s Health Initiative. J. Natl. Cancer Inst. 2014, 106, dju314. [Google Scholar] [CrossRef]
- Sharma, I.; Roebothan, B.; Zhu, Y.; Woodrow, J.; Parfrey, P.S.; Mclaughlin, J.R.; Wang, P.P. Hypothesis and data-driven dietary patterns and colorectal Cancer survival: Findings from Newfoundland and Labrador colorectal Cancer cohort. Nutr. J. 2018, 17, 55. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Wang, P.P.; Savas, S.; Woodrow, J.; Wish, T.; Jin, R.; Green, R.; Woods, M.; Roebothan, B.; et al. Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open 2013, 3, e002270. [Google Scholar] [CrossRef]
- Pelser, C.; Arem, H.; Pfeiffer, R.M.; Elena, J.W.; Alfano, C.M.; Hollenbeck, A.R.; Park, Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 2014, 120, 1540–1547. [Google Scholar] [CrossRef]
- Carr, P.R.; Jansen, L.; Walter, V.; Kloor, M.; Roth, W.; Bläker, H.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am. J. Clin. Nutr. 2016, 103, 192–200. [Google Scholar] [CrossRef]
- Yang, B.; McCullough, M.L.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; Campbell, P.T. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2014, 32, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Jacobs, E.J.; Campbell, P.T. Association between red and processed meat intake and mortality among colorectal cancer survivors. J. Clin. Oncol. 2013, 31, 2773–2782. [Google Scholar] [CrossRef]
- Lang, S.; Schimansky, S.; Beynon, R.; Penfold, C.; Davies, A.; Waylen, A.; Thomas, S.; Pring, M.; Pawlita, M.; Waterboer, T.; et al. Dietary behaviors and survival in people with head and neck cancer: Results from Head and Neck 5000. Head Neck 2019, 41, 2074–2084, Epub 2019 Jan 30. PMID: 30698303; PMCID: PMC7116031. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Font, R.; Mañós, M.; Dicenta, M.; Quintana, M.J.; Bosch, F.X.; Castellsagué, X. The role of vegetable and fruit consumption and other habits on survival following the diagnosis of oral cancer: A prospective study in Spain. Int. J. Oral. Maxillofac. Surg. 2009, 38, 31–39. [Google Scholar] [CrossRef]
- Chan, J.M.; Holick, C.N.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Giovannucci, E.L. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control. 2006, 17, 199–208. [Google Scholar] [CrossRef]
- Dikshit, R.P.; Boffetta, P.; Bouchardy, C.; Merletti, F.; Crosignani, P.; Cuchi, T.; Ardanaz, E.; Brennan, P. Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: A multicentric European study. Int. J. Cancer 2005, 117, 992–995. [Google Scholar] [CrossRef] [PubMed]



| Author, Year, Location | Cancer Site | Outcome of Interest | N, Age, Gender, Follow-Up Duration | Dietary Patterns (DPs) Used | Dietary Assessment Tools | Adjustment Factors |
|---|---|---|---|---|---|---|
| Breast cancer (BC) | ||||||
| Kroenke et al., 2005, USA [40] | Breast cancer | all-cause mortality | 2619 women, age at diagnosis by DP: Prudent = 58 years, Western = 58 years, median follow-up = 9 years | Prudent, Western | validated FFQ | Age, BMI, energy intake, smoking, PA, diet missing in 1986, 1990, 1994, age at menarche, OCU, MS, HRT, age at menopause, stage, tamoxifen, chemotherapy |
| Kwan et al., 2009, USA [41] | Breast cancer | all-cause mortality, BC mortality, mortality from causes other than BC, recurrence | 1901 women, age at diagnosis by DP: Prudent = 58.4 years, Western = 58.9 years, mean follow-up = 4.2 years | Prudent, Western | validated FFQ | Age, energy intake, race, BMI, PA, smoking, MS, weight change, stage, HRS, treatment |
| Kim et al., 2011, USA [42] | Breast cancer | all-cause mortality, BC mortality, mortality from causes other than BC | 2729 women, Age at baseline = 30–55 years, follow-up until 30 June 2004 | AHEI, DQIR, RFS, aMED | validated FFQ | Time since diagnosis, age, alcohol intake (for RFS only), energy, multivitamin use, BMI, weight change, OCU, smoking, PA, stage, treatment, age at first birth and parity, MS, HRT |
