Caloric Restriction: A Novel Conditioning Strategy to Improve the Survival of Ischemically Challenged Musculocutaneous Random Pattern Flaps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CR Regimen
2.3. Anesthesia
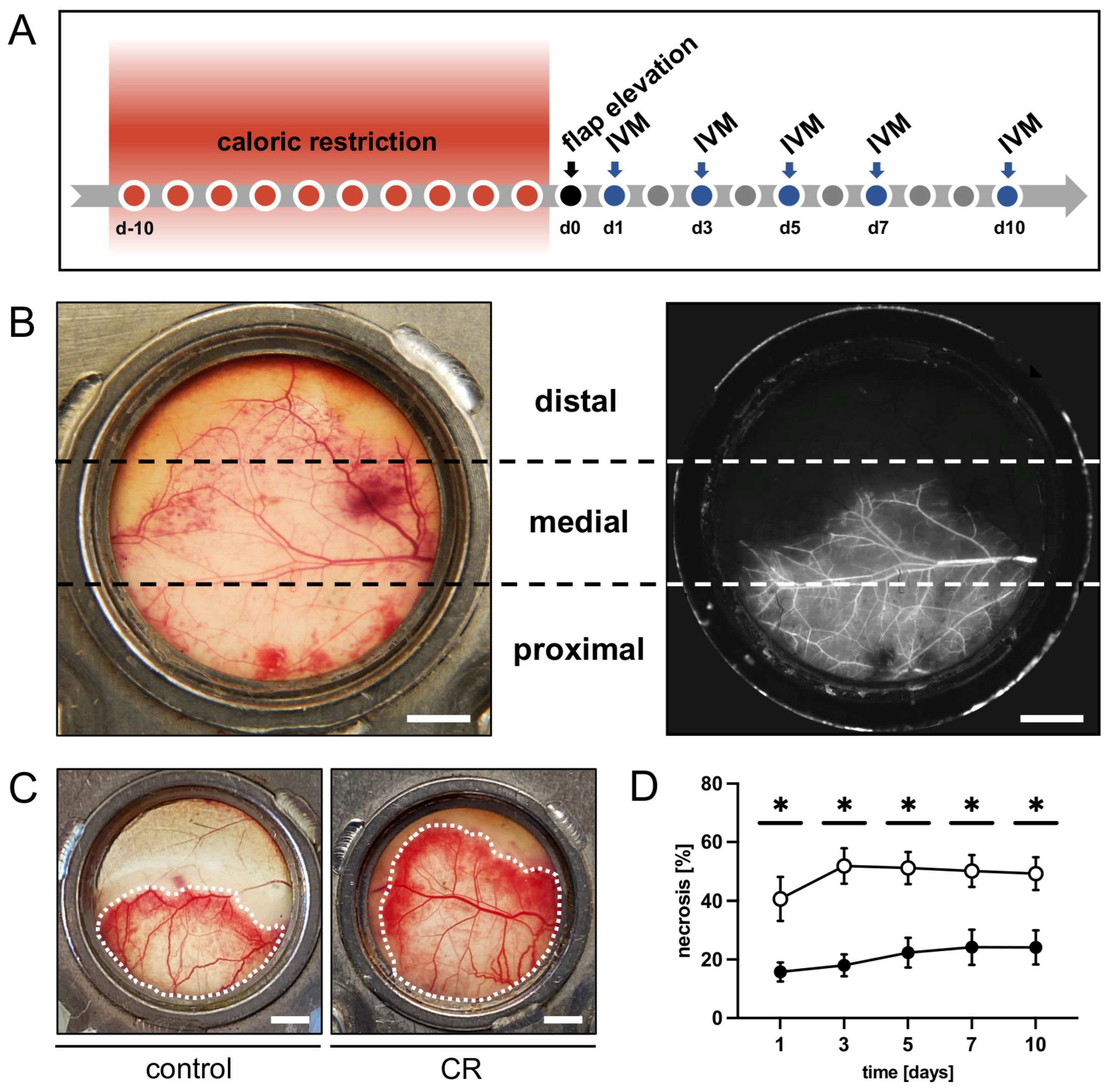
2.4. Flap Model
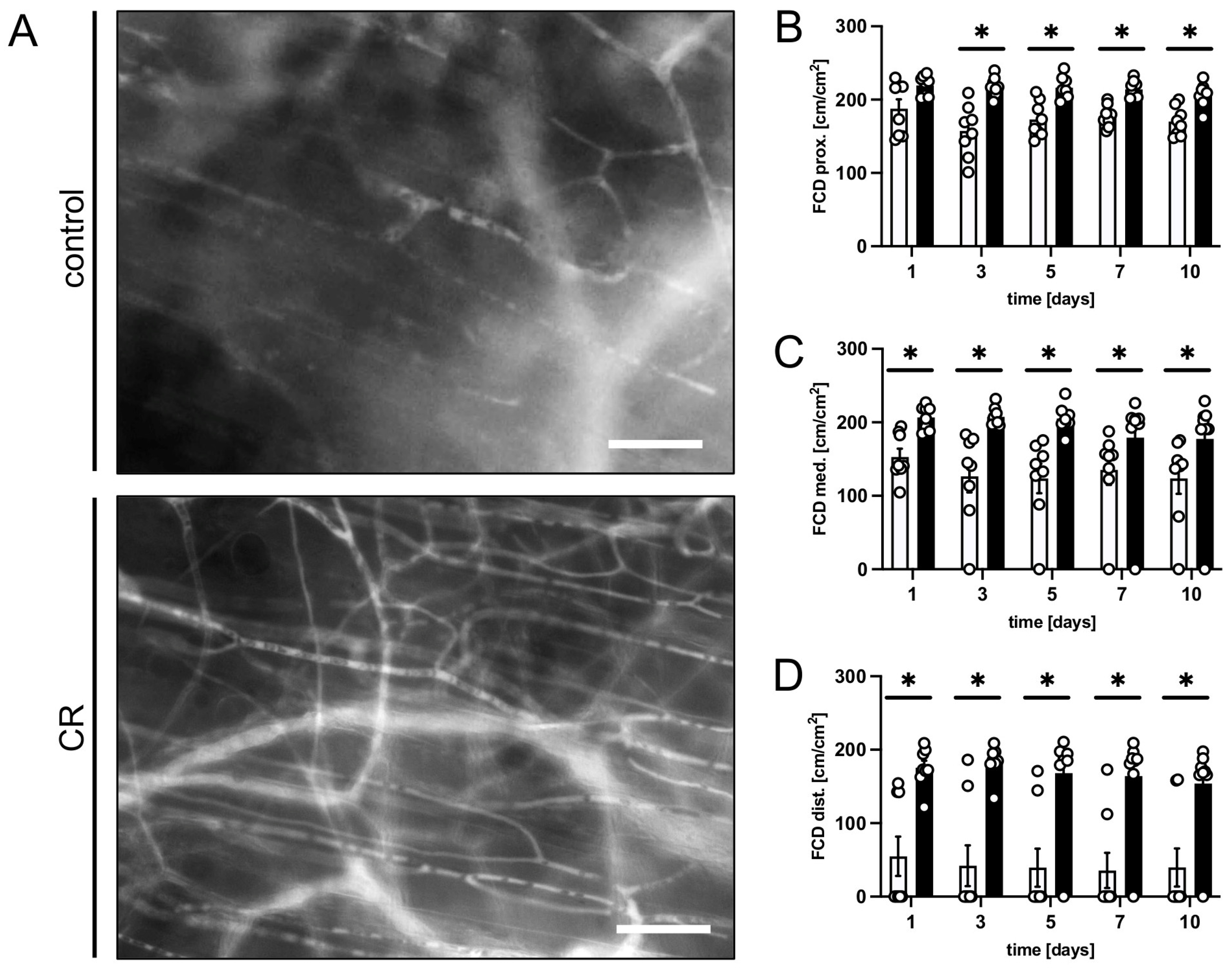
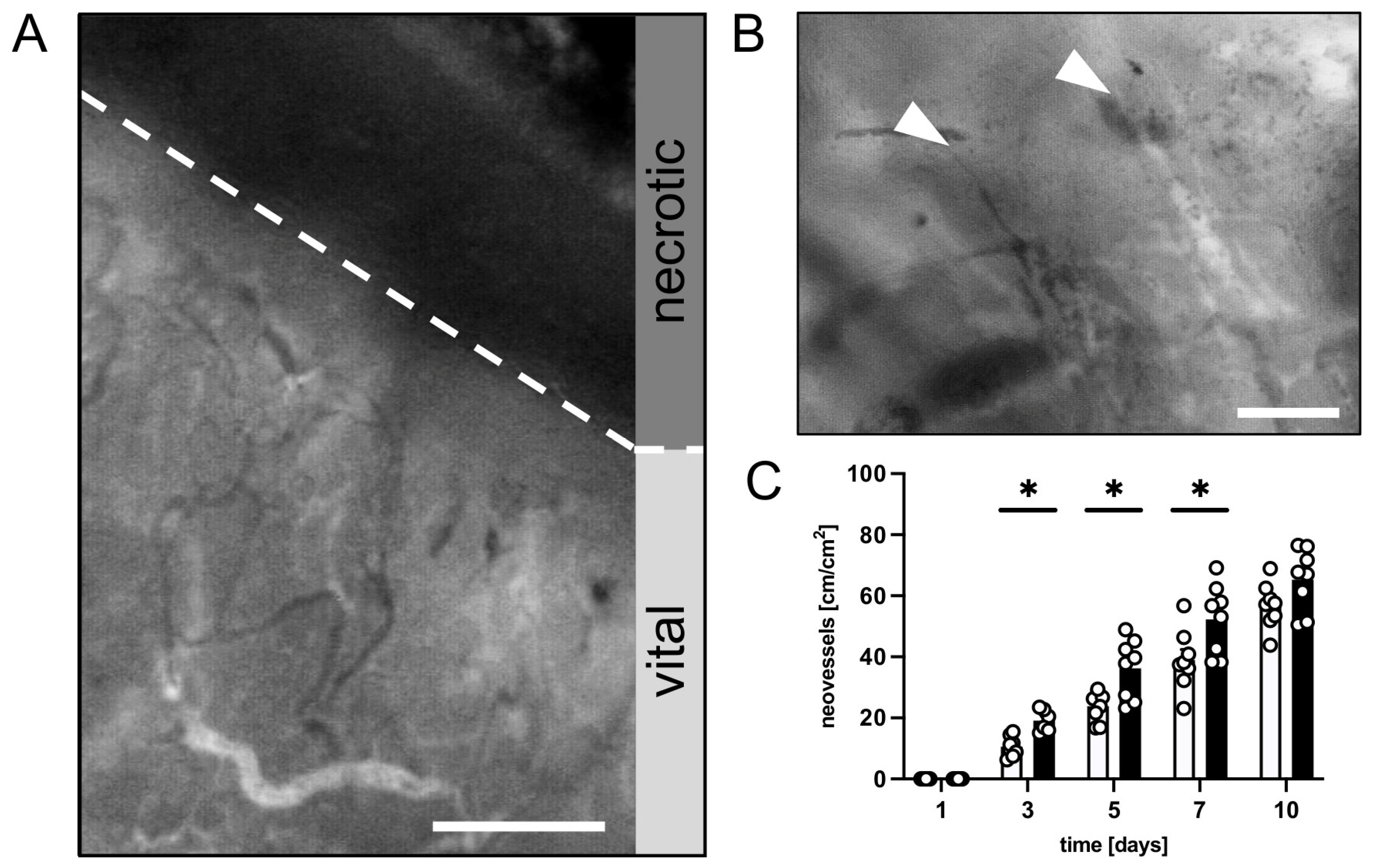
2.5. Intravital Fluorescence Microscopy
2.6. Histology and Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. In Vivo Experiments
3.2. Histological and Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofer, S.J.; Carmona-Gutierrez, D.; Mueller, M.I.; Madeo, F. The Ups and Downs of Caloric Restriction and Fasting: From Molecular Effects to Clinical Application. EMBO Mol. Med. 2022, 14, e14418. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, F.; Aujard, F. Caloric Restriction, Longevity and Aging: Recent Contributions from Human and Non-Human Primate Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109702. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S. Aging, Adiposity, and Calorie Restriction. JAMA 2007, 297, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; De Cabo, R. A Time to Fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The Role of Low-Calorie Diets and Intermittent Fasting in the Treatment of Obesity and Type-2 Diabetes. J. Physiol. Pharmacol. 2019, 69, 663–683. [Google Scholar]
- Müller, H.; De Toledo, F.W.; Resch, K.L. Fasting Followed by Vegetarian Diet in Patients with Rheumatoid Arthritis: A Sys-tematic Review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar]
- Hargraves, W.A.; Hentall, I.D. Analgesic Effects of Dietary Caloric Restriction in Adult Mice. Pain 2005, 114, 455–461. [Google Scholar] [CrossRef]
- Robertson, L.T.; Mitchell, J.R. Benefits of Short-Term Dietary Restriction in Mammals. Exp. Gerontol. 2013, 48, 1043–1048. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Müller, C.; de Jong, M.; et al. Short-Term Dietary Restriction and Fasting Precondition against Ischemia Reperfusion Injury in Mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, R.; Southard, J.H.; Belzer, F.O. Livers from Fasted Rats Acquire Resistance to Warm and Cold