Chlorogenic Acids, Acting via Calcineurin, Are the Main Compounds in Centella asiatica Extracts That Mediate Resilience to Chronic Stress in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Stock
2.2. Raw Centella asiatica Plant Materials and Pure Compounds
2.3. Preparation of Centella asiatica Water Extract (CAW)
2.4. Analysis of the CAW Extract
2.5. Supplementing Drosophila Food
2.6. Analysis of the Drosophila Food
2.7. Stress Protocol
2.8. Gap-Climbing Assays
2.9. Stop-for-Sweet (S4S) Assay
2.10. Sleep Experiments
2.11. Statistical Analyses
3. Results
3.1. CAW Is Stable in Standard Fly Food
3.2. CAW Ameliorates a Stress-Induced Depressive-like State in Drosophila
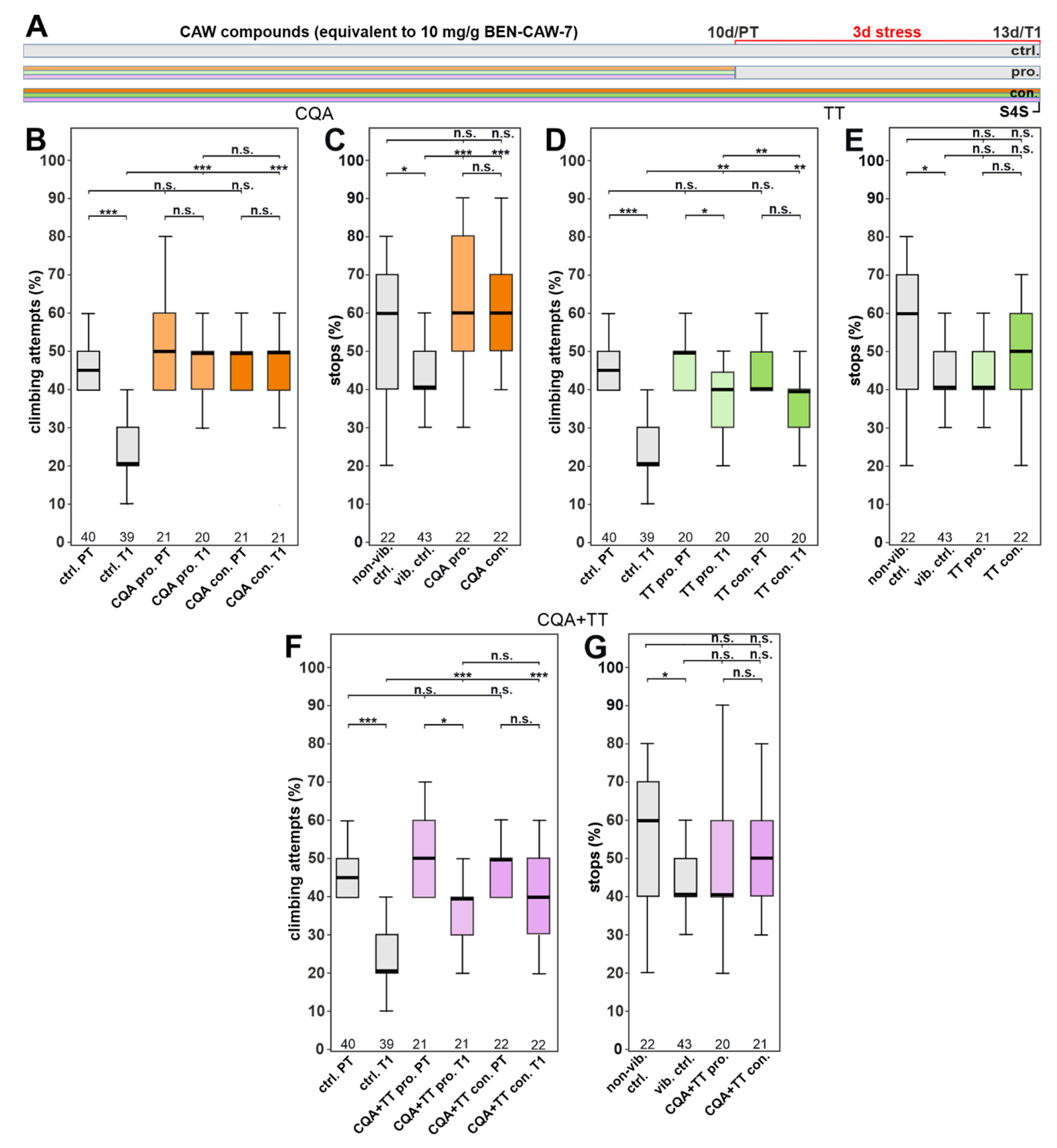
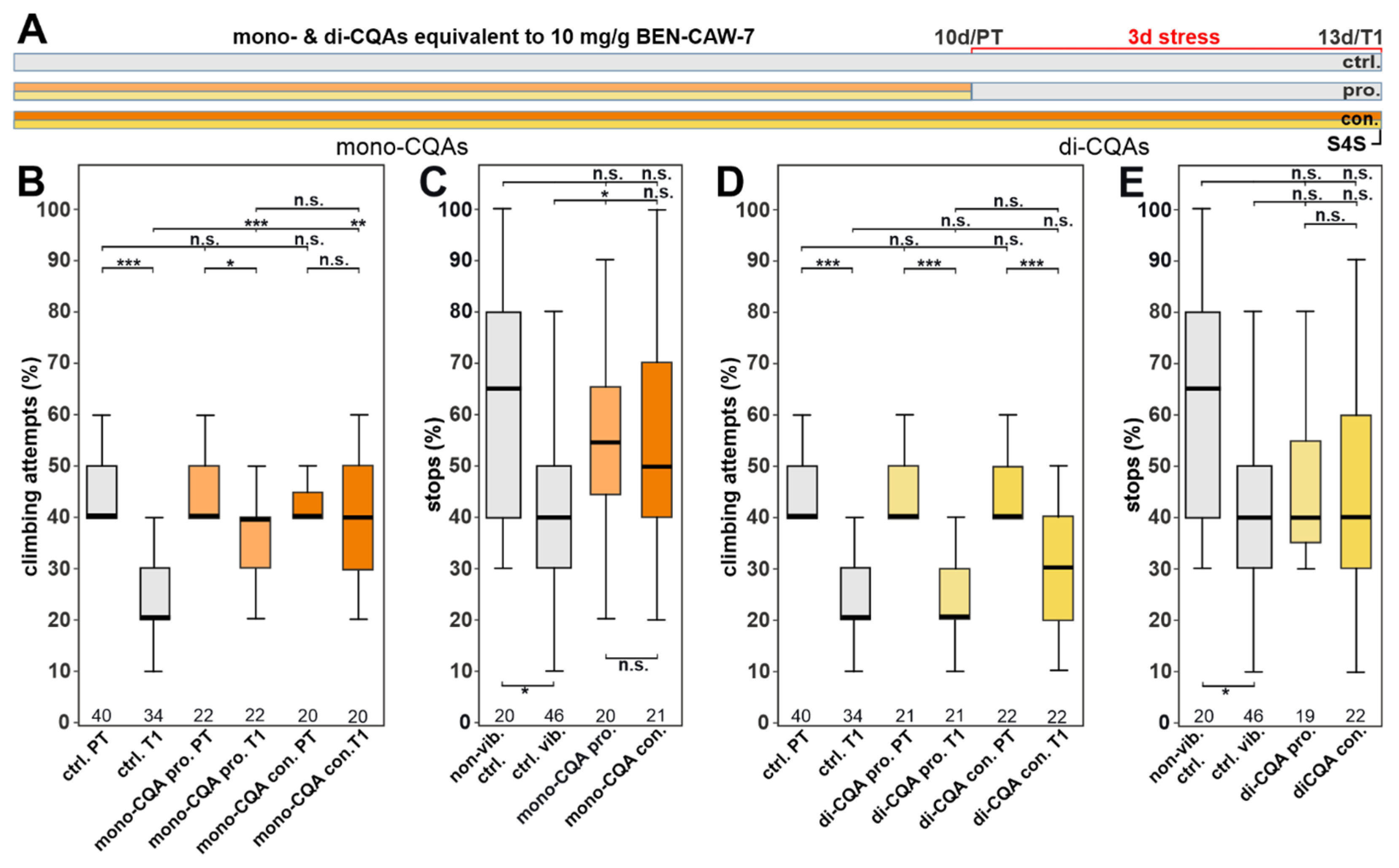
3.3. The Protective Effect of CAW Is Mainly Mediated by Mono-CQAs
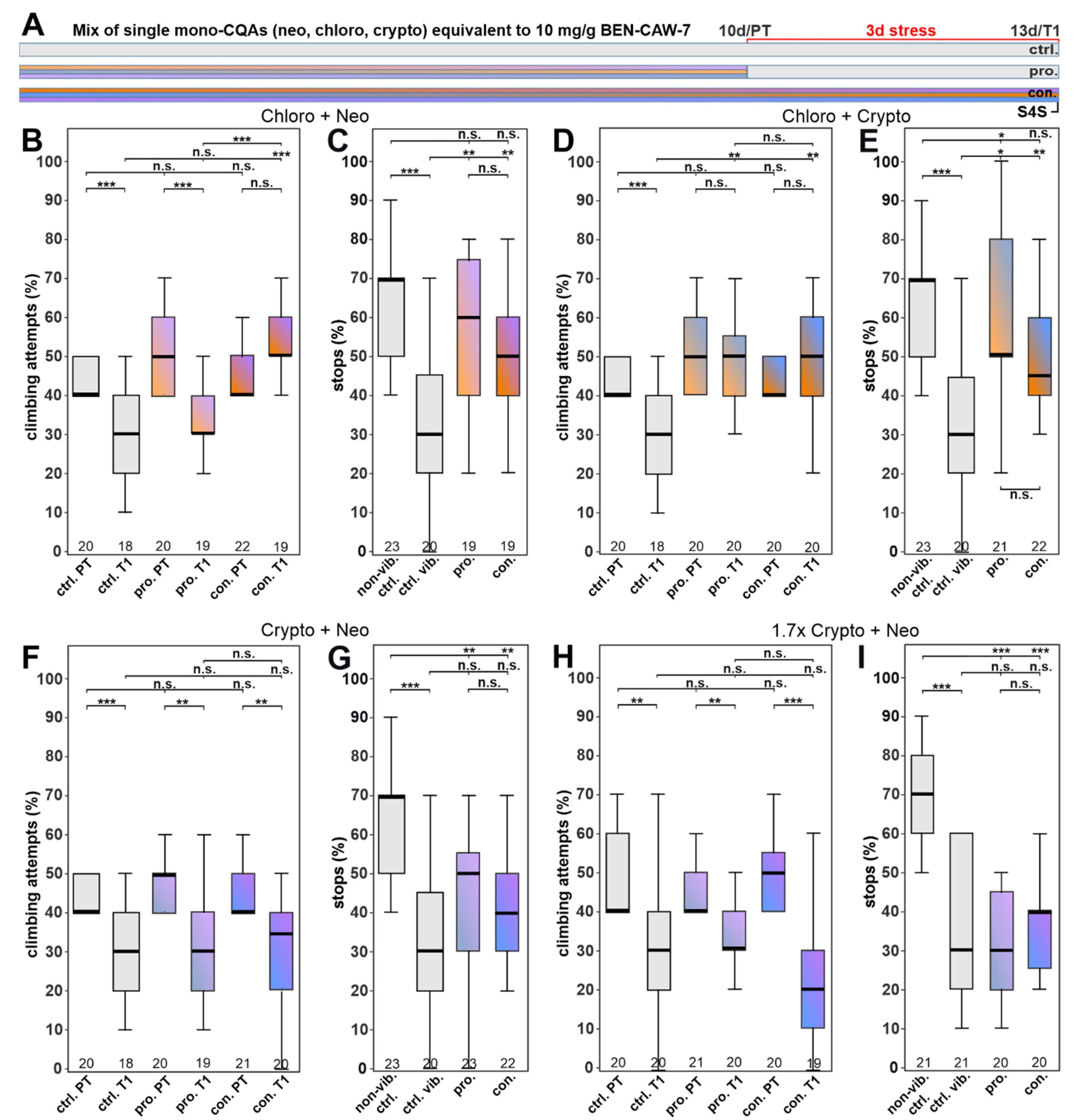
3.4. Single CQA Compounds Do Not Provide Resilience to Stress but a Combination of Chlorogenic Acid and One of Its Isomers Does
3.5. Increasing the Concentration of Chlorogenic Acid Can Overcome the Need for Synergistic Isomers
3.6. CAW Does Not Improve Sleep
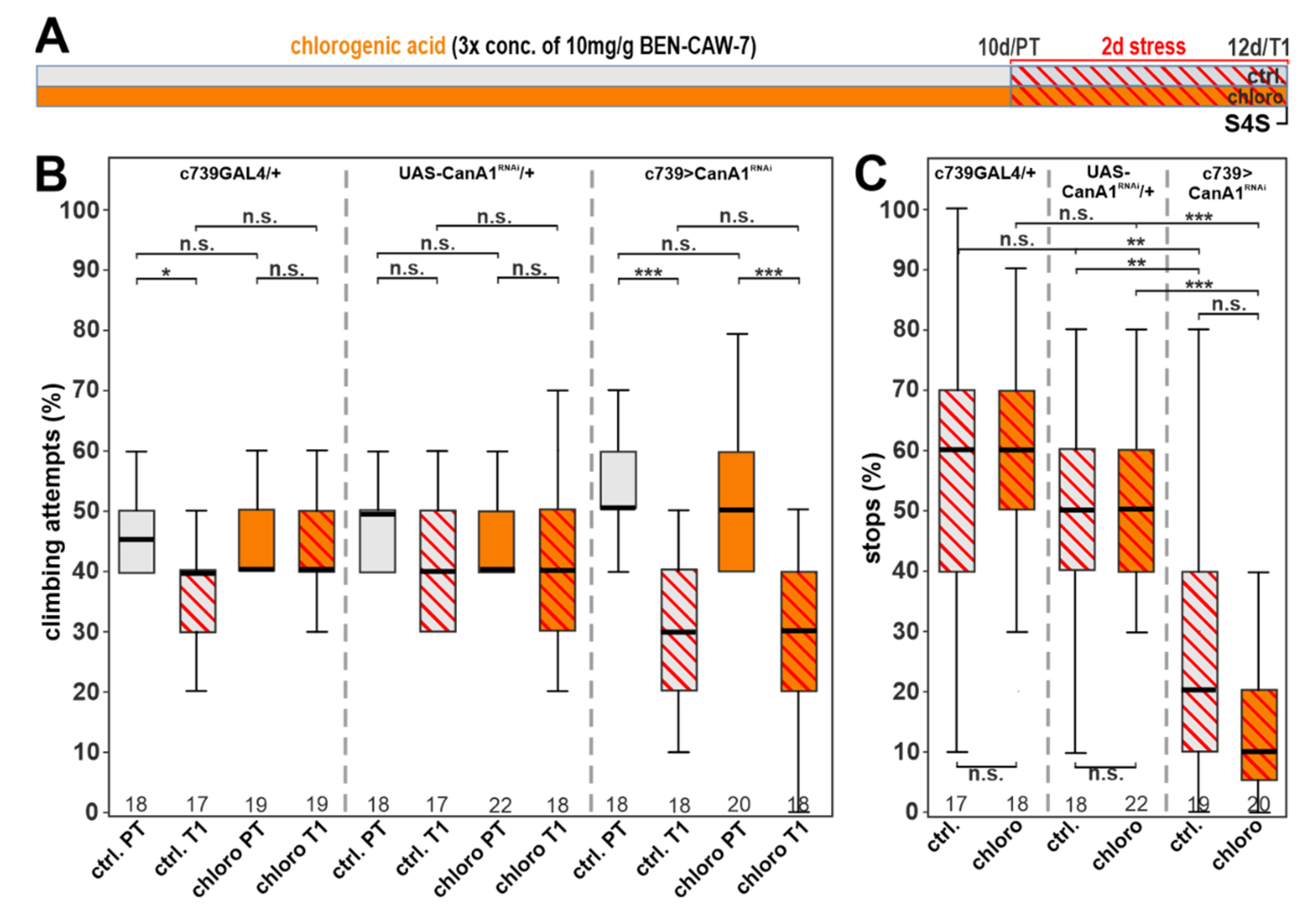
3.7. Calcineurin Depletion Prevents the Protective Function of Chlorogenic Acid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratt, L.A.; Druss, B.G.; Manderscheid, R.W.; Walker, E.R. Excess mortality due to depression and anxiety in the United States: Results from a nationally representative survey. Gen. Hosp. Psychiatry 2016, 39, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.D.; Dierker, L.C.; Wu, M.; Galea, S.; Hoven, C.W.; Weinberger, A.H. Trends in US depression prevalence from 2015 to 2020: The widening treatment gap. Am. J. Prev. Med. 2022, 63, 726–733. [Google Scholar] [CrossRef]
- Casey, D.A. Depression in Older Adults: A Treatable Medical Condition. Prim. Care 2017, 44, 499–510. [Google Scholar] [CrossRef]
- Almeida, O.P. Prevention of depression in older age. Maturitas 2014, 79, 136–141. [Google Scholar] [CrossRef]