| George et al., 2011, USA [43] | Breast cancer | all-cause mortality, BC mortality | 670 women, age at diagnosis = 18–64 years, mean follow-up = 6 years | HEI-2005 | validated FFQ | Energy intake, PA, race, stage, tamoxifen use, BMI |
| Izano et al., 2013, USA [44] | Breast cancer | BC mortality, death from causes other than BC | 4103 women, age at baseline = 30–55 years, median follow-up = 9.3 years | DASH, AHEI-2010 | validated FFQ | Age, energy intake, BMI, BMI change, age at first birth and parity, OCU, MS, HRT, smoking, stage, treatment, PA |
| George et al., 2014, USA [45] | Breast cancer | all-cause mortality, BC mortality, death from causes other than BC | 2317 women, age at screening for WHI: HEI-2005 Q1:63.6 years, Q2: 63.6 years, Q3: 63.4 years, Q4:63.9 years, median follow-up = 9.6 years | HEI-2005 | validated FFQ | Age, WHI component, ethnicity, income, education, stage, ER, PR, time since diagnosis, energy intake, PA, alcohol consumption, HRT |
| McCullough et al., 2016, USA [46] | Breast cancer | all-cause mortality, BC mortality, death from causes other than BC | 2152 women, age at diagnosis = 70.7 years, mean follow-up = 9.9 years | ACS score | validated FFQ | Age, year of diagnosis, stage, grade, ES, PR, initial treatment and the following assessed at the time of FFQ completion: BMI, smoking, PA and energy intake (Q) |
| Sun et al., 2018, USA [47] | Breast cancer | all-cause mortality, BC mortality | 2295 women, age at diagnosis = 66 years, mean follow-up = 12 years | HEI-2010 | validated FFQ | Age, energy intake, alcohol, smoking, PA, race, ethnicity, education, SES, stage, ER, PR, time from diagnosis to dietary intake assessment, postmenopausal hormone therapy use, BMI |
| Karavasiloglou et al., 2019, USA [48] | Breast cancer | all-cause mortality | 230 (110 breast cancer survivors and 120 gynecological cancer), age at diagnosis = 53.7 years, median follow-up = 8.6 years | HEI-2005, MDS | 24-h recall | Time between cancer diagnosis and completion of the NHANES III questionnaire, SES, marital status, BMI, PA, smoking, self-reported prevalent chronic diseases, daily energy intake, history of menopausal HRT, alcohol intake in the analyses for the HEI but not for the MDS |
| Wang et al., 2020, China [49] | Breast cancer | all-cause mortality, BC mortality, breast cancer-specific events | 3450 women, age at diagnosis = 59 years, follow-up duration = 10 years | CHFP-2007, CHFP-2016, DASH, HEI-2015 | validated FFQ | Age, intervals between diagnosis and 60-month survey, energy intake, income, education, marital status, MS, BMI, PA, ER, PR, HER2, TNM stages, comorbidity, chemotherapy, radiotherapy and immunotherapy |
| Ergas et al., 2021, USA [50] | Breast cancer | all-cause mortality, BC mortality, BC recurrence | 3660 women, age at diagnosis = 59.7 years, follow-up duration = 40 years | ACS, aMED, DASH, HEI-2015 | validated FFQ | Age, total energy intake, race, ethnicity, education, menopausal status, PA, smoking, stage, ER, PR, HER2, BMI, surgery type, chemotherapy, radiation and hormonal therapies |
| Colorectal cancer (CRC) | ||||||
| Meyerhardt et al., 2007, USA and Canada [51] | Colorectal cancer (stage III) | all-cause mortality, cancer recurrence | 1009 men, women, median age at diagnosis by DP: Prudent = 61 years, Western = 62 years median follow-up = 5.3 years | Prudent, Western | validated SFFQ | Age, sex, depth of invasion, positive lymph nodes, clinical perforation at surgery, bowel obstruction at surgery, baseline performance status, treatment, weight change, time-varying BMI, PA, total calories |