Ischemia Injury. Transplantation 1993, 55, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Verweij, M.; Van Ginhoven, T.M.; Mitchell, J.R.; Sluiter, W.; Engel, S.V.D.; Roest, H.P.; Torabi, E.; Ijzermans, J.N.M.; Hoeijmakers, J.H.J.; de Bruin, R.W.F. Preoperative Fasting Protects Mice against Hepatic Ischemia/Reperfusion Injury: Mechanisms and Effects on Liver Regeneration. Liver Transplant. 2010, 17, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Katare, R.G.; Kakinuma, Y.; Arikawa, M.; Yamasaki, F.; Sato, T. Chronic Intermittent Fasting Improves the Survival Following Large Myocardial Ischemia by Activation of BDNF/VEGF/PI3K Signaling Pathway. J. Mol. Cell Cardiol. 2009, 46, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Varendi, K.; Airavaara, M.; Anttila, J.; Vose, S.; Planken, A.; Saarma, M.; Mitchell, J.R.; Andressoo, J.-O. Short-Term Preoperative Dietary Restriction Is Neuroprotective in a Rat Focal Stroke Model. PLoS ONE 2014, 9, e93911. [Google Scholar] [CrossRef]
- Mitchell, S.E.; Delville, C.; Konstantopedos, P.; Hurst, J.; Derous, D.; Green, C.; Chen, L.; Han, J.J.; Wang, Y.; Promislow, D.E.; et al. The Effects of Graded Levels of Calorie Restriction: II. Impact of Short Term Calorie and Protein Restriction on Circulating Hormone Levels, Glucose Homeostasis and Oxidative Stress in Male C57BL/6 Mice. Oncotarget 2015, 6, 23213–23237. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Choi, M.; Karp, N.S. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation Using Magnetic Resonance Imaging. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1439. [Google Scholar] [CrossRef]
- Hashimoto, I.; Abe, Y.; Ishida, S.; Kashiwagi, K.; Mineda, K.; Yamashita, Y.; Yamato, R.; Toda, A.; Fukunaga, Y.; Yoshimoto, S.; et al. Development of Skin Flaps for Reconstructive Surgery: Random Pattern Flap to Perforator Flap. J. Med. Investig. 2016, 63, 159–162. [Google Scholar] [CrossRef]
- Harder, Y.; Contaldo, C.; Klenk, J.; Banic, A.; Jakob, S.; Erni, D. Improved Skin Flap Survival after Local Heat Preconditioning in Pigs1. J. Surg. Res. 2004, 119, 100–105. [Google Scholar] [CrossRef]
