Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice
Abstract
:1. Introduction
2. Materials and Methods
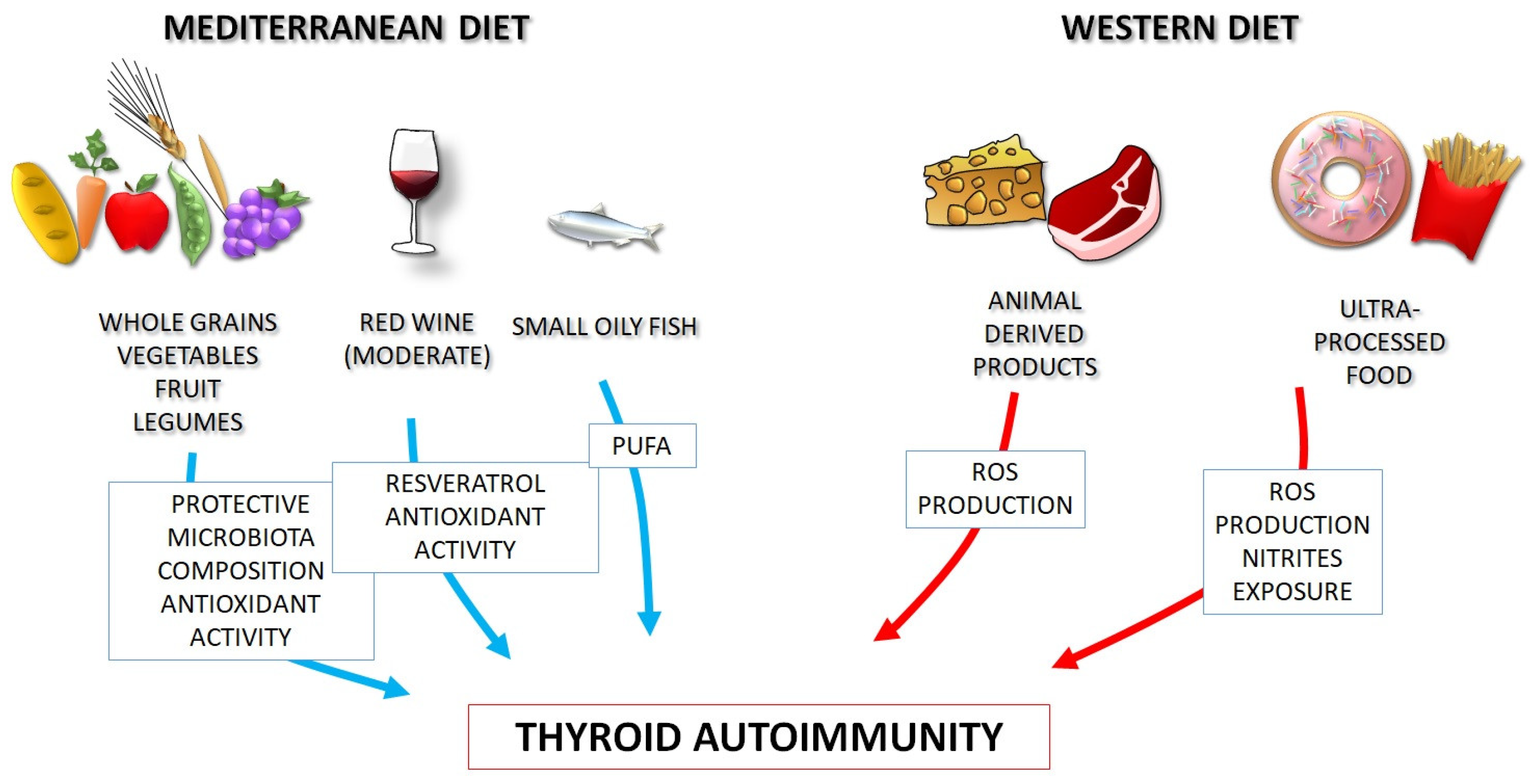
3. The Western Diet: Possible Links with Autoimmunity
4. The Mediterranean Diet as a Model of Healthy Eating
5. Dietary Habits and Thyroid Autoimmune Diseases
5.1. Evidence from Clinical Studies
5.2. Pathophysiological Bases of the Link between Dietary Components and Thyroid Autoimmune Diseases
5.2.1. Animal Products
5.2.2. Monounsaturated and Polyunsaturated Fatty Acids (Omega-3)
5.2.3. Extra Virgin Olive Oil (EVOO)
5.2.4. Phenolic Compounds of Wine (Resveratrol)
5.2.5. Fibers, Vitamins, and Trace Elements
- Iodine
- Selenium
- Iron and Zinc
- Vitamins
6. Role of Food Contaminants
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giuffrida, G.; Campennì, A. Autoimmune endocrine diseases. Minerva Endocrinol. 2018, 43, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Weetman, A.P. The pathogenesis of Hashimoto’s thyroiditis: Further developments in our understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology: Autoimmune thyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Masiello, E.; Magri, F.; Veronesi, G.; Bianconi, E.; Zerbini, F.; Gaiti, M.; Spreafico, E.; Gallo, D.; Premoli, P.; et al. The phenotype of newly diagnosed Graves’ disease in Italy in recent years is milder than in the past: Results of a large observational longitudinal study. J. Endocrinol. Investig. 2016, 39, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.; Tunbridge, W.M.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Grimley Evans, J.; Hasan, D.M.; Rodgers, H.; Tunbridge, F.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–256. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Trimarchi, F.; Giuffrida, G.; Certo, R.; Cama, E.; Campenni, A.; Alibrandi, A.; De Luca, F.; Wasniewska, M. Autoimmune comorbidities in Hashimoto’s thyroiditis: Different patterns of association in adulthood and childhood/adolescence. Eur. J. Endocrinol. 2017, 176, 133–141. [Google Scholar] [CrossRef]
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac. Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Selmi, C. The worldwide gradient of autoimmune conditions. Autoimmun. Rev. 2010, 9, A247–A250. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.D.M. Autoimmune disease: A modern epidemic? Molecular mimicry, the hygiene hypothesis, stealth infections, and other examples of disconnect between medical research and the practice of clinical medicine. N. Engl. J. Med. 2014, 347, 911–920. [Google Scholar] [CrossRef]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Thuesen, B.H.; Linneberg, A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015, 50, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Vicchio, T.M.; Certo, R.; Alibrandi, A.; Palmieri, O.; Campennì, A.; Cannavò, S.; Trimarchi, F.; Ruggeri, R.M. Vitamin D receptor gene poly0morphisms/haplotypes and serum 25(OH)D3 levels in Hashimoto’s thyroiditis. Endocrine 2017, 55, 599–606. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Trimarchi, F. Iodine nutrition optimization: Are there risks for thyroid autoimmunity? J. Endocrinol. Investig. 2021, 44, 1827–1835. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; D’Ascola, A.; Vicchio, T.M.; Campo, S.; Gianì, F.; Giovinazzo, S.; Frasca, F.; Cannavò, S.; Campennì, A.; Trimarchi, F. Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts. Endocrine 2020, 68, 151–162. [Google Scholar] [CrossRef]