| Fung et al., 2014, USA [52] | Colorectal cancer (stage I-III) | overall survival, CRC specific mortality | 1201 women, median age at diagnosis = 66.5 years, median follow-up = 11.2 years | DASH, aMED, AHEI-2010, Prudent, Western | SFFQ | Age, PA, BMI, weight change, grade, chemotherapy, smoking, energy intake, stage, tumor site and date of CRC diagnosis |
| Ratjen et al., 2017, Germany [53] | Colorectal cancer | all-cause mortality | 1404 patients, age at diet assessment = 69 years, median age at diagnosis = 62 years, median follow-up = 7 years | MMDS, HNFI | validated, web-based SFFQ | Age, sex, BMI, PA, survival time from CRC diagnosis until diet assessment, tumor location, occurrence at metastases, occurrence of other tumor, chemotherapy, smoking, energy intake, time x age, time x BMI, and time x metastases |
| Guinter et al., 2018, USA [54] | Colorectal cancer | all-cause mortality, CRC-specific mortality | Post-diagnosis sample: 1321 men, women, age at baseline = 64.6 years, age at diagnosis = 70.6 years, mean follow-up = 6.4 years | DASH, ACS score, Prudent, Western | validated FFQ | Age, year of diagnosis, sex, stage, total calorie intake, BMI, education, smoking, weight change since 1992, treatment |
| Ovarian Cancer (OC) | ||||||
| Al Ramadhani et al., 2020, Australia [57] | Ovarian cancer | OC mortality | 503 women, ages = 18–79 years, mean follow-up = 4.4 years | HEI-2010, AHEI-2010, ACS score, DGI | validated FFQ | Age, energy intake, smoking at 12 months (never/former/current), and FIGO stage, and stratified by PA at 12 months |
| Sasamoto et al., 2022, USA [58] | Ovarian cancer | all-cause mortality, OC mortality | 783 women, median age at diagnosis = 62 years, follow-up of NHS II = 6 years, follow-up of NHS = 20 years | EDIP, AHEI-2010 | validated FFQ | Age, year at diagnosis, histology, stage, smoking, BMI, energy intake, NSAID use |
| Prostate Cancer (PC) | ||||||
| Kenfield et al., 2014, USA [55] | Prostate cancer | all-cause mortality | 4538 men, age in 1990 by diet score 0 to 3:52.6 years, 4 to 5: 54.3 years, 6 to 9: 55.3 years, follow-up until 31 January 2010 | MDS | validated SFFQ | Age, time period, time since diagnosis to FFQ, energy intake, BMI, vigorous PA, smoking, stage, Gleason score, treatment, race, height, history of diabetes mellitus, family history of PC, multivitamin use |
| Yang et al., 2015, USA [56] | Prostate cancer (non-metastatic) | all-cause mortality | 926 men, age at diagnosis = 68.6 years, median follow-up = 9.9 years | Prudent Western | validated FFQ | Age, energy intake, BMI, smoking, vigorous PA, Gleason score, stage, prostate-specific antigen level, time interval between diagnosis and FFQ completion, initial treatment after diagnosis, family history of PC |
| A Priori Dietary Pattern | Main Characteristics of the Pattern (Reference) | Papers in this Meta-Analysis |
|---|---|---|
| Healthy Eating Index (HEI-2005) | -Measures adherence to the 2005 Dietary Guidelines for Americans -Includes 12 components that represent the major food groups found in MyPyramid -Diets that meet the least restrictive of the food-group recommendations (expressed on a per 1000 calorie basis) receive maximum scores for the nine adequacy components of the index: total vegetables (5 points), dark green and orange vegetables and legumes (5 points), total fruit (includes 100% juice) (5 points), whole fruit (5 points), total grains (5 points), whole grains (5 points), milk (10 points), meat and beans (10 points) and oils (10 points). In moderation: saturated fat, sodium, calories from solid fats, alcoholic beverages and added sugars (which serve as a proxy for discretionary calories) Ref: Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008;108:1896–901 [59] | George et al., 2011 [43]; George et al., 2014 [45]; Karavasiloglou et al., 2019 [48] |