- Tetsuka, S. Depression and dementia in older adults: A neuropsychological review. Aging Dis. 2021, 12, 1920. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, X.; Yang, D.; Xiong, C.; Xiong, Z. A comprehensive review and meta-analysis of neurological side effects related to second-generation antidepressants in individuals with major depressive disorder. Behav. Brain Res. 2023, 447, 114431. [Google Scholar] [CrossRef]
- Coupland, C.; Hill, T.; Morriss, R.; Moore, M.; Arthur, A.; Hippisley-Cox, J. Antidepressant use and risk of adverse outcomes in people aged 20–64 years: Cohort study using a primary care database. BMC Med. 2018, 16, 36. [Google Scholar] [CrossRef]
- Dickinson, A.; MacKay, D. Health habits and other characteristics of dietary supplement users: A review. Nutr. J. 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Majid, F.; Eckl, V.; Reynolds, C.M. Herbal supplement sales in US increase by record-breaking 17.3% in 2020. HerbalGram 2021, 131, 52–65. [Google Scholar]
- Lokanathan, Y.; Omar, N.; Ahmad Puzi, N.N.; Saim, A.; Hj Idrus, R. Recent Updates in Neuroprotective and Neuroregenerative Potential of Centella asiatica. Malays. J. Med. Sci. 2016, 23, 4–14. [Google Scholar] [PubMed]
- Chandrika, U.G.; Prasad Kumarab, P.A. Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits. Adv. Food Nutr. Res. 2015, 76, 125–157. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef]
- Vaidya, A. The status and scope of Indian medicinal plants acting on central nervous system. Indian J. Pharmacol. 1997, 29, 340. [Google Scholar]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Jaiswal, N.; Srivastava, M. A review on medicinal properties of Centella asiatica. Asian J. Pharm. Clin. Res. 2017, 10, 69–74. [Google Scholar] [CrossRef]
- Ganie, I.; Shahzad, A.; Ahmad, Z. Centella asiatica: Medicinal importance with special emphasis on its role in cancer and neuroprotection. BAOJ Biotechnol. 2022, 6, 1004. [Google Scholar]
- Jana, U.; Sur, T.; Maity, L.; Debnath, P.; Bhattacharyya, D. A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal. Med. Coll. J. 2010, 12, 8–11. [Google Scholar]
- Moulin, T.C.; Covill, L.E.; Itskov, P.M.; Williams, M.J.; Schiöth, H.B. Rodent and fly models in behavioral neuroscience: An evaluation of methodological advances, comparative research, and future perspectives. Neurosci. Biobehav. Rev. 2021, 120, 1–12. [Google Scholar] [CrossRef]
