Ameliorating Effects of Bifidobacterium longum subsp. infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder
Highlights
- The positive anti-obesity potential of FB3-14 is shown to improve dyslipidemia, liver function impairment, low-grade systemic inflammation, and lipid metabolism.
- FB3-14 significantly increases the abundance of Akkermansia, Lachnospiraceae_NK4A136_group and Bifidobacterim, which might enhance the secretion of PYY and GLP-1 by up-regulating butyric acid level mediating GPR41.
- Our study provides the evidence for the novel strain Bifidobacterium longum subsp. infantis FB3-14 to inhibit HFD-induced overweight and relieve gut microbiome dysregulation.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Strain
2.2. Animal Experiments
2.3. Serum Biochemical Analysis
2.4. Histological Examination
2.5. Quantification of Gene Expression
2.6. Short-Chain Fatty Acid (SCFAs) Determination
2.7. Gut Microbiota Profiling
2.8. Statistical Analysis
3. Results
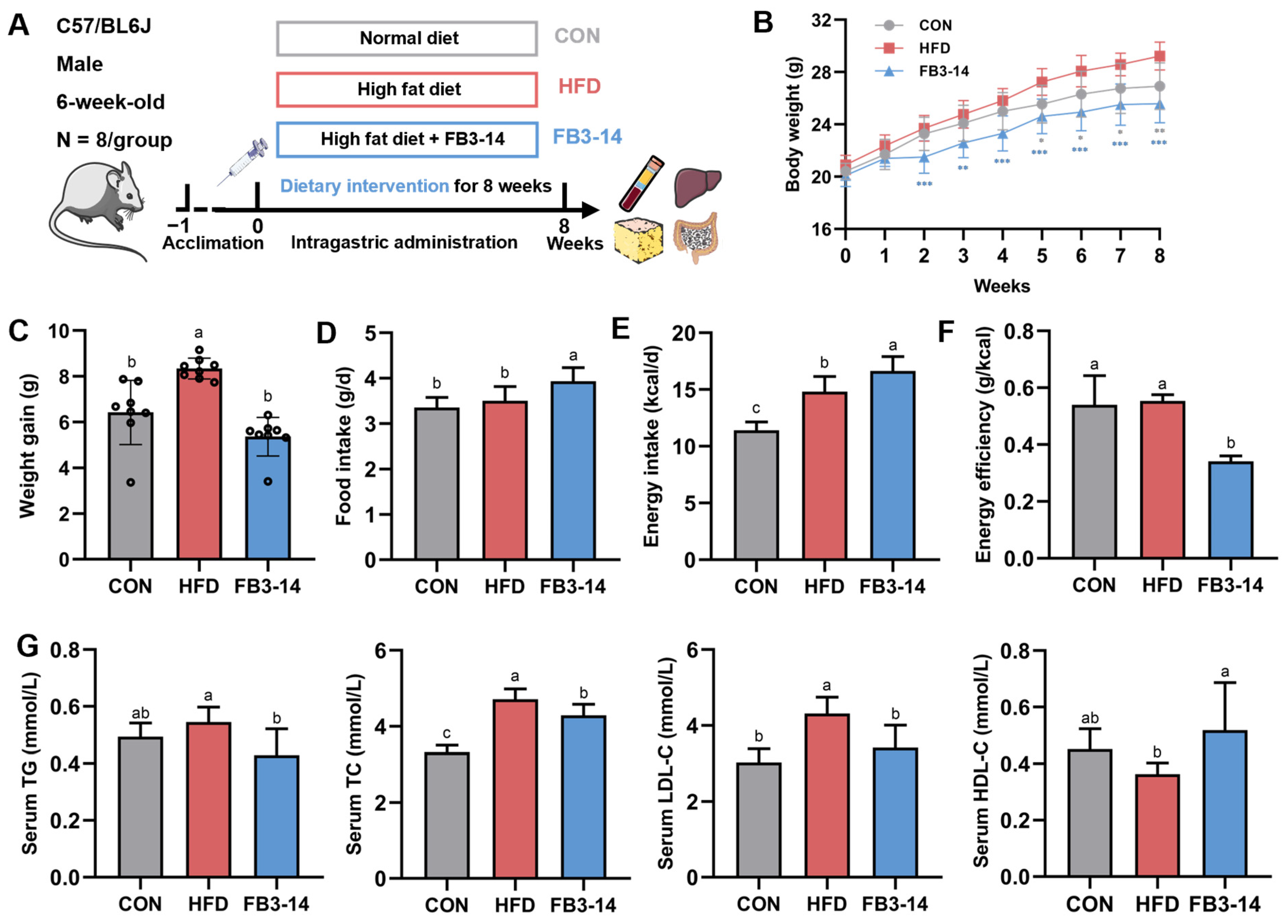
3.1. FB3-14 Inhibited Overweight and Dyslipidemia
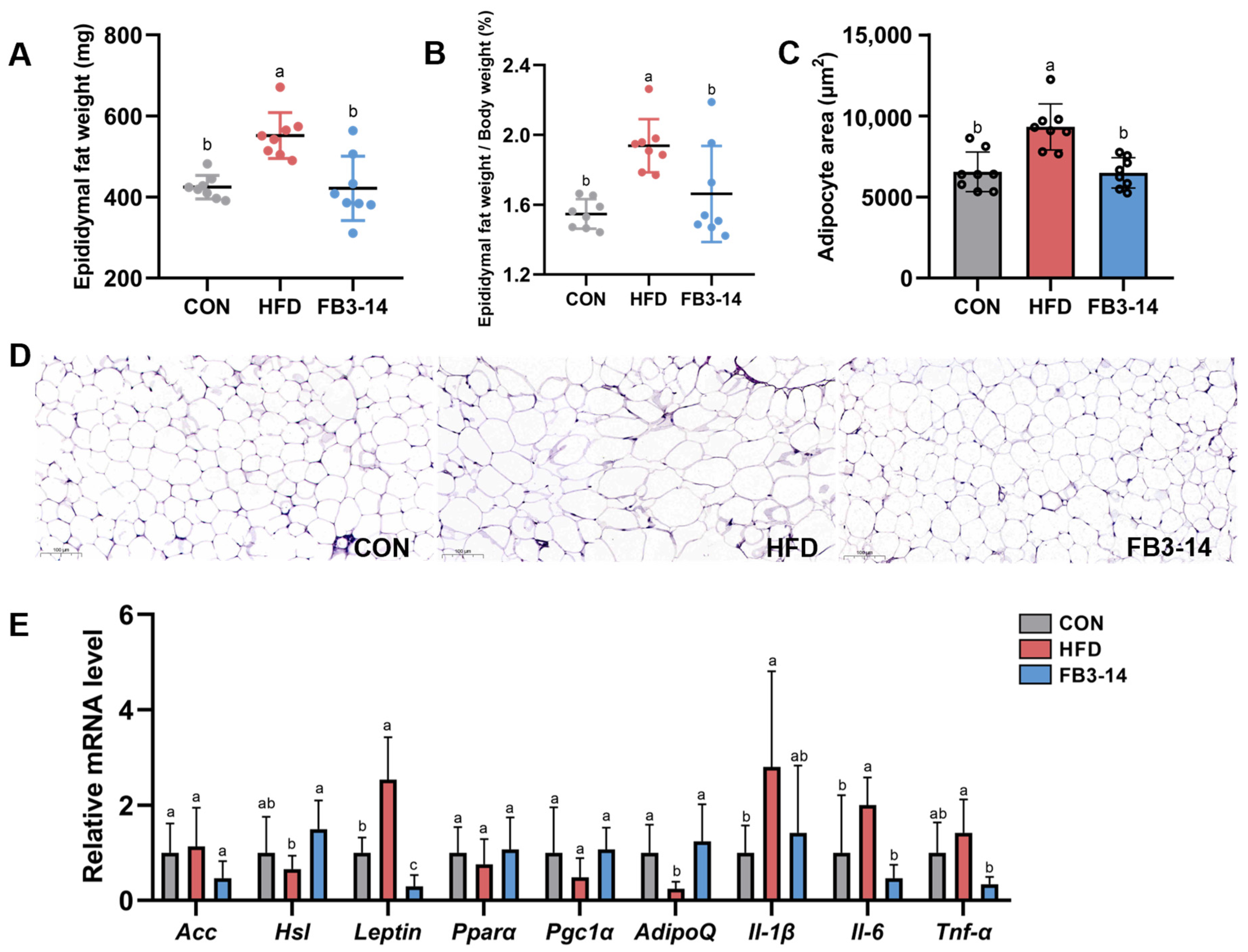
3.2. FB3-14 Alleviated Lipid Accumulation and Lipid Metabolism Disorders
3.3. FB3-14 Ameliorated Impaired Liver Function and Systemic Low-Grade Inflammation
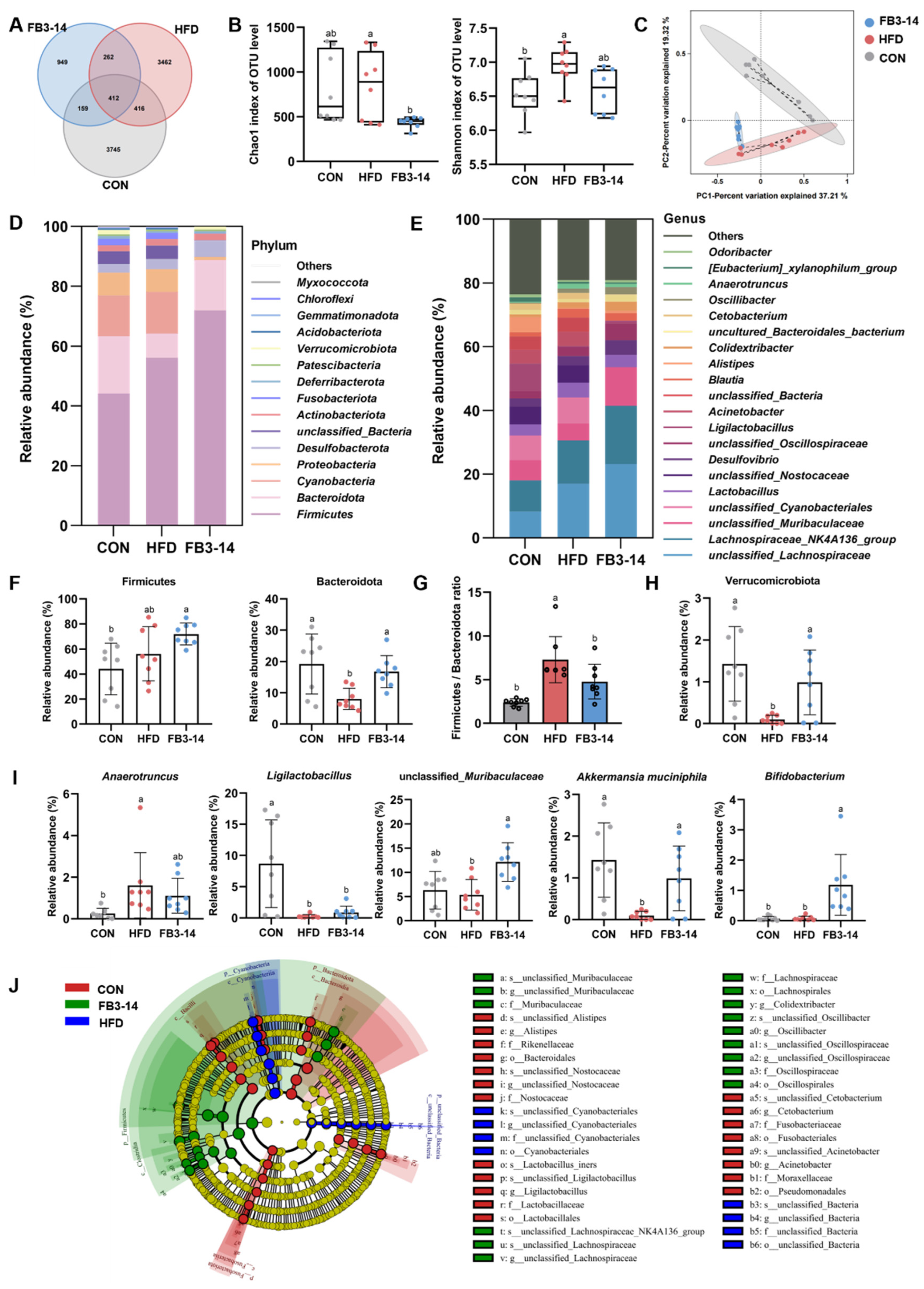
3.4. Effects of FB3-14 on Intestinal Flora Structure Disturbance
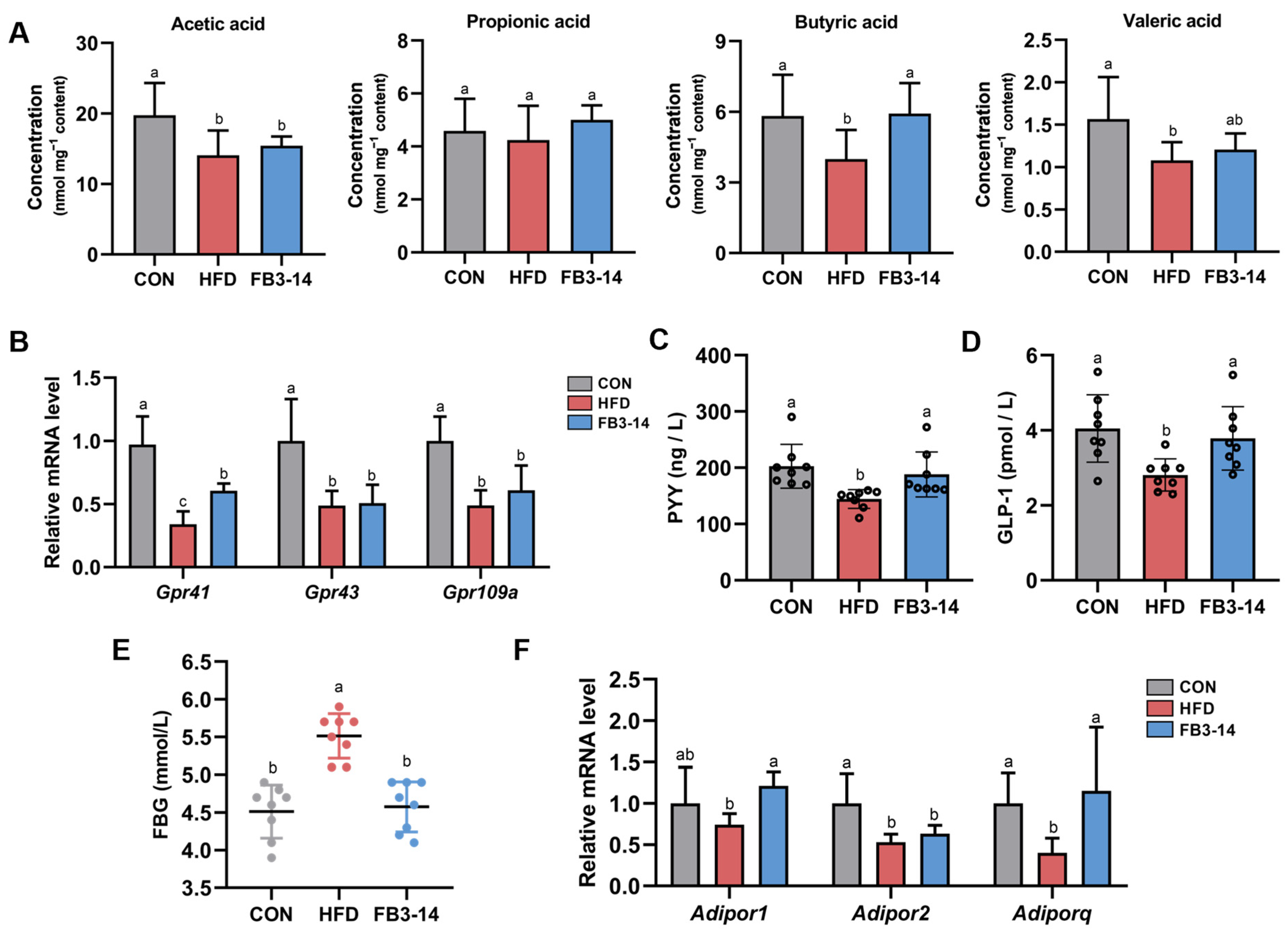
