Probiotics and the Potential of Genetic Modification as a Possible Treatment for Food Allergy
Abstract
:1. Introduction
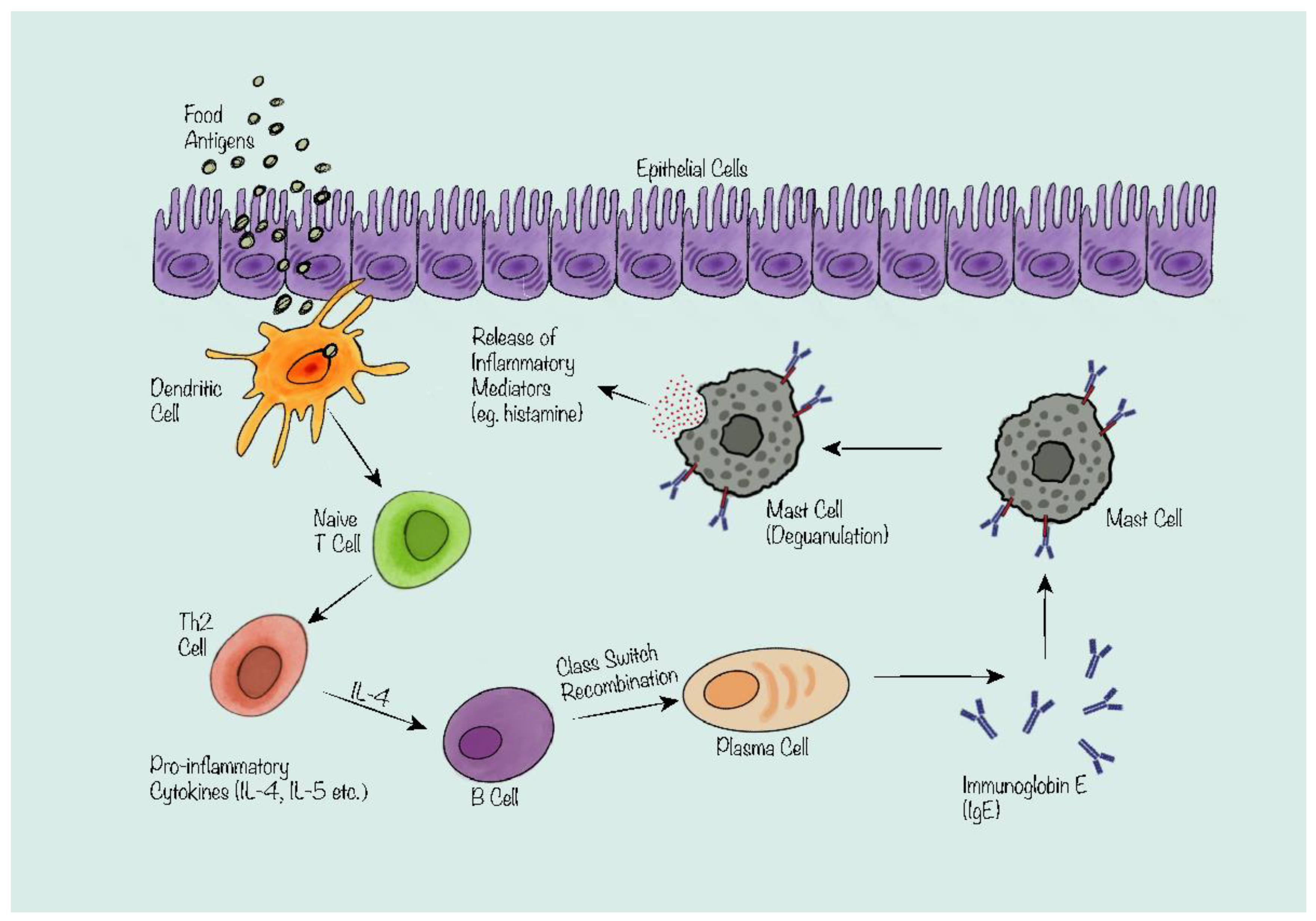
2. The Mechanism of IgE-Mediated Food Allergy
3. The Relationship of the Gut Microbiota and Food Allergy
4. Current Knowledge of Probiotics Treatment of Food Allergy
4.1. Animal Trials
| Author | Year of Publication | Title | Probiotics Strain | Journal |
|---|---|---|---|---|
| J. Yang et al. [27] | 2015 | Induction of Regulatory Dendritic Cells by Lactobacillus paracasei L9 Prevents Allergic Sensitization to Bovine β-Lactoglobulin in Mice | Lactobacillus paracasei L9 | J Microbiol Biotechnol |
| B. Yang et al. [34] | 2018 | Probiotics SOD inhibited food allergy via downregulation of STAT6-TIM4 signaling on DCs | Bifidobacterium infantis | Mol Immunol |
| L. Fu et al. [28] | 2020 | Lactobacillus casei Zhang Alleviates Shrimp Tropomyosin-Induced Food Allergy by Switching Antibody Isotypes through the NF-κB-Dependent Immune Tolerance | Lactobacillus casei Zhang | Mol Nutr Food Res |
| B. Y. Jin et al. [18] | 2021 | Probiotic Interventions Alleviate Food Allergy Symptoms Correlated With Cesarean Section: A Murine Model | Lactobacillus and Bifidobacterium | Front Immunol |
| C. Duan et al. [29] | 2023 | Oral administration of Lactobacillus plantarum JC7 alleviates OVA-induced murine food allergy through immunoregulation and restoring disordered intestinal microbiota | Lactobacillus plantarum JC7 | Eur J Nutr |
| X. Tian et al. [33] | 2023 | Probiotics Alleviate Food Protein Allergy in Mice by Activating TLR4 Signaling Pathway | Bifidobacterium animalis KV9, Lactobacillus vaginalis FN3 | Mol Nutr Food Res |
4.2. Clinical Trials
| Author | Year of Publication | Title | Probiotics Strain | Journal |
|---|---|---|---|---|
| M. Kalliomäki et al. [30] | 2001 | Probiotics in primary prevention of atopic disease: a randomized placebo-controlled trial | Lactobacillus GG | Lancet |
| A. Forsberg et al. [32] | 2013 | Pre- and post-natal Lactobacillus reuteri supplementation decreases allergen responsiveness in infancy | Lactobacillus reuteri | Clin Exp Allergy |
| D. J. Costa Et al. [40] | 2014 | Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN Study) | Lactobacillus paracasei LP-33 | Eur J Clin Nutr |
| R. Berni Canani et al. [41] | 2016 | Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants | Lactobacillus rhamnosus GG | Isme J |
| R. Berni Canani et al. [37] | 2017 | Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial | Lactobacillus rhamnosus GG | J Allergy Clin Immunol |
| B. Cukrowska et al. [35] | 2021 | The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study | Lactobacillus rhamnosus, Lactobacillus casei | Nutrients |