- van Alphen, B.; van Swinderen, B. Drosophila strategies to study psychiatric disorders. Brain Res. Bull. 2013, 92, 1–11. [Google Scholar] [CrossRef]
- Papanikolopoulou, K.; Mudher, A.; Skoulakis, E. An assessment of the translational relevance of Drosophila in drug discovery. Expert Opin. Drug Discov. 2019, 14, 303–313. [Google Scholar] [CrossRef]
- Su, T.T. Drug screening in Drosophila; why, when, and when not? Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e346. [Google Scholar] [CrossRef] [PubMed]
- Gospodaryov, D.V.; Yurkevych, I.S.; Jafari, M.; Lushchak, V.I.; Lushchak, O.V. Lifespan extension and delay of age-related functional decline caused by Rhodiola roseadepends on dietary macronutrient balance. Longev. Heal. 2013, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, H.; Long, D.M.; Law, A.; McClure, C.; Choi, J.; Yang, L.; Marney, L.; Poeck, B.; Strauss, R.; Stevens, J.F. Withania somnifera Extracts Promote Resilience against Age-Related and Stress-Induced Behavioral Phenotypes in Drosophila melanogaster; a Possible Role of Other Compounds besides Withanolides. Nutrients 2022, 14, 3923. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.-S.; Hermanns, T.; Poeck, B.; Strauss, R. Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nat. Commun. 2017, 8, 15738. [Google Scholar] [CrossRef]
- Hermanns, T.; Graf-Boxhorn, S.; Poeck, B.; Strauss, R. Octopamine mediates sugar relief from a chronic-stress-induced depression-like state in Drosophila. Curr. Biol. 2022, 32, 4048–4056.e3. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal models of depression: What can they teach us about the human disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2022, 19, 181–191. [Google Scholar] [CrossRef]
- Araujo, S.M.; Poetini, M.R.; Bortolotto, V.C.; de Freitas Couto, S.; Pinheiro, F.C.; Meichtry, L.B.; de Almeida, F.P.; Musachio, E.A.S.; de Paula, M.T.; Prigol, M. Chronic unpredictable mild stress-induced depressive-like behavior and dysregulation of brain levels of biogenic amines in Drosophila melanogaster. Behav. Brain Res. 2018, 351, 104–113. [Google Scholar] [CrossRef]