3.5. FB3-14 Restored Lipid Metabolism by Upregulating Butyric Aid Content
3.6. Correlation Analysis between Intestinal Microbiota and Metabolic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.-L.; Zhang, X.; Wang, K.-B.; Huang, J.-A.; Liu, Z.-H.; Zhu, M.-Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Young, G.; Koh, A.N.; Clay, F.; Semenkovich, J.I.G. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.-R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Longo, D.L.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Greiner, T.; Bäckhed, F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011, 22, 117–123. [Google Scholar] [CrossRef]
- Berger, K.; Burleigh, S.; Lindahl, M.; Bhattacharya, A.; Patil, P.; Stålbrand, H.; Nordberg Karlsson, E.; Hållenius, F.; Nyman, M.; Adlercreutz, P. Xylooligosaccharides Increase Bifidobacteria and Lachnospiraceae in Mice on a High-Fat Diet, with a Concomitant Increase in Short-Chain Fatty Acids, Especially Butyric Acid. J. Agric. Food Chem. 2021, 69, 3617–3625. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Arboleya, S.; Ruas-Madiedo, P.; Margolles, A.; Solís, G.; Salminen, S.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Characterization and in vitro properties of potentially probiotic Bifidobacterium strains isolated from breast-milk. Int. J. Food Microbiol. 2011, 149, 28–36. [Google Scholar] [CrossRef]