| P. Loke et al. [42] | 2022 | Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): a multicentre, randomised, phase 2b trial | Lactobacillus rhamnosus ATCC 53103 | Lancet Child Adolesc Health |
5. Future of Probiotics as a Treatment for Food Allergy
5.1. Multi-Strain and Synbiotic Formulations for Food Allergy
5.2. Precision Therapy with Synthetic Biology for Food Allergy
6. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Luo, J.; Zhang, Q.; Gu, Y.; Wang, J.; Liu, G.; He, T.; Che, H. Meta-Analysis: Prevalence of Food Allergy and Food Allergens-China, 2000–2021. China CDC Wkly 2022, 4, 766–770. [Google Scholar] [PubMed]
- Feng, H.; Xiong, X.; Chen, Z.; Xu, Q.; Zhang, Z.; Luo, N.; Wu, Y. Prevalence and Influencing Factors of Food Allergy in Global Context: A Meta-Analysis. Int. Arch. Allergy Immunol. 2023, 184, 320–352. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Mennini, M.; Arasi, S.; Artesani, M.C.; Fiocchi, A.G. Probiotics in food allergy. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, A.M.; Valsecchi, C.; Caimmi, S.; Licari, A.; Marseglia, A.; Leoni, M.C.; Caimmi, D.; del Giudice, M.M.; Leonardi, S.; La Rosa, M.; et al. Probiotics and food allergy. Ital. J. Pediatr. 2013, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, A.; Guo, T.; Sokolovska, A.; Miller, P.F.; Collins, J.J.; Lu, T.K.; Lora, J.M. Engineering living therapeutics with synthetic biology. Nat. Rev. Drug Discov. 2021, 20, 941–960. [Google Scholar] [CrossRef] [PubMed]
- König, I.R.; Fuchs, O.; Hansen, G.; von Mutius, E.; Kopp, M.V. What is precision medicine? Eur. Respir. J. 2017, 50, 1700391. [Google Scholar] [CrossRef]
- Vitte, J.; Vibhushan, S.; Bratti, M.; Montero-Hernandez, J.E.; Blank, U. Allergy, Anaphylaxis, and Nonallergic Hypersensitivity: IgE, Mast Cells, and Beyond. Med. Princ. Pr. 2022, 31, 501–515. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; Daanje, M.; Akdis, M.; Boyd, S.D.; van de Veen, W. Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy 2021, 76, 1707–1717. [Google Scholar] [CrossRef]
- Zhao, W.; Ho, H.E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Brody, H. The gut microbiome. Nature 2020, 577, S5. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Maddalena, Y.; Buono, A.; Bruno, C.; Voto, L.; Ercolini, D. Gut Microbiome as Target for Innovative Strategies Against Food Allergy. Front. Immunol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Wang, X.; Jin, Z.; Zhang, P.; Su, H.; Sun, X. Effect of Probiotics on Respiratory Tract Allergic Disease and Gut Microbiota. Front. Nutr. 2022, 9, 821900. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, Y.; Liu, L.; Li, Z. Association Between Breastmilk Microbiota and Food Allergy in Infants. Front. Cell Infect. Microbiol. 2021, 11, 770913. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.E.; Lange, N.E.; Zhou, Y.; O’Connor, G.T.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. Diet during Pregnancy and Infancy and the Infant Intestinal Microbiome. J. Pediatr. 2018, 203, 47–54.e4. [Google Scholar] [CrossRef]