- Langer, P. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front. Neuroendocrinol. 2010, 31, 497–518. [Google Scholar] [CrossRef]
- Brantley, P.J.; Myers, V.H.; Roy, H.J. Environmental and lifestyle influences on obesity. J. La. State Med. Soc. 2005, 157, S19–S27. [Google Scholar]
- Procaccini, C.; Carbone, F.; Galgani, M.; La Rocca, C.; De Rosa, V.; Cassano, S.; Matarese, G. Obesity and susceptibility to autoimmune diseases. Expert Rev. Clin. Immunol. 2011, 7, 287–294. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.M.; Pascoal, L.B.; Steigleder, K.M.; Siqueira, B.P.; Corona, L.P.; Ayrizono, M.L.S.; Milanski, M.; Leal, R.F. Role of diet and nutrition in inflammatory bowel disease. World J. Exp. Med. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; et al. Nutrition interventions in rheumatoid arthritis: The potential use of plant-based diets. A review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary habits and nutrition in rheumatoid arthritis: Can diet influence disease development and clinical manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, J.R.; Rothe, M.J.; Grant-Kels, J.M. Nutrition and psoriasis. Clin. Dermatol. 2010, 28, 615–626. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A. The share of ultra-processed foods and the overall nutritional quality of diets in the US: Evidence from a nationally representative cross-sectional study. Popul. Health Metr. 2017, 15, 6. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Levy, R.B.; Canella, D.S.; Da Costa Louzada, M.L.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef]
- Mazzucca, C.B.; Raineri, D.; Cappellano, G.; Chiocchetti, A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients 2021, 13, 3956. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Rezaei, N. (Eds.) Nutrition and Immunity; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- McCord, J.M. Human disease, free radicals, and the oxidant/antioxidant balance. Clin. Biochem. 1993, 26, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative stress and advanced glycation end products in Hashimoto’s thyroiditis. Thyroid 2016, 4, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Cristani, M.T.; Crupi, F.; Molonia, M.S.; Burduja, N.; Alibrandi, A.; Campennì, A.; Cannavò, S. Evaluation of paraoxonase activity and association with serum advanced glycation end products as reliable markers of oxidative stress in Hashimoto’s thyroiditis. Minerva Endocrinol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2021, 13, 7. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevic, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N. The seven countries study: 2289 deaths in 15 years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 3 August 2023).
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Barbalace, M.C.; Cannavò, S.; Ruggeri, R.M. Commentary: Fish and the thyroid: A Janus Bifrons relationship caused by pollutants and the omega-3 polyunsaturated fatty acids. Front. Endocrinol. 2023, 14, 1138245. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Ahluwalia, N.; Lassale, C.; Hercberg, S.; Fezeu, L.; Lairon, D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: A 6-year prospective study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 677–683. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Guevara, M.; Norat, T.; Langenberg, C.; Forouhi, N.G.; Sharp, S.; Slimani, N.; Schulze, M.B.; Buijsse, B.; Buckland, G.; et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: The InterAct project. Diabetes Care 2011, 34, 1913–1918. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19, Erratum in Diabetes Care 2018, 41, 2259–2260. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, D.; Barrea, L.; Muscogiuri, G.; Annunziata, G.; Colao, A.; Savastano, S. Breast cancer prevention in premenopausal women: Role of the Mediterranean diet and its components. Nutr. Res. Rev. 2020, 33, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimers Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Caunca, M.R. Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Burkitt, D.P. Some diseases characteristic of modern Western civilization. Br. Med. J. 1973, 1, 274–278. [Google Scholar] [CrossRef]
- Trowell, H.C.; Burkitt, D.P. Western Diseases, Their Emergence and Prevention; Harvard University Press: Cambridge, UK, 1981. [Google Scholar]
- McCarty, M.F. Upregulation of lymphocyte apoptosis as a strategy for preventing and treating autoimmune disorders: A role for whole-food vegan diets, fish oil and dopamine agonists. Med. Hypotheses. 2001, 57, 258–275. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. GCN2 and FGF21 are likely mediators of the protection from cancer, autoimmunity, obesity, and diabetes afforded by vegan diets. Med. Hypotheses. 2014, 83, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Hamideh, M.; Shah, R.; Sauk, J.S.; Jaffe, N. Dietary Patterns and Their Association with Symptoms Activity in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2022, 28, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.-A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.M. Dietary factors in the development of type 1 diabetes. Pediatr. Diabetes 2016, 22, 49–55. [Google Scholar] [CrossRef]
- Norris, J.M.; Yin, X.; Lamb, M.M.; Barriga, K.; Seifert, J.; Hoffman, M.; Orton, H.D.; Barón, A.E.; Clare-Salzler, M.; Chase, H.P.; et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007, 298, 1420–1428. [Google Scholar] [CrossRef]
- Tonstad, S.; Nathan, E.; Oda, K.; Fraser, G. Prevalence of hyperthyroidism according to type of vegetarian diet. Public Health Nutr. 2015, 18, 1482–1487. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Panza, F.; Lampignano, L.; Murro, I.; Di Noia, C.; Triggiani, V.; Giannelli, G.; Sardone, R.; De Pergola, G. Adherence to a Mediterranean Diet and Thyroid Function in Obesity: A Cross-Sectional Apulian Survey. Nutrients 2020, 12, 3173. [Google Scholar] [CrossRef]
- Liu, N.; Ma, F.; Feng, Y.; Ma, X. The Association between the Dietary Inflammatory Index and Thyroid Function in U.S. Adult Males. Nutrients 2021, 13, 3330. [Google Scholar] [CrossRef] [PubMed]
- Kaličanin, D.; Brčić, L.; Ljubetić, K.; Barić, A.; Gračan, S.; Brekalo, M.; Torlak Lovrić, V.; Kolčić, I.; Polašek, O.; Zemunik, T.; et al. Differences in food consumption between patients with Hashimoto’s thyroiditis and healthy individuals. Sci. Rep. 2020, 10, 10670. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.S.; Zhao, Y.F.; Song, Y.F.; Xu, C.; Yang, J.M.; Xuan, S.M.; Yan, H.L.; Yu, C.X.; Zhao, M.; Xu, J.; et al. Dietary high-fat lard intake induces thyroid dysfunction and abnormal morphology in rats. Acta Pharmacol. Sin. 2014, 35, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Shao, S.; Xu, G.; Song, Y.; Xu, C.; Gao, L.; Hu, C.; Zhao, J. A High-Fat Diet Rich in Saturated and Mono-Unsaturated Fatty Acids Induces Disturbance of Thyroid Lipid Profile and Hypothyroxinemia in Male Rats. Mol. Nutr. Food Res. 2018, 62, e1700599. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Kong, Y.; Zeng, L.; Wan, Q.; Hu, J.; Cai, Y. Effects of high-fat diet on thyroid autoimmunity in the female rat. BMC Endocr. Disord. 