- Tobalem, M.; Wettstein, R.; Pittet-Cuénod, B.; Vigato, E.; Machens, H.-G.; Lohmeyer, J.-A.; Rezaeian, F.; Harder, Y. Local Shockwave-Induced Capillary Recruitment Improves Survival of Musculocutaneous Flaps. J. Surg. Res. 2013, 184, 1196–1204. [Google Scholar] [CrossRef]
- Schmauss, D.; Weinzierl, A.; Weiss, F.; Egaña, J.T.; Rezaeian, F.; Hopfner, U.; Schmauss, V.; Machens, H.-G.; Harder, Y. Long-Term Pre- and Postconditioning with Low Doses of Erythropoietin Protects Critically Perfused Musculocutaneous Tissue from Necrosis. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 590–599. [Google Scholar] [CrossRef]
- Brandhorst, S.; Harputlugil, E.; Mitchell, J.R.; Longo, V.D. Protective Effects of Short-Term Dietary Restriction in Surgical Stress and Chemotherapy. Ageing Res. Rev. 2017, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Harputlugil, E.; Corpataux, J.-M.; Ozaki, C.K.; Mitchell, J.R. Is Overnight Fasting before Surgery Too Much or Not Enough? How Basic Aging Research Can Guide Preoperative Nutritional Recommendations to Improve Surgical Outcomes: A Mini-Review. Gerontology 2017, 63, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Beckman, J.A.; Nguyen, L.L.; Ozaki, C.K. Reducing Elective Vascular Surgery Perioperative Risk with Brief Preoperative Dietary Restriction. Surgery 2012, 153, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent Fasting in Type 2 Diabetes Mellitus and the Risk of Hypoglycaemia: A Randomized Controlled Trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, F.; De Bruin, R.W.F.; Pennings, J.L.A.; Payán-Gómez, C.; Engel, S.V.D.; Van Oostrom, C.T.; De Bruin, A.; Hoeijmakers, J.H.J.; Van Steeg, H.; Ijzermans, J.N.M.; et al. Preoperative Fasting Protects against Renal Ischemia-Reperfusion Injury in Aged and Overweight Mice. PLoS ONE 2014, 9, e100853. [Google Scholar] [CrossRef]
- Grundmann, F.; Müller, R.-U.; Hoyer-Allo, K.J.R.; Späth, M.R.; Passmann, E.; Becker, I.; Pfister, R.; Baldus, S.; Benzing, T.; Burst, V. Dietary Restriction for Prevention of Contrast-Induced Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Angiography: A Randomized Controlled Trial. Sci. Rep. 2020, 10, 5202. [Google Scholar] [CrossRef]