- Zhu, G.; Ma, F.; Wang, G.; Wang, Y.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria attenuate the development of metabolic disorders, with inter- and intra-species differences. Food Funct. 2018, 9, 3509–3522. [Google Scholar] [CrossRef]
- Yin, Y.-N. Effects of fourBifidobacteriaon obesity in high-fat diet induced rats. World J. Gastroenterol. 2010, 16, 3394. [Google Scholar] [CrossRef]
- Kim, G.; Yoon, Y.; Park, J.H.; Park, J.W.; Noh, M.-G.; Kim, H.; Park, C.; Kwon, H.; Park, J.-H.; Kim, Y.; et al. Bifidobacterial carbohydrate/nucleoside metabolism enhances oxidative phosphorylation in white adipose tissue to protect against diet-induced obesity. Microbiome 2022, 10, 188. [Google Scholar] [CrossRef]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Zhang, X.; Kou, R.; Chen, M.; Zhang, B.; Liu, J.; Peng, B.; Zhang, Y.; Wang, S. Cold-Brewed Jasmine Tea Attenuates High-Fat Diet-Induced Obesity and Gut Microbial Dysbiosis. Nutrients 2022, 14, 5359. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Wang, J.; Ma, H.; Zhang, B.; Wang, S. The Protective Effects of 2′-Fucosyllactose against E. coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion. Nutrients 2020, 12, 1284. [Google Scholar] [CrossRef]