- Yang, L.; Marney, L.; Magana, A.A.; Choi, J.; Wright, K.; McFerrin, J.; Gray, N.E.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Quantification of Caffeoylquinic Acids and Triterpenes as Targeted Bioactive Compounds of Centella asiatica in Extracts and Formulations by Liquid Chromatography Mass Spectrometry. J. Chromatogr. Open 2023, 4, 100091. [Google Scholar] [CrossRef]
- Cassar, M.; Law, A.D.; Chow, E.S.; Giebultowicz, J.M.; Kretzschmar, D. Disease-associated mutant tau prevents circadian changes in the cytoskeleton of central pacemaker neurons. Front. Neurosci. 2020, 14, 232. [Google Scholar] [CrossRef]
- Alcazar Magana, A.; Wright, K.; Vaswani, A.; Caruso, M.; Reed, R.L.; Bailey, C.F.; Nguyen, T.; Gray, N.E.; Soumyanath, A.; Quinn, J. Integration of mass spectral fingerprinting analysis with precursor ion (MS1) quantification for the characterisation of botanical extracts: Application to extracts of Centella asiatica (L.) Urban. Phytochem. Anal. 2020, 31, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Cabey, K.; Long, D.M.; Law, A.; Gray, N.E.; McClure, C.; Caruso, M.; Lak, P.; Wright, K.M.; Stevens, J.F.; Maier, C.S.; et al. Withania somnifera and Centella asiatica Extracts Ameliorate Behavioral Deficits in an In Vivo Drosophila melanogaster Model of Oxidative Stress. Antioxidants 2022, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Franzen, P.L.; Buysse, D.J. Sleep disturbances and depression: Risk relationships for subsequent depression and therapeutic implications. Dialogues Clin. Neurosci. 2008, 10, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhou, J.; Ma, J.; Chang, J.; Qiu, Y.; Zhuang, Z.; Xiao, H.; Zeng, L. Sleep disturbance mediates the relationship between depressive symptoms and cognitive function in older adults with mild cognitive impairment. Geriatr. Nurs. 2021, 42, 1019–1023. [Google Scholar] [CrossRef]
- Gomes, S.R.B.S.; von Schantz, M.; Leocadio-Miguel, M. Predicting depressive symptoms in middle-aged and elderly adults using sleep data and clinical health markers: A machine learning approach. Sleep Med. 2023, 102, 123–131. [Google Scholar] [CrossRef]
- Krystal, A.; Fava, M.; Rubens, R.; Wessel, T.; Caron, J.; Wilson, P.; Roth, T.; McCall, W.V. Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J. Clin. Sleep Med. 2007, 3, 48–55. [Google Scholar] [PubMed]