| Alternate Healthy Eating Index (AHEI) | -Designed to target food choices associated with reduced risk for chronic diseases -Based on 1992 Food Guide Pyramid and 1995 Dietary Guidelines for Americans -Consists of 9 components: vegetables (no potatoes included), fruits, nuts and soy, fiber cereals, white/red meat ratio, trans fatty acids, polyunsaturated/saturated fatty acids ratio, alcohol -Each component takes 0 to 10 points (zero to max adherence). Multivitamin use duration was scored dichotomously: 7.5 points for ≥5 y regular use, 2.5 points for all others, to avoid overly emphasizing this component. -Score ranges from 2.5 (lower) to 87.5 (highest) Ref: McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002; 76:1261–1271. [60] | Kim et al., 2011 [42]; Sasamoto et al., 2022 [58] |
| Healthy Eating Index-2010 (HEI-2010) | -The HEI-2010 retains several features of the HEI-2005: (1) Consists of 12 components, including 9 adequacy components: total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids and 3 moderation components: grains, sodium and empty calories; (2) Density approach to setting standards: e.g., per 1000 calories. Least--restrictive standards, i.e., those that are easiest to achieve among recommendations that vary by energy level, sex and/or age. -Changes to the index include: (1) Dark green and orange vegetables and legumes replaced greens and beans; (2) plant proteins and seafood have been added; (3) a ratio of poly- and mono-unsaturated to saturated fatty acids replaced oils and saturated fat; and (4) a moderation component, refined grains, replaced the adequacy component total grains Ref: Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013 Apr;113(4):569-80. Erratum in: J. Acad. Nutr. Diet. 2016 Jan;116(1):170. PMID: 23415502; PMCID: PMC3810369. [61] | Sun et al., 2018 [47]; Al Ramadhani et al., 2020 [57] |
| Alternate Healthy Eating Index-2010 (AHEI-2010) | -Components were chosen based on their association with chronic diseases -Points were awarded for higher consumption of vegetables (excluding potatoes), whole grains, nuts, whole fruit and legumes, long chain omega-3 fatty acids and polyunsaturated fatty acids -Lower consumption for red/processed meat, sugar-sweetened beverages, sodium, trans fatty acids and moderate alcohol intake -Each food group could take a value from 0 to 10 points (maximum overall score: 110 points). Ref:Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012 Jun;142(6):1009-18. [62] | Izano et al., 2013 [44]; Fung et al., 2014 [52]; Al Ramadhani et al., 2020 [57] |
| Healthy Eating Index (HEI-2015) | -Consists of 13 components: total vegetables, greens and beans, total fruits, whole fruits, dairy, seafood and plant protein, refined grains, total protein, added sugars, fatty acids, saturated fatty acids and sodium and -Each component scored a maximum of 10 points; for components divided into two, each subcomponent was allocated 5 points. -For the HEI-2015, only the 1200 to 2400 kcal patterns were used (compared with the range of 1000 to 3200 kcal, used for some components in prior versions). Ref: Kirkpatrick SI, Reedy J, Krebs-Smith SM, Pannucci TE, Subar AF, Wilson MM, Lerman JL, Tooze JA. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet. 2018 Sep;118(9):1603-1621. [63] | Wang et al., 2020 [49]; Ergas et al., 2021 [50] |
| Diet Quality Index-Revised (DQIR) | -Consists of 10 components: vegetables, fruits, grains, total fat, saturated fat, cholesterol, iron, calcium, diet diversity, added fat and sugar moderation -Each component scores from 0 to 10 based on the recommended range of intakes. -The maximum possible score is 100. Ref: Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index revised: a measurement instrument for populations. J. Am. Diet. Assoc. 1999 Jun;99(6):697-704. [64] | Kim et al., 2011 [42] |
