Efficacy and Safety of Intravenous Ferric Carboxymaltose Treatment of Iron Deficiency Anaemia in Patients with Corpus Atrophic Gastritis: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Intravenous FCM Administration and Biochemical and Clinical Follow-Up
2.3. Definitions
2.4. Statistical Analysis
3. Results
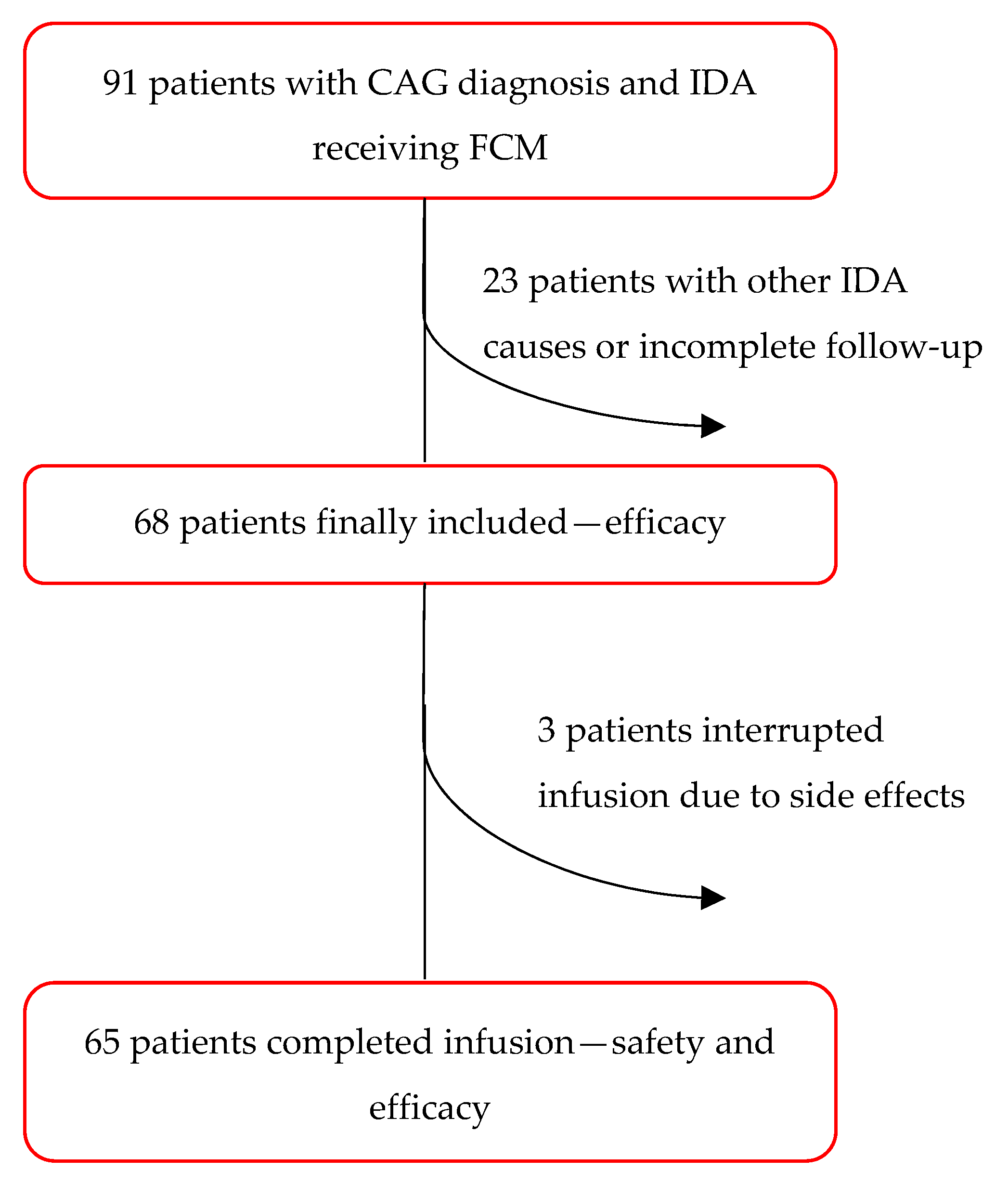
3.1. Study Population
3.2. Timing and Dosage of Intravenous FCM Treatment
3.3. Safety
3.4. Efficacy
3.4.1. Anaemia and Iron Storage Recovery
3.4.2. Relapse and Long-Term Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lahner, E.; Zagari, R.M.; Zullo, A.; Di Sabatino, A.; Meggio, A.; Cesaro, P.; Lenti, M.V.; Annibale, B.; Corazza, G.R. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig. Liver Dis. 2019, 51, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Ronson, A.; Souroujon, M.; Maschler, I.; Heyd, J.; Patz, J. Variable hematologic presentation of autoimmune gastritis: Age-related progression from iron deficiency to cobalamin depletion. Blood 2006, 107, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Dottori, L.; Pivetta, G.; Ligato, I.; Dilaghi, E.; Lahner, E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients 2022, 14, 1672. [Google Scholar] [CrossRef]
- Lenti, M.V.; Lahner, E.; Bergamaschi, G.; Miceli, E.; Conti, L.; Massironi, S.; Cococcia, S.; Zilli, A.; Caprioli, F.; Vecchi, M.; et al. Cell Blood Count Alterations and Patterns of Anaemia in Autoimmune Atrophic Gastritis at Diagnosis: A Multicentre Study. J. Clin. Med. 2019, 8, 1992. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- DeLoughery, T.G. Safety of Oral and Intravenous Iron. Acta Haematol. 2019, 142, 8–12. [Google Scholar] [CrossRef]
- Annibale, B.; Capurso, G.; Chistolini, A.; D’ambra, G.; DiGiulio, E.; Monarca, B.; DelleFave, G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am. J. Med. 2001, 111, 439–445. [Google Scholar] [CrossRef]
- Hershko, C.; Hoffbrand, A.V.; Keret, D.; Souroujon, M.; Maschler, I.; Monselise, Y.; Lahad, A. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica 2005, 90, 585–595. [Google Scholar] [CrossRef]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef] [PubMed]
- Geisser, P.; Burckhardt, S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011, 3, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Geisser, P.; Rumyantsev, V. The pharmacology and safety profile of ferric carboxymaltose (Ferinject®): Structure/reactivity relationships of iron preparations. Port. J. Nephrol. Hypert. 2009, 23, 11–16. [Google Scholar]
- Funk, F.; Ryle, P.; Canclini, C.; Neiser, S.; Geisser, P. The new generation of intravenous iron: Chemistry, pharmacology, and toxicology of ferric carboxymaltose. Arzneimittelforschung 2010, 60, 345–353. [Google Scholar] [CrossRef]
- Vifor Pharma UK Limited. Ferinject (Ferric Carboxymaltose): UK Summary of Product Characteristics. 2017. Available online: http://www.medicines.org.uk (accessed on 9 February 2018).