- Koh, K.; Evans, J.M.; Hendricks, J.C.; Sehgal, A. A Drosophila model for age-associated changes in sleep: Wake cycles. Proc. Natl. Acad. Sci. USA 2006, 103, 13843–13847. [Google Scholar] [CrossRef]
- Wu, H.-Z.; Luo, J.; Yin, Y.-X.; Wei, Q. Effects of chlorogenic acid, an active compound activating calcineurin, purified from Flos Lonicerae on macrophage. Acta Pharmacol. Sin. 2004, 25, 1685–1692. [Google Scholar]
- Yin, Y.; Xie, M.; Wu, H.; Jiang, M.; Zheng, J.; Wei, Q. Interaction of calcineurin with its activator, chlorogenic acid revealed by spectroscopic methods. Biochimie 2009, 91, 820–825. [Google Scholar] [CrossRef]
- Tong, L.; Song, Y.; Jia, Z.; Zhang, W.; Wei, Q. Calmodulin-dependent activation of calcineurin by chlorogenic acid. IUBMB Life 2007, 59, 402–407. [Google Scholar] [CrossRef]
- Saraf, J.; Bhattacharya, P.; Kalia, K.; Borah, A.; Sarmah, D.; Kaur, H.; Dave, K.R.; Yavagal, D.R. A friend or foe: Calcineurin across the gamut of neurological disorders. ACS Cent. Sci. 2018, 4, 805–819. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Grübel, K.; Busch, S.; Friedrich, A.B.; Siwanowicz, I.; Tanimoto, H. The Mushroom Body of Adult Drosophila Characterized by GAL4 Drivers. J. Neurogenet. 2009, 23, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Malík, M.; Tlustoš, P. Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers. Plants 2023, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Golla, P.; Tirupathi, H. To evaluate and compare antidepressant activity of Centella asiatica in mice by using forced swimming test. Int. J. Basic Clin. Pharmacol. 2016, 5, 2017–2020. [Google Scholar] [CrossRef]
- Jagadeesan, S.; Chiroma, S.M.; Baharuldin, M.T.H.; Taib, C.N.M.; Amom, Z.; Adenan, M.I.; Moklas, M.A.M. Centella asiatica prevents chronic unpredictable mild stress-induced behavioral changes in rats. Biomed. Res. Ther. 2019, 6, 3233–3243. [Google Scholar] [CrossRef]
- Cao, B.; Zhu, J.; Zuckerman, H.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Subramanieapillai, M.; Park, C.; Lee, Y.; McIntyre, R.S. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 109–117. [Google Scholar] [CrossRef]
- Su, Y.-A.; Si, T. Progress and challenges in research of the mechanisms of anhedonia in major depressive disorder. Gen. Psychiatry 2022, 35, e100724. [Google Scholar] [CrossRef]