- He, M.M.; Fang, Z.; Hang, D.; Wang, F.; Polychronidis, G.; Wang, L.; Lo, C.H.; Wang, K.; Zhong, R.; Knudsen, M.D.; et al. Circulating liver function markers and colorectal cancer risk: A prospective cohort study in the UK Biobank. Int. J. Cancer 2020, 148, 1867–1878. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, W.; Li, J.; Du, L.; Lv, O.; Zhao, S.; Li, J. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol. Nutr. Food Res. 2019, 63, 1800773. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H.; Torres-Fuentes, C.; van de Wouw, M.; Long-Smith, C.M.; Mitchell, A.; Strain, C.; Berding, K.; Bastiaanssen, T.F.S.; Rea, K.; Golubeva, A.V.; et al. Bifidobacterium longum counters the effects of obesity: Partial successful translation from rodent to human. EBioMedicine 2021, 63, 103176. [Google Scholar] [CrossRef]
- Sohn, M.; Jung, H.; Lee, W.S.; Kim, T.H.; Lim, S. Effect of Lactobacillus plantarum LMT1-48 on Body Fat in Overweight Subjects A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. J. 2021, 47, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jeong, D.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Seo, K.-H. Dual function ofLactobacillus kefiriDH5 in preventing high-fat-diet-induced obesity: Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Zeng, Z.; Qin, Y.; Shen, Q.; Li, P. Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J. Funct. Foods 2020, 67, 103843. [Google Scholar] [CrossRef]
