Examining the Triple Burden of Malnutrition: Insights from a Community-Based Comprehensive Nutrition Survey among Indigenous Tribal Children (0–19 Years) in the Western Ghats Hills of India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Sample Size
2.2. Selection of Villages and Households
2.3. Data Collection
2.4. Ethics
2.5. Data Analysis
3. Results
3.1. Socio-Economic Characteristics of the Study Households
3.2. Anthropometric Malnutrition
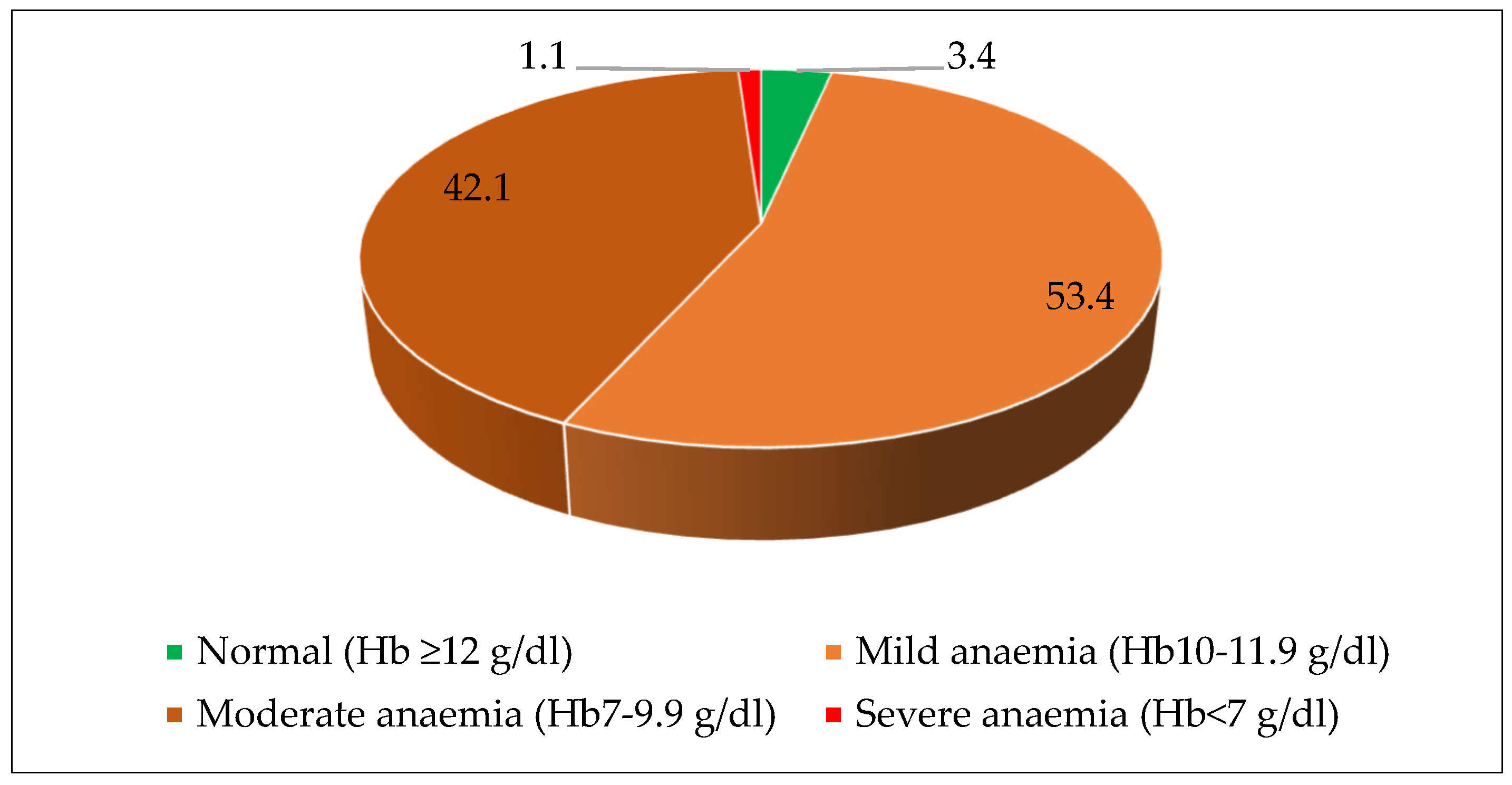
3.3. Anemia and Micronutrient Deficiencies
3.4. Food and Nutrient Intake of the Population
4. Discussion
5. Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, J.; Lovett, R.; Kukutai, T.; Jones, C.; Henry, D. Indigenous health data and the path to healing. Lancet 2017, 390, 2022–2023. [Google Scholar] [CrossRef]
- Wylie, L.; McConkey, S. Insiders’ Insight: Discrimination against Indigenous Peoples through the Eyes of Health Care Professionals. J. Racial Ethn. Health Disparities 2019, 6, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kirmayer, L.J.; Brass, G. Addressing global health disparities among Indigenous peoples. Lancet 2016, 388, 105–106. [Google Scholar] [CrossRef]
- Durie, M.H. The health of indigenous peoples. BMJ 2003, 326, 510–511. [Google Scholar] [CrossRef]
- Harfield, S.G.; Davy, C.; McArthur, A.; Munn, Z.; Brown, A.; Brown, N. Characteristics of Indigenous primary health care service delivery models: A systematic scoping review. Glob. Health 2018, 14, 12. [Google Scholar] [CrossRef]
- Davy, C.; Harfield, S.; McArthur, A.; Munn, Z.; Brown, A. Access to primary health care services for Indigenous peoples: A framework synthesis. Int. J. Equity Health 2016, 15, 163. [Google Scholar] [CrossRef]
- Durey, A.; Thompson, S.C. Reducing the health disparities of Indigenous Australians: Time to change focus. BMC Health Serv. Res. 2012, 12, 151. [Google Scholar] [CrossRef]