2022, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A.; Kabir, A.; Azizi, F.; Hadaegh, F. Is dietary nitrate/nitrite exposure a risk factor for development of thyroid abnormality? A systematic review and meta-analysis. Nitric Oxide 2015, 47, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Famà, F.; Perdichizzi, L.G.; Antonelli, A.; Brenta, G.; Vermiglio, F.; Moleti, M. Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids. Front. Endocrinol. 2022, 13, 891233. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; De Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153, S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, A.; Zeyda, M.; Mascher, D.; Itariu, B.K.; Murano, I.; Leitner, L.; Hochbrugger, E.E.; Fraisl, P.; Cinti, S.; Serhan, C.N.; et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 2013, 62, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Escribano, B.M.; Luque, E.; Feijóo, M.; Caballero-Villarraso, J.; Valdelvira, M.E.; Ochoa-Sepúlveda, J.J.; Lillo, R.; Paz, E.; Santamaría, A.; et al. Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models. Nutrients 2019, 11, 2448. [Google Scholar] [CrossRef] [PubMed]
- Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Rosillo, M.Á.; González-Benjumea, A.; Alarcón-de-la-Lastra, C. Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice. Antioxidants 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Montserrat-de la Paz, S.; Sanchez-Hidalgo, M.; Cardeno, A.; Bermudez, B.; Muriana, F.; Alarcon-de-la-Lastra, C. Virgin olive oil and its phenol fraction modulate monocyte/macrophage functionality: A potential therapeutic strategy in the treatment of systemic lupus erythematosus. Br. J. Nutr. 2018, 120, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cardeno, A.; Rosillo, M.Á.; Sánchez-Fidalgo, S.; Utrilla, J.; Martín-Lacave, I.; Alarcon-de-la-Lastra, C. Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-κB and MAPK activation. J. Nutr. Biochem. 2016, 27, 278–288. [Google Scholar] [CrossRef]
- Pang, K.L.; Lumintang, J.N.; Chin, K.-Y. Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients 2021, 13, 529. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The fluid aspect of the Mediterranean diet in the prevention and management of cardiovascular disease and diabetes: The role of polyphenol content in moderate consumption of wine and olive oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Blanquer-Rosselló, M.; Hernández-López, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D. Physiological effects of resveratrol. BioFactors 2010, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Kantham, S.; Rao, V.; Palanivelu, M.; Pham, H.; Shaw, P.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.R.; Villegas, I.; La Casa, C.; de La Lastra, C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004, 67, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.W.; Gao, J.S.; Li, L.; Xie, X. Resveratrol inhibits TNF-a-induced IL-1b, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Chien, A.; Jialal, I.; Devaraj, S. Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: Mechanistic insights. J. Nutr. Biochem. 2012, 23, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Jørgensen, T.; Laurberg, P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: A population-based case-control study. Eur. J. Endocrinol. 2012, 167, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Stramazzo, I.; Centanni, M. Gut microbiome and thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101506. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Iodine nutrition in pregnancy and lactation. Endocrinol. Metab. Clin. N. Am. 2011, 40, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Amato, G.; Biondi, B.; Mazziotti, G.; Del Buono, A.; Rotonda Nicchio, M.; Balzano, S.; Bellastella, A.; Glinoer, D.; Carella, C. Parity as a thyroid size-determining factor in areas with moderate iodine deficiency. J. Clin. Endocrinol. Metab. 2000, 85, 4534–4537. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The remarkable impact of iodisation programmes on global public health. Proc. Nutr. Soc. 2023, 82, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Impact of seaweed intake on health. Eur. J. Clin. Nutr. 2021, 75, 877–889. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Abdelhamid, A.; Jennings, A.; Hayhoe, R.P.G.; Awuzudike, V.E.; Welch, A.A. High variability of food and nutrient intake exists across the Mediterranean Dietary Pattern—A systematic review. Food Sci. Nutr. 2020, 8, 4907–4918. [Google Scholar] [CrossRef]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef]
- Bracci, E.L.; Keogh, J.B.; Milte, R.; Murphy, K.J. A comparison of dietary quality and nutritional adequacy of popular energy-restricted diets against the Australian Guide to Healthy Eating and the Mediterranean Diet. Br. J. Nutr. 2022, 128, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Borak, J. Iodine Deficiency in Vegetarian and Vegan Diets: Evidence-Based Review of the World’s Literature on Iodine Content in Vegetarian Diets. In Comprehensive Handbook of Iodine, 1st ed.; Elsevier Science Publishing Co., Ltd.: Amsterdam, The Netherlands, 2009; pp. 521–531. [Google Scholar]
- Rayman, M.P.; Bath, S.C. The new emergence of iodine deficiency in the UK: Consequences for child neurodevelopment. Ann. Clin. Biochem. 2015, 52, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple nutritional factors and the risk of Hashimoto’s thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Nettore, I.C.; De Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium supplementation modulates apoptotic processes in thyroid follicular cells. Biofactors 2017, 43, 415–423. [Google Scholar] [CrossRef]
- Wang, W.; Xue, H.; Li, Y.; Hou, X.; Fan, C.; Wang, H.; Zhang, H.; Shan, Z.; Teng, W. Effects of selenium supplementation on spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. Thyroid 2015, 25, 1137–1144. [Google Scholar] [CrossRef]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 6, 542–550. [Google Scholar] [CrossRef]
- Esposito, D.; Rotondi, M.; Accardo, G.; Vallone, G.; Conzo, G.; Docimo, G.; Selvaggi, F.; Cappelli, C.; Chiovato, L.; Giugliano, D.; et al. Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: Clinical results of a blinded placebo-controlled randomized prospective trial. J. Endocrinol. Investig. 2017, 40, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Liang, S.-S.; Ren, J.-J.; Wang, Z.-Y.; Deng, X.-X.; Liu, W.-D.; Yan, Y.-L.; Song, G.-H.; Li, X.-X. The Effects of Selenium Supplementation in the Treatment of Autoimmune Thyroiditis: An Overview of Systematic Reviews. Nutrients 2023, 15, 3194. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Terry, E.N.; Diamond, A.M. Selenium. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 568–587. [Google Scholar]