| Recommended Food Score (RFS) | -Consists of 23 components: apples/pears; oranges/grapefruit; cantaloupes; orange/grapefruit juice; grapefruit; other fruit juices; dried beans; tomatoes; broccoli; spinach; mustard, turnip/collard greens; carrots/mixed vegetables with carrots; green salad; sweet potatoes, yams/other potatoes; baked or stewed chicken or turkey; baked or broiled fish; dark breads; cornbread, tortillas and grits; high-fiber cereals; cooked cereals; 2% milk and beverages with 2% milk; and 1% milk or skimmed milk. -The score is calculated by summing each of these 23 items that was consumed at least once a week, for a maximum score of 23. Ref: Mai V, Kant AK, Flood A, Lacey JV Jr, Schairer C, Schatzkin A. Diet quality and subsequent cancer incidence and mortality in a prospective cohort of women. Int. J. Epidemiol. 2005 Feb;34(1):54-60. [65] | Kim et al., 2011 [42] |
| Mediterranean Diet Score (MDS) | -Consists of 9 components: vegetables, fruit and nuts, legumes, cereals, fish and seafood, meat and meat products, dairy, ratio of monounsaturated to saturated fatty acids and alcohol intake. -Participants with consumption of legumes, vegetables, fruit and nuts, cereals, and fish and seafood above the sex-specific population median received 1 point (per component), whereas consumption of meat and meat products, dairy products and a ratio of monounsaturated to saturated fats lower than the population median received 1 point (per component). Ethanol consumption of 5–25 g/day received 1 point and 0 points otherwise. -The MDS takes values from 0 to 9. Ref: Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003 Jun 26;348(26):2599-608. [66] | Kenfield et al., 2014 [55]; Karavasiloglou et al., 2019 [48] |
| Alternate Mediterranean Diet Score (aMED) | -This score adapts the principles of the traditional Mediterranean diet to non-Mediterranean countries -The Mediterranean diet score was changed as follows: potato products were excluded from the vegetable group, fruits and nuts formed 2 separate groups, the grain group included only whole-grain products, the meat group included only red and processed meat. Participants received 1 point when they consumed > than the median intake of vegetables, legumes, fruits, nuts, whole grains, fish and monounsaturated/saturated fat ratio, and otherwise received 0 points for the particular food group. Participants received 1 point for consuming less than the median intake of meat and dairy. For ethanol: 1 point was assigned for intake between 5 and 15 g/d. -The score ranges from 0 to 9. Ref: Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. American Journal of Clinical Nutrition. 2005;82:163–173. [67] | Kim et al., 2011 [42]; Fung et al., 2014 [52]; Ergas et al., 2021 [50] |
| Modified Mediterranean Diet Score (MMDS) | -This score modified the original MDS by Trichopoulou et al. to be applied to the non-Mediterranean countries -Consists of 9 components: vegetables, legumes, fruit and nuts, cereals, fish and seafood, meat and meat products, dairy products, unsaturated/saturated fatty acids ratio and alcohol. -A value of 1 for an intake at or above the sex-specific median of 5 components (vegetables, fruits and nuts, legumes, cereals and fish) and for an intake below the sex-specific median of 2 components (meat products and dairy products); otherwise, a value of 0 was assigned. For ethanol, a value of 1 was assigned to men who consumed between 10 and 50 g/d and to women who consumed between 5 and 25 g/d; otherwise, a value of 0 was assigned. -For fat intake, the ratio of unsaturated lipids (monounsaturated and polyunsaturated lipids) to saturated lipids was calculated. Individuals with this ratio at or above the sex-specific median were assigned a value of 1, and otherwise were assigned a value of 0. -The total score ranges from 0 (minimum adherence) to 9 (maximum adherence). Ref: Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocke MC, Peeters PH, van der Schouw YT, Boeing H, Hoffmann K, Boffetta P, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005;330:991. [68] | Ratjen et al., 2017 [53] |