- Wang, L.; Guo, T.; Guo, Y.; Xu, Y. Asiaticoside produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice, involving reversion of inflammation and the PKA/pCREB/BDNF signaling pathway. Mol. Med. Rep. 2020, 22, 2364–2372. [Google Scholar] [CrossRef]
- Gray, N.E.; Morré, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica protect against amyloid-β toxicity. J. Alzheimer’s Dis. 2014, 40, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, Y.; Kurisu, M.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Bioorganic Med. Chem. 2012, 20, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Park, J.; Jung, J.; Kim, S.H.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Han, D.; Lee, C.; Lee, J. Dicaffeoylquinic acids alleviate memory loss via reduction of oxidative stress in stress-hormone-induced depressive mice. Pharmacol. Res. 2020, 161, 105252. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Lucas, M.; Mirzaei, F.; Pan, A.; Okereke, O.I.; Willett, W.C.; O’Reilly, J.; Koenen, K.; Ascherio, A. Coffee, caffeine, and risk of depression among women. Arch. Intern. Med. 2011, 171, 1571–1578. [Google Scholar] [CrossRef]
- Tenore, G.C.; Daglia, M.; Orlando, V.; D’Urso, E.; Saadat, S.H.; Novellino, E.; Nabavi, S.F.; Nabavi, S.M. Coffee and Depression: A Short Review of Literature. Curr. Pharm. Des. 2015, 21, 5034–5040. [Google Scholar] [CrossRef]
- Park, R.J.; Moon, J.D. Coffee and depression in Korea: The fifth Korean National Health and Nutrition Examination Survey. Eur. J. Clin. Nutr. 2015, 69, 501–504. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, Y.-B.; Han, P.-L.; Lee, J.-K.; Suh, H.-W. Antidepressant-like effect of chlorogenic acid isolated from Artemisia capillaris Thunb. Anim. Cells Syst. 2010, 14, 253–259. [Google Scholar] [CrossRef]
- Murlanova, K.; Cohen, N.; Pinkus, A.; Vinnikova, L.; Pletnikov, M.; Kirby, M.; Gorelick, J.; Drori, E.; Pinhasov, A. Antidepressant-like effects of a chlorogenic acid- and cynarine-enriched fraction from Dittrichia viscosa root extract. Sci. Rep. 2022, 12, 3647. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Miyazaki, S.; Fujita, Y.; Oikawa, H.; Takekoshi, H.; Soya, H.; Ogata, M.; Fujikawa, T. Combination of syringaresinol-di-O-β-D-glucoside and chlorogenic acid shows behavioral pharmacological anxiolytic activity and activation of hippocampal BDNF-TrkB signaling. Sci. Rep. 2020, 10, 18177. [Google Scholar] [CrossRef]