- Simopoulos, A.P. The traditional diet of Greece and cancer. Eur. J. Cancer Prev. 2004, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Tang, J.; Gillings, R.; Perfecto, A.; Dutton, J.; Speakman, J.; Fraser, W.D.; Nicoletti, C.; Berendsen, A.A.M.; de Groot, L.C.P.G.M.M.; et al. Changing from a Western to a Mediterranean-style diet does not affect iron or selenium status: Results of the New Dietary Strategies Addressing the Specific Needs of the Elderly Population for Healthy Aging in Europe (NU-AGE) 1-year randomized clinical trial in elderly Europeans. Am. J. Clin. Nutr. 2020, 111, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, S.; Gelal, B.; Baral, N.; Lamsal, M. Association between iron status and thyroid function in Nepalese children. Thyroid Res. 2016, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Office of Dietary Supplements: Iron Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 3 August 2023).
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, A.R.S.; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The role of zinc in thyroid hormones metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Licastro, F.; Mocchegiani, E.; Masi, M.; Fabris, N. Modulation of the neuroendocrine system and immune functions by zinc supplementation in children with Down’s syndrome. J. Trace Elem. Electrolytes Health Dis. 1993, 7, 237–239. [Google Scholar] [PubMed]
- Bucci, I.; Napolitano, G.; Giuliani, C.; Lio, S.; Minnucci, A.; Di Giacomo, F.; Calabrese, G.; Sabatino, G.; Palka, G.; Monaco, F. Zinc sulfate supplementation improves thyroid function in hypozincemic Down children. Biol. Trace Elem. Res. 1999, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kandhro, G.A.; Kazi, T.G.; Afridi, H.I.; Kazi, N.; Baig, J.A.; Arain, M.B.; Sirajuddin; Shah, A.Q.; Sarfraz, R.A.; Jamali, M.K.; et al. Effect of zinc supplementation on the zinc level in serum and urine and their relation to thyroid hormone profile in male and female goitrous patients. Clin. Nutr. 2009, 28, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Licastro, F.; Mocchegiani, E.; Masi, M.; Fabris, N. Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: A randomized double-blind controlled trial. J. Am. Coll. Nutr. 2015, 34, 391–399. [Google Scholar]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.J.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet 2016, 116, 1266–1282, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B12, Folic Acid, and the Immune System. In Nutrition and Immunity; Mahmoudi, M., Rezaei, N., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kacharava, T.; Giorgadze, E.; Janjgava, S.; Lomtadze, N.; Taboridze, I. Correlation Between Vitamin B12 Deficiency and Autoimmune Thyroid Diseases. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 86–94. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Mele, C.; Caputo, M.; Bisceglia, A.; Samà, M.T.; Zavattaro, M.; Aimaretti, G.; Pagano, L.; Prodam, F.; Marzullo, P. Immunomodulatory Effects of Vitamin D in Thyroid Diseases. Nutrients 2020, 12, 1444. [Google Scholar] [CrossRef]
- Rotondi, M.; Chiovato, L. Vitamin D deficiency in patients with Graves’ disease: Probably something more than a casual association. Endocrine 2013, 43, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernandez, R.; Alonso, M.; Segura, C.; Muñoz, I.; García-Caballero, T.; Diguez, C. Vitamin D receptor gene expression in human pituitary gland. Life Sci. 1997, 60, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.P.; Liane, K.M.; Bjørhovde, S.B.; Bjøro, T.; Torjesen, P.A.; Haug, E. Vitamin D receptor binding and biological effects of cholecalciferol analogues in rat thyroid cells. J. Steroid Biochem. Mol. Biol. 1994, 50, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, A.; Kowalczyk, M.J.; Herman, W.; Czyżyk, A.; Kowalska, M.; Żaba, R.; Łącka, K. Vitamin D Receptor Gene Polymorphisms and Autoimmune Thyroiditis: Are They Associated with Disease Occurrence and Its Features? BioMed Res. Int. 2019, 2019, 8197580. [Google Scholar] [CrossRef] [PubMed]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef]