| Dietary Approaches to Stop Hypertension (DASH) | -Consists of 8 components: vegetables, fruits, nuts and legumes, low-fat dairy products, whole grains, red and processed meat, sweets, and sodium -For fruits, vegetables, nuts and legumes, low-fat dairy products and whole grains, the lowest quintile of intake was given a score of 1, and highest quintile a score of 5. For sweets, red and processed meat, and sodium, for which a low intake is recommended, the scoring was reversed. -The score ranges from 8 (non-adherence) to 40 (perfect adherence). Ref: Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Archives of internal medicine. 2008; 168(7):713–720.10.1001/archinte.168.7.713 [69] | Izano et al., 2013 [44]; Fung et al., 2014 [52]; Guinter et al., 2018 [54]; Wang et al., 2020 [49]; Ergas et al., 2021 [50] |
| American Cancer Society nutrition score (ACS) | -The score was developed to evaluate the association of the ACS Nutrition and Physical Activity Guidelines for Cancer Prevention with cause-specific mortality. -Consists of 5 components: fruits, vegetables, whole grains, red and processed meat- It ranges from 0 to 9. It sums three key food-based recommendations (each contributing 0–3 points) with a score of 3 reflecting optimal adherence for each: “consume 5+ servings of a variety of fruits and vegetables”, “choose whole grains in preference to processed, refined grains”, and “limit consumption of red and processed meats” (quartiles of total red and processed meat, reverse-scored) Ref: Kushi LH, Doyle C, McCullough M et al American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012; 62:30–67 [70] | McCullough et al., 2016 [46]; Guinter et al., 2018 [54]; Al Ramadhani et al., 2020 [57]; Ergas et al., 2021 [50] |
| Empirical dietary inflammatory pattern score (EDIP) | -EDIP assesses the inflammatory potential of an individual’s diet. -It is a weighted sum of 18 food groups that are predictive of circulating inflammatory biomarkers, with higher (more positive) scores indicating more proinflammatory diets and lower (more negative) scores indicating anti-inflammatory diets. Ref: Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and Validation of an Empirical Dietary Inflammatory Index. J Nutr. 2016 Aug;146(8):1560-70. [71] | Sasamoto et al., 2022 [58] |
| Australian Dietary Guideline Index (DGI) | -Consists of 15 components: vegetables and legumes, fruit, total cereals, meat and alternatives, total dairy, saturated fat, beverages, alcoholic beverages, sodium, and added sugars. Diet quality was incorporated using indicators relating to whole-grain cereals, lean meat, reduced/low fat dairy and dietary variety. Ref: Sarah A. McNaughton, Kylie Ball, David Crawford, Gita D. Mishra, An Index of Diet and Eating Patterns Is a Valid Measure of Diet Quality in an Australian Population, The Journal of Nutrition, Volume 138, Issue 1, January 2008, Pages 86–93 [72] | Al Ramadhani et al., 2020 [57] |
| Chinese Food Pagoda-2007 (CHFP-2007), Chinese Food Pagoda-2016 (CHFP-2016) | -Consists of 10 components: salt, fats and oil, dairy products, beans, meat and poultry, fish, eggs, vegetables, fruits, grains -CHFP-2007 recommended intake amounts: salt (<6 g/d), fats and oils (<30 g/d), dairy products (>300 g/d), beans (>30 g/d), meat and poultry (<100 g/d), fish (>50 g/d), eggs (<50 g/d), vegetables (>400 g/d), fruits (>100 g/d), grains (>300 g/d) -CHFP-2016 recommended intake amounts: fats and oils (25–30 g), beans (25–35 g), meat and poultry (40–75 g), fish 40–75 g), eggs (40–50 g), vegetables (300–500 g), fruits (200–400 g), grains (250–400 g), the other components the same amounts as the CHFP-2007 -CHFP scores range from 0 to 45 points (lowest to highest adherence). Ref: Wang SS, Lay S, Yu HN, Shen SR. Dietary Guidelines for Chinese Residents (2016): comments and comparisons. J Zhejiang Univ Sci B. 2016;17(9):649-656. [73] Yuan Y-Q, Li F, Wu H, Wang Y-C, Chen J-S, He G-S, Li S-G, Chen B. Evaluation of the Validity and Reliability of the Chinese Healthy Eating Index. Nutrients. 2018; 10(2):114. [74] | Wang et al., 2020 [49] |