- Kozhimannil, K.B.; Interrante, J.D.; Tofte, A.N.; Admon, L.K. Severe Maternal Morbidity and Mortality Among Indigenous Women in the United States. Obstet. Gynecol. 2020, 135, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.J.; Varcoe, C.; Lavoie, J.; Smye, V.; Wong, S.T.; Krause, M.; Tu, D.; Godwin, O.; Khan, K.; Fridkin, A. Enhancing health care equity with Indigenous populations: Evidence-based strategies from an ethnographic study. BMC Health Serv. Res. 2016, 16, 544. [Google Scholar] [CrossRef]
- ORGI. Census of India; ORGI: New Delhi, India, 2011. [Google Scholar]
- Shirisha, P. Socioeconomic determinants of nutritional status among ‘Baiga’ tribal children In Balaghat district of Madhya Pradesh: A qualitative study. PLoS ONE 2019, 14, e0225119. [Google Scholar] [CrossRef]
- Ghosh, S.; Varerkar, S.A. Undernutrition among tribal children in Palghar district, Maharashtra, India. PLoS ONE 2019, 14, e0212560. [Google Scholar] [CrossRef]
- IIPS; ICF. National Family Health Survey—5; International Institute of Population Sciences: Mumbai, India, 2021. [Google Scholar]
- Ratcliffe, J. Social justice and the demographic transition: Lessons from India’s Kerala State. Int. J. Health Serv. 1978, 8, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.J.D. Public intervention and poverty alleviation: A study of the declining incidence of rural poverty in Kerala, India. Dev. Chang. 1995, 26, 701–728. [Google Scholar] [CrossRef]
- Drèze, J.; Sen, A. Indian Development: Selected Regional Perspectives; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Haddad, S.; Mohindra, K.S.; Siekmans, K.; Màk, G.; Narayana, D. “Health divide” between indigenous and non-indigenous populations in Kerala, India: Population based study. BMC Public Health 2012, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, S. Middlemen Flourish as Attappadi Infants Die of Malnutrition; The New Indian Express: Palakkad, Indian, 2021. [Google Scholar]
- Bloem, M. The 2006 WHO child growth standards. BMJ 2007, 334, 705–706. [Google Scholar] [CrossRef] [PubMed]
- WHO. Growth References 0–19 Year; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Document Reference WHO; NMH/NHD/MNM/11.1. 2011. Available online: http://www.who.int/entity/vmnis/indicators/haemoglobin (accessed on 25 March 2022).
- Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr. Bull. 2007, 28 (Suppl. S4), S621–S627. [CrossRef]
- WHO. Vitamin and Mineral Nutrition Information System (VMNIS) Micronutrients Database; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline; National Academy of Sciences: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- ICMR, Expert Group. Recommended Dietary Intakes for Indians; Indian Council of Medical Research: New Delhi, India, 1981. [Google Scholar]
- ICMR. Report of the Expert Group of the ICMR: Nutrient Requirements and Recommended Dietary Allowances for Indians; Indian Council of Medical Research: New Delhi, India, 2020. [Google Scholar]
- Quamme, S.H.; Iversen, P.O. Prevalence of child stunting in Sub-Saharan Africa and its risk factors. Clin. Nutr. Open Sci. 2022, 42, 49–61. [Google Scholar] [CrossRef]
- UNICEF; WHO; The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Priest, N.; Paradies, Y.; Stewart, P.; Luke, J. Racism and health among urban Aboriginal young people. BMC Public Health 2011, 11, 568. [Google Scholar] [CrossRef]
- Williams, D.R.; Mohammed, S.A. Discrimination and racial disparities in health: Evidence and needed research. J. Behav. Med. 2009, 32, 20–47. [Google Scholar] [CrossRef]
- UNPFII. State of the World’s Indigenous Peoples 2010; UN Permanent Forum on Indigenous Issues (UNPFII): New York, NY, USA, 2010. [Google Scholar]
- George, M.S.; Davey, R.; Mohanty, I.; Upton, P. “Everything is provided free, but they are still hesitant to access healthcare services”: Why does the indigenous community in Attapadi, Kerala continue to experience poor access to healthcare? Int. J. Equity Health 2020, 19, 105. [Google Scholar] [CrossRef]
- Short, D. Cultural genocide and indigenous peoples: A sociological approach. Int. J. Hum. Rights 2010, 14, 833–848. [Google Scholar] [CrossRef]
- Sahu, M.; Chattopadhyay, B.; Das, R.; Chaturvedi, S. Measuring Impact of Climate Change on Indigenous Health in the Background of Multiple Disadvantages: A Scoping Review for Equitable Public Health Policy Formulation. J. Prev. 2023, 44, 421–456. [Google Scholar] [CrossRef]




| Background Characteristics | N = 480 | Per Cent |
|---|---|---|
| Type of Tribe | ||
| Irula | 372 | 77.5 |
| Kurumba | 64 | 13.3 |
| Muduga | 43 | 9.0 |
| Others | 1 | 0.2 |
| Religion | ||
| Hindu | 475 | 98.9 |
| Christian | 5 | 1.1 |
| Type of family | ||
| Nuclear | 356 | 74.2 |
| Extended Nuclear | 90 | 18.8 |
| Joint | 34 | 7.0 |
| Household size | ||
| 2–3 members | 101 | 21.0 |
| 4–6 members | 345 | 71.9 |
| 7+ members | 34 | 7.1 |
| Mean household size [95% CI] | 4.5 [4.4–4.6] | |
| Landholding (Acres) | ||
| No Land | 159 | 33.1 |
| Marginal landholding (<2.5 acres) | 234 | 48.8 |