- Ma, J.; Wu, D.; Li, C.; Fan, C.; Chao, N.; Liu, J.; Li, Y.; Wang, R.; Miao, W.; Guan, H. Lower serum 25-Hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine 2015, 94, e1639. [Google Scholar] [CrossRef]
- Planck, T.; Shahida, B.; Malm, J.; Manjer, J. Vitamin D in Graves’ Disease: Levels, Correlation with Laboratory and Clinical Parameters, and Genetics. Eur. Thyroid J. 2018, 7, 27–33. [Google Scholar] [CrossRef]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M. Tackling inadequate vitamin D intakes within the population: Fortification of dairy products with vitamin D may not be enough. Endocrine 2016, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Filmann, N.; Mann, W.A. Vitamin D Status and Thyroid Autoantibodies in Autoimmune Thyroiditis. Horm. Metab. Res. 2019, 51, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Florek, E.; Pietrończyk, K.; Sawicka-Gutaj, N.; Ruchała, M.; Ronen, O.; Nixon, I.J.; Shaha, A.R.; Rodrigo, J.P.; Tufano, R.P.; et al. The Role of Vitamin D in Autoimmune Thyroid Diseases: A Narrative Review. J. Clin. Med. 2023, 12, 1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Influence of the Mediterranean Diet on 25-Hydroxyvitamin D Levels in Adults. Nutrients 2020, 12, 1439. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Muscogiuri, G.; Paganitsa, G.; Parvouleskou, G.; Syriou, V.; Karagkoynis, P.; Stratigou, T.; Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; et al. Adherence to the Mediterranean diet is an independent predictor of circulating vitamin D levels in normal weight and non-smoker adults: An observational cross-sectional study. Int. J. Food Sci. Nutr. 2021, 72, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Environmental exposures and autoimmune thyroid disease. Thyroid 2010, 20, 755–761. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol. 2021, 11, 612320. [Google Scholar] [CrossRef]
- Bodin, J.; Stene, L.C.; Nygaard, U.C. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? BioMed Res. Int. 2015, 2015, 208947. [Google Scholar] [CrossRef]
- Radford, S. Sources of Contamination in Food. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E., Jock, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 518–522. [Google Scholar] [CrossRef]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Melough, M.M.; Maffini, M.V.; Otten, J.J.; Sathyanarayana, S. Diet quality and exposure to endocrine-disrupting chemicals among US adults. Environ. Res. 2022, 211, 113049. [Google Scholar] [CrossRef]


| Items | Specification |
| Date of Search | 31 May 2023 |
| Databases and other sources searched | MEDLINE (via PubMed) and Scopus |
| Search terms used | First-step search: “autoimmune thyroid disease”, “Diet” Second-step search: “autoimmunity”, “Hashimoto’s thyroiditis”, “hypothyroidism”, “hyperthyroidism”, “dietary regimens”, “Mediterranean diet”, “western-style diet”, “vegetarianism”, “vegan diet”, “Oxidative stress” |
| Timeframe | No restrictions |
| Inclusion and exclusion criteria (study type, language restrictions) | Type of studies included: Clinical trials, meta-analyses, randomized controlled trials, narrative and systemic reviews Type of studies excluded: Case reports and case series, opinions Language restrictions: Abstract in English. Articles were excluded for irrelevance to the topic in question, duplicates, or for presence of original articles on the same topic that are more recent and/or with larger number of cases. |