- Grundmann, F.; Müller, R.; Reppenhorst, A.; Hülswitt, L.; Späth, M.R.; Kubacki, T.; Scherner, M.; Faust, M.; Becker, I.; Wahlers, T.; et al. Preoperative Short-Term Calorie Restriction for Prevention of Acute Kidney Injury After Cardiac Surgery: A Randomized, Controlled, Open-Label, Pilot Trial. J. Am. Heart Assoc. 2018, 7, e008181. [Google Scholar] [CrossRef]
- Jung, A.P.; Curtis, T.S.; Turner, M.J.; Lightfoot, J.T. Physical Activity and Food Consumption in High- and Low-Active Inbred Mouse Strains. Med. Sci. Sports Exerc. 2010, 42, 1826–1833. [Google Scholar] [CrossRef]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric Restriction and Aging: Studies in Mice and Monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.A.; de Groot, M.H.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J.S. Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab. 2017, 26, 267–277.e2. [Google Scholar] [CrossRef] [PubMed]
- Mezhnina, V.; Pearce, R.; Poe, A.; Velingkaar, N.; Astafev, A.; Ebeigbe, O.P.; Makwana, K.; Sandlers, Y.; Kondratov, R.V. CR Reprograms Acetyl-CoA Metabolism and Induces Long-Chain Acyl-CoA Dehydrogenase and CrAT Expression. Aging Cell 2020, 19, e13266. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.T.; Treviño-Villarreal, J.H.; Mejia, P.; Grondin, Y.; Harputlugil, E.; Hine, C.; Vargas, D.; Zheng, H.; Ozaki, C.K.; Kristal, B.S.; et al. Protein and Calorie Restriction Contribute Additively to Protection from Renal Ischemia Reperfusion Injury Partly via Leptin Reduction in Male Mice. J. Nutr. 2015, 145, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Harder, Y.; Amon, M.; Erni, D. Menger Evolution of Ischemic Tissue Injury in a Random Pattern Flap: A New Mouse Model Using Intravital Microscopy. J. Surg. Res. 2004, 121, 197–205. [Google Scholar] [CrossRef]
- Harder, Y.; Schmauss, D.; Wettstein, R.; Egaña, J.T.; Weiss, F.; Weinzierl, A.; Schuldt, A.; Machens, H.-G.; Menger, M.D.; Rezaeian, F. Ischemic Tissue Injury in the Dorsal Skinfold Chamber of the Mouse: A Skin Flap Model to Investigate Acute Persistent Ischemia. J. Vis. Exp. 2014, 93, e51900. [Google Scholar] [CrossRef]