- Ray, M.; Hor, P.K.; Ojha, D.; Soren, J.P.; Singh, S.N.; Mondal, K.C. Bifidobacteria and its rice fermented products on diet induced obese mice: Analysis of physical status, serum profile and gene expressions. Benef. Microbes 2018, 9, 441–452. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef] [PubMed]
- Falcão-Pires, I.; Castro-Chaves, P.; Miranda-Silva, D.; Lourenço, A.P.; Leite-Moreira, A.F. Physiological, pathological and potential therapeutic roles of adipokines. Drug Discov. Today 2012, 17, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ji, Y.; Jung, H.-Y.; Park, H.; Kang, J.; Choi, S.-H.; Shin, H.; Hyun, C.-K.; Kim, K.-T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2016, 101, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Han, S.K.; Kim, J.K.; Oh, S.J.; Jang, H.B.; Kim, D.H. Lactobacillus sakei Alleviates High-Fat-Diet-Induced Obesity and Anxiety in Mice by Inducing AMPK Activation and SIRT1 Expression and Inhibiting Gut Microbiota-Mediated NF-κB Activation. Mol. Nutr. Food Res. 2019, 63, 1800978. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef]
- Langin, D.; Arner, P. Importance of TNFα and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol. Metab. 2006, 17, 314–320. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, M.; Zhou, M.; He, Y.; Li, Y.; Lang, H.; Wei, X.; Yan, L.; Xu, H. Hibiscus manihot L improves obesity in mice induced by a high-fat diet. J. Funct. Foods 2022, 89, 104953. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.