- Lim, D.W.; Yoo, G.; Lee, C. Dried Loquat Fruit Extract Containing Chlorogenic Acid Prevents Depressive-like Behaviors Induced by Repeated Corticosteroid Injections in Mice. Molecules 2023, 28, 5612. [Google Scholar] [CrossRef]
- Crozatier, C.; Farley, S.; Mansuy, I.M.; Dumas, S.; Giros, B.; Tzavara, E.T. Calcineurin (protein phosphatase 2B) is involved in the mechanisms of action of antidepressants. Neuroscience 2007, 144, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Turrillas, R.; Frechilla, D.; Del Río, J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology 2002, 43, 1230–1237. [Google Scholar] [CrossRef]
- Martínez-Turrillas, R.; Del Río, J.; Frechilla, D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology 2005, 49, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Solà, C.; Tusell, J.M.; Serratosa, J. Comparative study of the distribution of calmodulin kinase II and calcineurin in the mouse brain. J. Neurosci. Res. 1999, 57, 651–662. [Google Scholar] [CrossRef]
- Kipanyula, M.J.; Kimaro, W.H.; Etet, P.F.S. The Emerging Roles of the Calcineurin-Nuclear Factor of Activated T-Lymphocytes Pathway in Nervous System Functions and Diseases. J. Aging Res. 2016, 2016, 5081021. [Google Scholar] [CrossRef]
- He, J.G.; Zhou, H.Y.; Wang, F.; Chen, J.G. Dysfunction of Glutamatergic Synaptic Transmission in Depression: Focus on AMPA Receptor Trafficking. Biol. Psychiatry Glob. Open Sci. 2023, 3, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hara, H.; Kimura, H. Role of the AMPA receptor in antidepressant effects of ketamine and potential of AMPA receptor potentiators as a novel antidepressant. Neuropharmacology 2023, 222, 109308. [Google Scholar] [CrossRef]





| Compound Group and Name | Structural Information | Concentration (mg/mL) Equivalent to CAW 100 mg/mL |
|---|---|---|
| CQAs: | ||
| Chlorogenic acid | 3-caffeoylquinic acid | 0.750 ± 0.017 |
| Cryptochlorogenic acid | 4-caffeoylquinic acid | 0.298 ± 0.004 |