- Jin, B.Y.; Li, Z.; Xia, Y.N.; Li, L.X.; Zhao, Z.X.; Li, X.Y.; Li, Y.; Li, B.; Zhou, R.C.; Fu, S.C.; et al. Probiotic Interventions Alleviate Food Allergy Symptoms Correlated With Cesarean Section: A Murine Model. Front. Immunol. 2021, 12, 741371. [Google Scholar] [CrossRef]
- Wu, B.B.; Yang, Y.; Xu, X.; Wang, W.P. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J. Pediatr. 2016, 12, 177–182. [Google Scholar] [CrossRef]
- Campbell, E.; Hesser, L.A.; Nagler, C.R. B cells and the microbiota: A missing connection in food allergy. Mucosal Immunol. 2021, 14, 4–13. [Google Scholar] [CrossRef]
- Maynard, C.L.; Craig, L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nanan, R.; Macia, L.; Tan, J.; Sominsky, L.; Quinn, T.P.; O’Hely, M.; Ponsonby, A.L.; Tang, M.L.K.; Collier, F.; et al. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J. Allergy Clin. Immunol. 2021, 148, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Regulation of the T cell response. Clin. Exp. Allergy 2006, 36, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; de Foy, J.M.P.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, F.; Zhang, H.; Jiang, L.; Hao, Y.; Luo, X. Induction of Regulatory Dendritic Cells by Lactobacillus paracasei L9 Prevents Allergic Sensitization to Bovine β-Lactoglobulin in Mice. J. Microbiol. Biotechnol. 2015, 25, 1687–1696. [Google Scholar] [CrossRef]
- Fu, L.; Xie, M.; Wang, C.; Qian, Y.; Huang, J.; Sun, Z.; Zhang, H.; Wang, Y. Lactobacillus Casei Zhang Alleviates Shrimp Tropomyosin-Induced Food Allergy by Switching Antibody Isotypes through the NF-κB-Dependent Immune Tolerance. Mol. Nutr. Food Res. 2020, 64, e1900496. [Google Scholar] [CrossRef]
- Duan, C.; Ma, L.; Yu, J.; Sun, Y.; Liu, L.; Ma, F.; Li, X.; Li, D. Oral administration of Lactobacillus plantarum JC7 alleviates OVA-induced murine food allergy through immunoregulation and restoring disordered intestinal microbiota. Eur. J. Nutr. 2023, 62, 685–698. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Wang, D.; Ma, W.; She, R.; Sun, Q.; Liu, Y.; Hu, Y.; Liu, L.; Yang, Y.; Peng, K. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult. Sci. 2009, 88, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, A.; Abrahamsson, T.R.; Björkstén, B.; Jenmalm, M.C. Pre- and post-natal Lactobacillus reuteri supplementation decreases allergen responsiveness in infancy. Clin. Exp. Allergy 2013, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liang, X.; He, H.; Cui, Q.; Liu, Q.; Fan, R.; Liu, T.; Yi, H.; Gong, P.; Wang, Q.; et al. Probiotics Alleviate Food Protein Allergy in Mice by Activating TLR4 Signaling Pathway. Mol. Nutr. Food Res. 2023, e2200579. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Luo, Y.; Liu, Z.; Yang, P.; Gui, Y. Probiotics SOD inhibited food allergy via downregulation of STAT6-TIM4 signaling on DCs. Mol. Immunol. 2018, 103, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Cheng, L.; Wang, J.; Raghavan, V. Gut microbiome modulation by probiotics, prebiotics, synbiotics and postbiotics: A novel strategy in food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef]
- Bird, J.A.; Spergel, J.M.; Jones, S.M.; Rachid, R.; Assa’ad, A.H.; Wang, J.; Leonard, S.A.; Laubach, S.S.; Kim, E.H.; Vickery, B.P.; et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J. Allergy Clin. Immunol. Pr. 2018, 6, 476–485.e3. [Google Scholar] [CrossRef]
- Costa, D.J.; Marteau, P.; Amouyal, M.; Poulsen, L.K.; Hamelmann, E.; Cazaubiel, M.; Housez, B.; Leuillet, S.; Stavnsbjerg, M.; Molimard, P.; et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: A double-blind, randomized, placebo-controlled trial (GA2LEN Study). Eur. J. Clin. Nutr. 2014, 68, 602–607. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Loke, P.; Orsini, F.; Lozinsky, A.C.; Gold, M.; O’Sullivan, M.D.; Quinn, P.; Lloyd, M.; Ashley, S.E.; Pitkin, S.; Axelrad, C.; et al. Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): A multicentre, randomised, phase 2b trial. Lancet Child Adolesc. Health 2022, 6, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, A.; West, C.E.; Prescott, S.L.; Jenmalm, M.C. Pre- and probiotics for allergy prevention: Time to revisit recommendations? Clin. Exp. Allergy 2016, 46, 1506–1521. [Google Scholar] [CrossRef] [PubMed]
- Vadala, B.S.; Kumar, P.; Dwivedi, M.K. Chapter 14-Probiotics in mitigation of food allergies and lactose intolerance. In Probiotics in the Prevention and Management of Human Diseases; Kumar Dwivedi, M., Amaresan, N., Sankaranarayanan, A., Helen Kemp, E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 213–221. [Google Scholar]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Rout, A.; Jalan, K.; Ghosh, N.; Das, A.; Bagchi, D. Chapter 25-Prebiotics and probiotics in prevention of food allergy. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Bagchi, D., Ohia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 419–444. [Google Scholar]