- Harder, Y.; Amon, M.; Georgi, M.; Banic, A.; Erni, D.; Menger, M.D. Evolution of a “Falx Lunatica” in Demarcation of Critically Ischemic Myocutaneous Tissue. Am. J. Physiol. Circ. Physiol. 2005, 288, H1224–H1232. [Google Scholar] [CrossRef]
- Menger, M.D.; Lehr, H.-A. Scope and Perspectives of Intravital Microscopy—Bridge over from in Vitro to in Vivo. Immunol. Today 1993, 14, 519–522. [Google Scholar] [CrossRef]
- Il’Yasova, D.; Fontana, L.; Bhapkar, M.; Pieper, C.F.; Spasojevic, I.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kraus, W.E. The CALERIE Study Investigators Effects of 2 Years of Caloric Restriction on Oxidative Status Assessed by Urinary F2-Isoprostanes: The CALERIE 2 Randomized Clinical Trial. Aging Cell 2018, 17, e12719. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Tarabra, E.; Toledo, M.; Garcia-Macia, M.; Sahu, S.; Coletto, L.; Batista-Gonzalez, A.; Barzilai, N.; Pessin, J.E.; Schwartz, G.J.; et al. System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab. 2017, 26, 856–871.e5. [Google Scholar] [CrossRef]
- Li, J.; Bao, G.; Alyafeai, E.; Ding, J.; Li, S.; Sheng, S.; Shen, Z.; Jia, Z.; Lin, C.; Zhang, C.; et al. Betulinic Acid Enhances the Viability of Random-Pattern Skin Flaps by Activating Autophagy. Front. Pharmacol. 2019, 10, 1017. [Google Scholar] [CrossRef]
- Schneider, C.A.; Taegtmeyer, H. Fasting in Vivo Delays Myocardial Cell Damage after Brief Periods of Ischemia in the Isolated Working Rat Heart. Circ. Res. 1991, 68, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Bralet, A.-M.; Gueldry, S.; Bralet, J. Fasting Prior to Transient Cerebral Ischemia Reduces Delayed Neuronal Necrosis. Metab. Brain Dis. 1990, 5, 65–75. [Google Scholar] [CrossRef]
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-Dependent Differential Stress Resistance Protects Normal but Not Cancer Cells against High-Dose Chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220. [Google Scholar] [CrossRef] [PubMed]
- Soñanez-Organis, J.G.; Vázquez-Medina, J.P.; Crocker, D.E.; Ortiz, R.M. Prolonged Fasting Activates Hypoxia inducible Factors-1α, -2α and -3α in a Tissue-Specific Manner in Northern Elephant Seal Pups. Gene 2013, 526, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Kondo, M.; Shibata, R.; Miura, R.; Shimano, M.; Kondo, K.; Li, P.; Ohashi, T.; Kihara, S.; Maeda, N.; Walsh, K.; et al. Caloric Restriction Stimulates Revascularization in Response to Ischemia via Adiponectin-Mediated Activation of Endothelial Nitric-Oxide Synthase. Perspect. Surg. 2009, 284, 1718–1724. [Google Scholar] [CrossRef]