| Neochlorogenic acid | 5-caffeoylquinic acid | 0.339 ± 0.006 |
| 1,3-Dicaffeoylquinic acid | 1,3-dicaffeoylquinic acid | 0.258 ± 0.011 |
| 1,5-Dicaffeoylquinic acid | 1,5-dicaffeoylquinic acid | 0.389 ± 0.006 |
| Isochlorogenic acid A | 3,5-dicaffeoylquinic acid | 0.177 ± 0.004 |
| Isochlorogenic acid B | 3,4-dicaffeoylquinic acid | 0.229 ± 0.006 |
| Isochlorogenic acid C | 4,5-dicaffeoylquinic acid | 0.195 ± 0.007 |
| Triterpenes: | ||
| Asiatic acid | Triterpene aglycone | 0.042 ± 0.003 |
| Madecassic acid | Triterpene aglycone | 0.075 ± 0.001 |
| Asiaticoside | Asiatic acid glycoside | 1.475 ± 0.014 |
| Madecassoside | Madecassic acid glycoside | 3.589 ± 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holvoet, H.; Long, D.M.; Yang, L.; Choi, J.; Marney, L.; Poeck, B.; Maier, C.S.; Soumyanath, A.; Kretzschmar, D.; Strauss, R. Chlorogenic Acids, Acting via Calcineurin, Are the Main Compounds in Centella asiatica Extracts That Mediate Resilience to Chronic Stress in Drosophila melanogaster. Nutrients 2023, 15, 4016. https://doi.org/10.3390/nu15184016
Holvoet H, Long DM, Yang L, Choi J, Marney L, Poeck B, Maier CS, Soumyanath A, Kretzschmar D, Strauss R. Chlorogenic Acids, Acting via Calcineurin, Are the Main Compounds in Centella asiatica Extracts That Mediate Resilience to Chronic Stress in Drosophila melanogaster. Nutrients. 2023; 15(18):4016. https://doi.org/10.3390/nu15184016
Chicago/Turabian StyleHolvoet, Helen, Dani M. Long, Liping Yang, Jaewoo Choi, Luke Marney, Burkhard Poeck, Claudia S. Maier, Amala Soumyanath, Doris Kretzschmar, and Roland Strauss. 2023. "Chlorogenic Acids, Acting via Calcineurin, Are the Main Compounds in Centella asiatica Extracts That Mediate Resilience to Chronic Stress in Drosophila melanogaster" Nutrients 15, no. 18: 4016. https://doi.org/10.3390/nu15184016
APA StyleHolvoet, H., Long, D. M., Yang, L., Choi, J., Marney, L., Poeck, B., Maier, C. S., Soumyanath, A., Kretzschmar, D., & Strauss, R. (2023). Chlorogenic Acids, Acting via Calcineurin, Are the Main Compounds in Centella asiatica Extracts That Mediate Resilience to Chronic Stress in Drosophila melanogaster. Nutrients, 15(18), 4016. https://doi.org/10.3390/nu15184016