| Small landholding (2.5–5.0 acres) | 51 | 10.6 |
| Large landholding (≥5.0 acres) | 36 | 7.5 |
| Type of house | ||
| Kutcha | 98 | 20.4 |
| Semi-Pucca | 146 | 30.4 |
| Pucca | 236 | 49.2 |
| Source of drinking water | ||
| Open well | 41 | 8.5 |
| Tube well | 20 | 4.2 |
| Tap | 214 | 44.6 |
| Pond or tank | 40 | 8.3 |
| Stream/river/canal | 165 | 34.4 |
| Cooking fuel used | ||
| Firewood | 330 | 68.8 |
| Liquefied Petroleum Gas | 147 | 30.6 |
| Kerosene | 2 | 0.4 |
| Biogas | 1 | 0.2 |
| Availability of electricity | 403 | 83.7 |
| Availability of Sanitary Latrines | ||
| Present, and using | 357 | 74.4 |
| Present, but not using | 48 | 10.0 |
| No facility | 75 | 15.6 |
| Availability of separate kitchen | ||
| Yes | 418 | 87.1 |
| No | 62 | 12.9 |
| Weight-For-Age | |||||
|---|---|---|---|---|---|
| Age Group (Months) | N | Severe Underweight [WAZ < −3SD] | Moderate Underweight [WAZ −3SD to −2SD] | Total Underweight [WAZ < −2SD] | Normal [WAZ ≥ −2SD] |
| <12 | 77 | 11.7 [6.1–21.2] | 26.0 [17.2–37.1] | 37.7 [27.4–49.2] | 62.3 [58.8–72.6] |
| 12–35 | 209 | 16.3 [11.8–21.9] | 32.5 [26.4–39.2] | 48.8 [42.0–55.6] | 51.2 [44.4–58.0] |
| 36–59 | 230 | 16.2 [11.9–21.6] | 35.4 [29.4–41.8] | 51.5 [45.0–58.0] | 48.4 [42.0–55.0] |
| 0–59 | 516 | 15.5 [12.6–19.0] | 32.8 [28.9–37.0] | 48.3 [44.0–52.7] | 51.7 [47.3–56.0] |
| Height-For-Age | |||||
| Age Group (Months) | N | Severe Stunting [HAZ < −3SD] | Moderate Stunting [HAZ − 3SD to −2SD] | Total Stunting [HAZ < −2SD] | Normal [HAZ ≥ −2SD] |
| <12 | 77 | 7.8 [3.5–16.5] | 15.6 [8.9–25.7] | 23.4 [15.1–34=3] | 76.6 [65.6–84.9] |
| 12–35 | 209 | 20.6 [15.6–26.6] | 32.5 [26.5–39.2] | 53.1 [46.3–59.8] | 46.9 [40.2–53.7] |
| 36–59 | 230 | 7.8 [5.0–12.1] | 27.8 [22.4–34.0] | 35.6 [29.7–42.1] | 64.4 [57.9–70.3] |
| 0–59 | 516 | 13.0 [10.4–16.2] | 27.9 [24.2–32.0] | 40.9 [36.8–45.3] | 59.1 [54.7–63.2] |
| Weight-For-Height | |||||
| Age Group (Months) | N | Severe Wasting [WHZ < −3SD] | Moderate Wasting [WHZ − 3SD to −2SD] | Total Wasting [WHZ < −2SD] | Normal [WHZ ≥ −2SD] |
| <12 | 77 | 9.1 [4.3–18.8] | 13.0 [7.0–22.7] | 22.1 [14.0–32.9] | 77.9 [67.0–85.9] |
| 12–35 | 209 | 5.7 [3.3–10.0] | 19.6 [14.7–25.6] | 25.3 [20.0–31.7] | 74.7 [68.2–80.1] |
| 36–59 | 230 | 8.7 [5.7–13.2] | 22.3 [17.3–28.2] | 31.0 [25.3–37.3] | 69.0 [62.7–74.7] |
| 0–59 | 516 | 7.6 [5.6–10.2] | 19.8 [16.6–23.5] | 27.4 [23.7–31.4] | 72.6 [68.6–76.3] |
| Age Group (Months) | N | Severe [Hb < 7 g/dL] | Moderate [Hb 7–9.9 g/dL] | Mild [Hb 10–10.9 g/dL] | Total [Hb < 11 g/dL] | No Anemia [Hb ≥ 11 g/dL] |
|---|---|---|---|---|---|---|
| 12–23 | 84 | 16.7 | 66.7 | 14.3 | 97.7 | 2.3 |
| 24–35 | 115 | 2.6 | 67.0 | 26.1 | 95.7 | 4.3 |