- Walsh, M.E.; Shi, Y.; Van Remmen, H. The Effects of Dietary Restriction on Oxidative Stress in Rodents. Free Radic. Biol. Med. 2013, 66, 88–99. [Google Scholar] [CrossRef]
- Carden, D.L.; Smith, J.K.; Korthuis, R.J. Neutrophil-Mediated Microvascular Dysfunction in Postischemic Canine Skeletal Muscle. Role of Granulocyte Adherence. Circ. Res. 1990, 66, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Puhr-Westerheide, D.; Schink, S.J.; Fabritius, M.; Mittmann, L.; Hessenauer, M.E.T.; Pircher, J.; Zuchtriegel, G.; Uhl, B.; Holzer, M.; Massberg, S.; et al. Neutrophils Promote Venular Thrombosis by Shaping the Rheological Environment for Platelet Aggregation. Sci. Rep. 2019, 9, 15932. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef]
- Nakai, M.; Ribeiro, R.V.; Stevens, B.R.; Gill, P.; Muralitharan, R.R.; Yiallourou, S.; Muir, J.; Carrington, M.; Head, G.A.; Kaye, D.M.; et al. Essential Hypertension Is Associated with Changes in Gut Microbial Metabolic Pathways: A Multisite Analysis of Ambulatory Blood Pressure. Hypertension 2021, 78, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.M.; Li, J.; Stevens, B.R.; Pepine, C.J.; Raizada, M.K. Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 2022, 130, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, S.; Jia, W. Calorie Restriction and Its Impact on Gut Microbial Composition and Global Metabolism. Front. Med. 2018, 12, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Ianiro, G.; Laterza, L.; Lopetuso, L.R.; Ponziani, F.R.; Gasbarrini, A.; Mele, M.C. Gut Microbiota during Dietary Restrictions: New Insights in Non-Communicable Diseases. Microorganisms 2020, 8, 1140. [Google Scholar] [CrossRef]
- Anderson, E.M.; Rozowsky, J.M.; Fazzone, B.J.; Schmidt, E.A.; Stevens, B.R.; O’malley, K.A.; Scali, S.T.; Berceli, S.A. Temporal Dynamics of the Intestinal Microbiome Following Short-Term Dietary Restriction. Nutrients 2022, 14, 2785. [Google Scholar] [CrossRef]