- Ramaswami, R.; Bayer, R.; Galea, S. Precision Medicine from a Public Health Perspective. Annu. Rev. Public. Health 2018, 39, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, A.; van der Windt, D.; Riley, R.; Abrams, D.; Moons, K.; Steyerberg, E.; Schroter, S.; Sauerbrei, W.; Altman, D.; Hemingway, H.; et al. Prognosis research strategy (PROGRESS) 4: Stratified medicine research. BMJ 2013, 346, e5793. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.; Collins, J.; Romesberg, F. The Future Is Synthetic Biology. Cell 2018, 175, 895–897. [Google Scholar]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8(+) T cell immunity. Cell Metab. 2023, 35, 943–960.e9. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Li, B.; Wang, Y.; Xie, Z.; Liu, D.; Yuan, Y. Significant research progress in synthetic biology. Synth. Biol. J. 2020, 1, 7–28. [Google Scholar]
- Fredens, J.; Wang, K.; de la Torre, D.; Funke, L.F.H.; Robertson, W.E.; Christova, Y.; Chia, T.; Schmied, W.H.; Dunkelmann, D.L.; Beránek, V.; et al. Total synthesis of Escherichia coli with a recoded genome. Nature 2019, 569, 514–518. [Google Scholar] [CrossRef]
- Sanmarco, L.M.; Rone, J.M.; Polonio, C.M.; Fernandez Lahore, G.; Giovannoni, F.; Ferrara, K.; Gutierrez-Vazquez, C.; Li, N.; Sokolovska, A.; Plasencia, A.; et al. Lactate limits CNS autoimmunity by stabilizing HIF-1α in dendritic cells. Nature 2023, 620, 881–889. [Google Scholar] [CrossRef]
- Benhamou, A.H.; Koehli, A.; Rochat, I.; Inci, D.; Moeller, A.; Taramarcaz, P.; Lauener, R.P.; Eigenmann, P.A. Exhaled nitric oxide decreases after positive food-allergen challenge. Clin. Transl. Allergy 2011, 1, 14. [Google Scholar] [CrossRef]
- Ogulur, I.; Pat, Y.; Ardicli, O.; Barletta, E.; Cevhertas, L.; Fernandez-Santamaria, R.; Huang, M.; Bel Imam, M.; Koch, J.; Ma, S.; et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021, 76, 3659–3686. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Peng, J.; Wang, S.; Ding, Z.; Chen, G.; Sun, J. Probiotics and the Potential of Genetic Modification as a Possible Treatment for Food Allergy. Nutrients 2023, 15, 4159. https://doi.org/10.3390/nu15194159
Wei Y, Peng J, Wang S, Ding Z, Chen G, Sun J. Probiotics and the Potential of Genetic Modification as a Possible Treatment for Food Allergy. Nutrients. 2023; 15(19):4159. https://doi.org/10.3390/nu15194159
Chicago/Turabian StyleWei, Yuqiu, Jing Peng, Siyu Wang, Zheng Ding, Guixi Chen, and Jiazeng Sun. 2023. "Probiotics and the Potential of Genetic Modification as a Possible Treatment for Food Allergy" Nutrients 15, no. 19: 4159. https://doi.org/10.3390/nu15194159
APA StyleWei, Y., Peng, J., Wang, S., Ding, Z., Chen, G., & Sun, J. (2023). Probiotics and the Potential of Genetic Modification as a Possible Treatment for Food Allergy. Nutrients, 15(19), 4159. https://doi.org/10.3390/nu15194159