-Y. Beneficial Effects of Dietary Polyphenols on High-Fat Diet-Induced Obesity Linking with Modulation of Gut Microbiota. J. Agric. Food Chem. 2019, 68, 33–47. [Google Scholar] [CrossRef]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted Distribution of the Butyrate Kinase Pathway among Butyrate-Producing Bacteria from the Human Colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, R.; Wang, J.; Li, A.; Wang, Y.; Zhang, B.; Liu, J.; Sun, Y.; Wang, S. Ameliorating Effects of Bifidobacterium longum subsp. infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder. Nutrients 2023, 15, 4104. https://doi.org/10.3390/nu15194104
Kou R, Wang J, Li A, Wang Y, Zhang B, Liu J, Sun Y, Wang S. Ameliorating Effects of Bifidobacterium longum subsp. infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder. Nutrients. 2023; 15(19):4104. https://doi.org/10.3390/nu15194104
Chicago/Turabian StyleKou, Ruixin, Jin Wang, Ang Li, Yuanyifei Wang, Bowei Zhang, Jingmin Liu, Yi Sun, and Shuo Wang. 2023. "Ameliorating Effects of Bifidobacterium longum subsp. infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder" Nutrients 15, no. 19: 4104. https://doi.org/10.3390/nu15194104
APA StyleKou, R., Wang, J., Li, A., Wang, Y., Zhang, B., Liu, J., Sun, Y., & Wang, S. (2023). Ameliorating Effects of Bifidobacterium longum subsp. infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder. Nutrients, 15(19), 4104. https://doi.org/10.3390/nu15194104