- Zhu, C.; Song, K.; Shen, Z.; Quan, Y.; Tan, B.; Luo, W.; Wu, S.; Tang, K.; Yang, Z.; Wang, X. Roseburia Intestinalis Inhibits Interleukin-17 Excretion and Promotes Regulatory T Cells Differentiation in Colitis. Mol. Med. Rep. 2018, 17, 7567–7574. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Zhang, X.; Ma, M.; Xie, Z.; Pan, Q.; Ma, Z.; Peppelenbosch, M.P. Remodeling of the Gut Microbiome during Ramadan-Associated Intermittent Fasting. Am. J. Clin. Nutr. 2021, 113, 1332–1342. [Google Scholar] [CrossRef]
- Kip, P.; Trocha, K.M.; Tao, M.; O’leary, J.J.; Ruske, J.; Giulietti, J.M.; Trevino-Villareal, J.H.; MacArthur, M.R.; Bolze, A.; Burak, M.F.; et al. Insights from a Short-Term Protein–Calorie Restriction Exploratory Trial in Elective Carotid Endarterectomy Patients. Vasc. Endovasc. Surg. 2019, 53, 470–476. [Google Scholar] [CrossRef]
- Kip, P.; Sluiter, T.J.; Moore, J.K.; Hart, A.; Ruske, J.; O’leary, J.; Jung, J.; Tao, M.; MacArthur, M.R.; Heindel, P.; et al. Short-Term Pre-Operative Protein Caloric Restriction in Elective Vascular Surgery Patients: A Randomized Clinical Trial. Nutrients 2021, 13, 4024. [Google Scholar] [CrossRef]
- Jongbloed, F.; De Bruin, R.W.F.; Klaassen, R.A.; Beekhof, P.; Van Steeg, H.; Dor, F.J.M.F.; Van der Harst, E.; Dollé, M.E.T.; Ijzermans, J.N.M. Short-Term Preoperative Calorie and Protein Restriction Is Feasible in Healthy Kidney Donors and Morbidly Obese Patients Scheduled for Surgery. Nutrients 2016, 8, 306. [Google Scholar] [CrossRef]
- Adawi, M.; Watad, A.; Brown, S.; Aazza, K.; Aazza, H.; Zouhir, M.; Sharif, K.; Ghanayem, K.; Farah, R.; Mahagna, H.; et al. Ramadan Fasting Exerts Immunomodulatory Effects: Insights from a Systematic Review. Front. Immunol. 2017, 8, 1144. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.A.-I.E.; Kacimi, S.; Al-Kurd, R.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent Fasting during Ramadan Attenuates Proinflammatory Cytokines and Immune Cells in Healthy Subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef] [PubMed]

| Volumetric Blood Flow [pL/s] | d1 | d3 | d5 | d7 | d10 | |
|---|---|---|---|---|---|---|
| Arterioles | ||||||