| Healthy Nordic Food Index (HNFI) | -Consists of 6 components typically consumed in Nordic countries: cabbage, root vegetables, rye bread, oatmeal, apples and pears, and fish and shellfish -A value of 1 is given for an intake at or above the sex-specific median of the sample and a value of 0 is given if the intake is below the sex-specific median for each item and each participant. -Score ranges between 0 and 6 (minimum to maximum adherence) Ref: Olsen A, Egeberg R, Halkjaer J, Christensen J, Overvad K, Tjonneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr 2011;141:639–44. [75] | Ratjen et al., 2017 [53] |
| Study | Bias due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Exposures | Bias due to Deviations from Intended Exposures | Bias due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | ||||||||
| 2005; Kroenke [40] | Low | Low | Low | Low | Low | Low | Low | Low |
| 2009; Kwan [41] | Low | Low | Low | Low | NI | Low | Low | Low |
| 2011; Kim [42] | Low | Low | Low | Low | NI | Low | Low | Low |
| 2011; George [43] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| 2013; Izano [44] | Low | Low | Low | Low | NI | Low | Low | Low |
| 2014; George [45] | Low | Low | Low | Low | Low | Low | Low | Low |
| 2016; McCullough [46] | Low | Low | Low | Low | Low | Low | Low | Low |
| 2018; Sun [47] | Low | Low | Low | Low | Low | Low | Low | Low |
| 2019; Karavasiloglou [48] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| 2020; Wang [49] | Moderate | Low | Low | Moderate | NI | Low | Low | Moderate |
| 2021; Ergas [50] | Low | Low | Low | Low | Low | Low | Low | Low |
| Prostate cancer | ||||||||
| 2014; Kenfield [55] | Low | Low | Low | Low | NI | Low | Low | Low |
| 2015; Yang [56] | Low | Low | Moderate | Low | Low | Low | Low | Moderate |
| Colon cancer | ||||||||
| 2007; Meyerhardt [51] | Low | Low | Moderate | Low | Low | Low | Low | Moderate |
| 2014; Fung [52] | Low | Low | Low | Low | Low | Low | Low | Low |
| 2017; Ratjen [53] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| 2018; Guinter [54] | Moderate | Low | Low | Low | NI | Low | Low | Moderate |
| Ovarian cancer | ||||||||
| 2022; Sasamoto [58] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| 2020; Al Ramadhani [57] | Moderate | Moderate | Low | Low | NI | Low | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spei, M.-E.; Bellos, I.; Samoli, E.; Benetou, V. Post-Diagnosis Dietary Patterns among Cancer Survivors in Relation to All-Cause Mortality and Cancer-Specific Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2023, 15, 3860. https://doi.org/10.3390/nu15173860
Spei M-E, Bellos I, Samoli E, Benetou V. Post-Diagnosis Dietary Patterns among Cancer Survivors in Relation to All-Cause Mortality and Cancer-Specific Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients. 2023; 15(17):3860. https://doi.org/10.3390/nu15173860
Chicago/Turabian StyleSpei, Maria-Eleni, Ioannis Bellos, Evangelia Samoli, and Vassiliki Benetou. 2023. "Post-Diagnosis Dietary Patterns among Cancer Survivors in Relation to All-Cause Mortality and Cancer-Specific Mortality: A Systematic Review and Meta-Analysis of Cohort Studies" Nutrients 15, no. 17: 3860. https://doi.org/10.3390/nu15173860
APA StyleSpei, M.-E., Bellos, I., Samoli, E., & Benetou, V. (2023). Post-Diagnosis Dietary Patterns among Cancer Survivors in Relation to All-Cause Mortality and Cancer-Specific Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients, 15(17), 3860. https://doi.org/10.3390/nu15173860