| Selection process | A 2-step selection process conducted by 3 reviewers (RMR, MCB, LC) independently of each other. Articles were selected on the basis of relevance of title and abstract in the topic. |
| Study | Dietary Habits | Design of the Study | Thyroid Effects |
| Tonstad et al. [72] | Vegetarian diets vs. omnivorous diets | Observational clinical study on 65,981 subjects, members of the Seventh-day Adventist church | Lower risk of prevalent hyperthyroidism in vegan (OR = 0.49; 95% CI 0.33–0·72), lacto-ovo (OR = 0.65; 95% CI 0.53–0.81), and pesco-vegetarian (OR = 0.74; 95% CI 0.56–1.00) diets than in omnivorous diets |
| Zupo et al. [73] | Adherence to the MD (PREDIMED score) EVOO consumption | Observational study on a cohort of 324 euthyroid overweight/obese subjects (228 F and 96 M, aged 14–72 years) | Inverse relation with serum fT3 (p < 0.01) and fT4 (p < 0.01) levels; no effect on serum TSH |
| Liu et al. [74] | Dietary inflammatory potential (DIP) score | Cross-sectional study including 2346 U.S. male subjects aged ≥ 20 years (data from NHANES) | Positive association with serum TT4 (β = 0.07; p = 0.0044); no effect of serum fT3, fT4 or TSH |
| Kaličanin et al. [75] | Food group consumption frequency | Observational study including 491 HT patients and 433 controls | ↑ consumption of animal fat (OR 1.55, p < 0.0001) and processed meat (OR 1.16, p = 0.0012) in HT pts. ↑ consumption of red meat (OR 0.80, p < 0.0001), non-alcoholic beverages (OR 0.82, p < 0.0001), whole grains (OR 0.82, p < 0.0001), and plant oil (OR 0.87, p < 0.0001) in controls Association of plant oil consumption with increased fT3 levels in HT patients (β = 0.07, p < 0.0001) |
| Ruggeri et al. [37] | Food group consumption frequency Adherence to the MD (PREDIMED score) | Observational study including 81 (71 F, 10 M) HT patients and 119 (102 F, 17 M) controls | ↑ intake frequencies of animal foods (meat, p = 0.0001; fish, p = 0.0001; dairy products, p = 0.004) in HT pts ↑ intake frequencies of plant foods (legumes, p = 0.001; fruits and vegetables, p = 0.030; nuts, p = 0.0005) in controls Lower adherence to the Mediterranean diet in HT patients compared to controls (p = 0.0001) PREDIMED score was a predictor of TPOAb positivity (OR 0.192, 95% CI 0.074–0.500, p = 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggeri, R.M.; Barbalace, M.C.; Croce, L.; Malaguti, M.; Campennì, A.; Rotondi, M.; Cannavò, S.; Hrelia, S. Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients 2023, 15, 3953. https://doi.org/10.3390/nu15183953
Ruggeri RM, Barbalace MC, Croce L, Malaguti M, Campennì A, Rotondi M, Cannavò S, Hrelia S. Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients. 2023; 15(18):3953. https://doi.org/10.3390/nu15183953
Chicago/Turabian StyleRuggeri, Rosaria Maddalena, Maria Cristina Barbalace, Laura Croce, Marco Malaguti, Alfredo Campennì, Mario Rotondi, Salvatore Cannavò, and Silvana Hrelia. 2023. "Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice" Nutrients 15, no. 18: 3953. https://doi.org/10.3390/nu15183953
APA StyleRuggeri, R. M., Barbalace, M. C., Croce, L., Malaguti, M., Campennì, A., Rotondi, M., Cannavò, S., & Hrelia, S. (2023). Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients, 15(18), 3953. https://doi.org/10.3390/nu15183953