| prox. | control | 474 ± 67 | 699 ± 147 | 848 ± 143 | 974 ± 173 | 1363 ± 248 |
| CR | 791 ± 113 * | 931 ± 159 | 1089 ± 164 | 1626 ± 235 * | 1586 ± 211 | |
| med. | control | 375 ± 109 | 502 ± 115 | 556 ± 93 | 822 ± 193 | 1060 ± 211 |
| CR | 573 ± 109 | 813 ± 138 | 871 ± 139 | 1140 ± 174 | 1160 ± 195 | |
| dist. | control | 208 ± 42 | 414 ± 120 | 681 ± 135 | 480 ± 205 | 867 ± 446 |
| CR | 377 ± 66 | 524 ± 84 | 821 ± 167 | 899 ± 98 | 1125 ± 161 | |
| Capillaries | ||||||
| prox. | control | 1 ± 0 | 2 ± 0 | 3 ± 1 | 5 ± 1 | 7 ± 1 |
| CR | 2 ± 0 * | 3 ± 0 * | 5 ± 0 | 8 ± 1 * | 11 ± 1 | |
| med. | control | 2 ± 0 | 2 ± 0 | 3 ± 0 | 5 ± 1 | 6 ± 1 |
| CR | 2 ± 0 | 3 ± 0 * | 5 ± 0 * | 7 ± 1 | 9 ± 1 | |
| dist. | control | 1 ± 0 | 2 ± 0 | 3 ± 1 | 6 ± 1 | 6 ± 1 |
| CR | 2 ± 0 | 3 ± 0 | 4 ± 1 | 7 ± 1 | 10 ± 2 | |
| Venules | ||||||
| prox. | control | 595 ± 146 | 921 ± 188 | 1459 ± 272 | 2048 ± 438 | 2440 ± 416 |
| CR | 1538 ± 332 * | 1800 ± 374 | 2199 ± 456 | 3470 ± 801 | 3789 ± 926 | |
| med. | control | 607 ± 159 | 1208 ± 519 | 1453 ± 283 | 1934 ± 443 | 2469 ± 413 |
| CR | 1116 ± 277 | 1431 ± 326 | 1905 ± 337 | 2629 ± 754 | 3240 ± 796 | |
| dist. | control | 125 ± 47 | 589 ± 119 | 762 ± 328 | 1508 ± 479 | 1522 ± 93 |
| CR | 361 ± 86 | 703 ± 214 | 1272 ± 301 | 1505 ± 127 | 1903 ± 362 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinzierl, A.; Coerper, M.; Harder, Y.; Menger, M.D.; Laschke, M.W. Caloric Restriction: A Novel Conditioning Strategy to Improve the Survival of Ischemically Challenged Musculocutaneous Random Pattern Flaps. Nutrients 2023, 15, 4076. https://doi.org/10.3390/nu15184076
Weinzierl A, Coerper M, Harder Y, Menger MD, Laschke MW. Caloric Restriction: A Novel Conditioning Strategy to Improve the Survival of Ischemically Challenged Musculocutaneous Random Pattern Flaps. Nutrients. 2023; 15(18):4076. https://doi.org/10.3390/nu15184076
Chicago/Turabian StyleWeinzierl, Andrea, Maximilian Coerper, Yves Harder, Michael D. Menger, and Matthias W. Laschke. 2023. "Caloric Restriction: A Novel Conditioning Strategy to Improve the Survival of Ischemically Challenged Musculocutaneous Random Pattern Flaps" Nutrients 15, no. 18: 4076. https://doi.org/10.3390/nu15184076