| 36–47 | 95 | 2.1 | 50.5 | 33.7 | 86.3 | 13.7 |
| 48–59 | 125 | 2.4 | 48.8 | 35.2 | 86.4 | 13.6 |
| 12–59 | 419 | 5.2 | 57.8 | 28.2 | 91.2 | 8.8 |
| Deficiency of Micronutrients | 12–23 Months | 24–35 Months | 36–47 Months | 48–59 Months | Pooled (12–59 Months) | |
|---|---|---|---|---|---|---|
| Iron deficiency (Serum ferritin < 12 ng/mL) | N | 65 | 109 | 90 | 123 | 387 |
| n (%) | 42 (64.6) | 64 (58.7) | 38 (42.2) | 49 (39.8) | 193 (49.9) | |
| Folate deficiency (Serum erythrocyte folate < 151 ng/mL) | N | 73 | 109 | 90 | 123 | 395 |
| n (%) | 11 (15.1) | 11 (10.1) | 16 (17.8) | 24 (19.5) | 62 (15.7) | |
| Vitamin-B12 deficiency (Serum Vit B12 < 203 pg/nL) | N | 70 | 106 | 90 | 122 | 388 |
| n (%) | 24 (34.3) | 34 (32.1) | 31 (34.4) | 45 (36.9) | 134 (34.6) | |
| Vitamin-D deficiency (Serum 25 (OH) concentration < 12 ng/mL) | N | 74 | 109 | 91 | 124 | 398 |
| n (%) | 13 (17.6) | 17 (15.6) | 17 (18.7) | 32 (25.8) | 79 (19.9) | |
| Vitamin-A deficiency (Serum retinol concentration < 20 mg/dL) | N | 74 | 109 | 91 | 124 | 398 |
| n (%) | 10 (13.5) | 11 (10.1) | 12 13.2) | 14 (11.3) | 47 (11.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaleel, A.; Arlappa, N.; Ramakrishna, K.S.; Sunu, P.V.; Jayalakshmi, G.; Neeraja, G.; Narasimhulu, D.; Kumar, T.S.; Kumar, S.B. Examining the Triple Burden of Malnutrition: Insights from a Community-Based Comprehensive Nutrition Survey among Indigenous Tribal Children (0–19 Years) in the Western Ghats Hills of India. Nutrients 2023, 15, 3995. https://doi.org/10.3390/nu15183995
Jaleel A, Arlappa N, Ramakrishna KS, Sunu PV, Jayalakshmi G, Neeraja G, Narasimhulu D, Kumar TS, Kumar SB. Examining the Triple Burden of Malnutrition: Insights from a Community-Based Comprehensive Nutrition Survey among Indigenous Tribal Children (0–19 Years) in the Western Ghats Hills of India. Nutrients. 2023; 15(18):3995. https://doi.org/10.3390/nu15183995
Chicago/Turabian StyleJaleel, Abdul, N. Arlappa, K. Sree Ramakrishna, P. V. Sunu, G. Jayalakshmi, G. Neeraja, D. Narasimhulu, T. Santhosh Kumar, and Senthil B. Kumar. 2023. "Examining the Triple Burden of Malnutrition: Insights from a Community-Based Comprehensive Nutrition Survey among Indigenous Tribal Children (0–19 Years) in the Western Ghats Hills of India" Nutrients 15, no. 18: 3995. https://doi.org/10.3390/nu15183995
APA StyleJaleel, A., Arlappa, N., Ramakrishna, K. S., Sunu, P. V., Jayalakshmi, G., Neeraja, G., Narasimhulu, D., Kumar, T. S., & Kumar, S. B. (2023). Examining the Triple Burden of Malnutrition: Insights from a Community-Based Comprehensive Nutrition Survey among Indigenous Tribal Children (0–19 Years) in the Western Ghats Hills of India. Nutrients, 15(18), 3995. https://doi.org/10.3390/nu15183995