- Lyseng-Williamson, K.A.; Keating, G.M. Ferric carboxymaltose: A review of its use in iron-deficiency anaemia. Drugs 2009, 69, 739–756. [Google Scholar] [CrossRef]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853.e2. [Google Scholar] [CrossRef]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT_) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef]
- Evstatiev, R.; Alexeeva, O.; Bokemeyer, B.; Chopey, I.; Felder, M.; Gudehus, M.; Iqbal, T.; Khalif, I.; Marteau, P.; Stein, J.; et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 269–277. [Google Scholar] [CrossRef]
- Befrits, R.; Wikman, O.; Blomquist, L.; Hjortswang, H.; Hammarlund, P.; Bajor, A.; Klintman, D.; Blom, H. Anemia and iron deficiency in inflammatory bowel disease: An open, prospective, observational study on diagnosis, treatment with ferric carboxymaltose and quality of life. Scand. J. Gastroenterol. 2013, 48, 1027–1032. [Google Scholar] [CrossRef]
- Stein, J.; Dignass, A.; Weber-Mangal, S.; Hartmann, F. Improvement in hematological status and symptoms in IBD patients using ferric carboxymaltose (Ferinject_): A German multicenter non-interventional study (abstract no. P157). J. Crohns Colitis 2011, 5, S77–S78. [Google Scholar]
- Stein, J.; Aksan, A.; Klemm, W.; Nip, K.; Weber-Mangal, S.; Dignass, A. Safety and Efficacy of Ferric Carboxymaltose in the Treatment of Iron Deficiency Anaemia in Patients with Inflammatory Bowel Disease, in Routine Daily Practice. J. Crohn’s Colitis 2018, 12, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: Expert review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef]
- Esposito, G.; Dilaghi, E.; Cazzato, M.; Pilozzi, E.; Conti, L.; Carabotti, M.; Di Giulio, E.; Annibale, B.; Lahner, E. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig. Liver Dis. 2021, 53, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. The updated Sydney System. In-ternational Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 27 July 2020).
- Ko, C.W.; Siddique, S.M.; Patel, A.; Harris, A.; Sultan, S.; Altayar, O.; Falck-Ytter, Y. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1085–1094. [Google Scholar] [CrossRef]
- Bermejo, F.; García-López, S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J. Gastroenterol. 2009, 15, 4638. [Google Scholar] [CrossRef]
- Bregman, D.B.; Goodnough, L.T. Experience with intravenous ferric carboxymaltose in patients with iron deficiency anemia. Ther. Adv. Hematol. 2014, 5, 48–60. [Google Scholar] [CrossRef]
- Keating, G.M. Ferric carboxymaltose: A review of its use in iron deficiency. Drugs 2015, 75, 101–127. [Google Scholar] [CrossRef]
- Scott, L.J. Ferric carboxymaltose: A review in iron deficiency. Drugs 2018, 78, 479–493. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, X.; Li, X.; Zhang, H.; Wu, M.; Liu, J.; Palmen, M.; Roubert, B.; Li, C. Pharmacokinetic, Pharmacodynamic, and Safety Profiles of Ferric Carboxymaltose in Chinese Patients with Iron-deficiency Anemia. Clin. Ther. 2020, 42, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Onken, J.E.; Bregman, D.B.; Harrington, R.A.; Morris, D.; Buerkert, J.; Hamerski, D.; Iftikhar, H.; Mangoo-Karim, R.; Martin, E.R.; Martinez, C.O.; et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: The REPAIR-IDA trial. Nephrol. Dial. Transplant. 2014, 29, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Barish, C.F.; Koch, T.; Butcher, A.; Morris, D.; Bregman, D.B. Safety and efficacy of intravenous ferric carboxymaltose (750 mg) in the treatment of iron deficiency anemia: Two randomized, controlled trials. Anemia 2012, 2012, 172104. [Google Scholar] [CrossRef] [PubMed]
- Onken, J.E.; Bregman, D.B.; Harrington, R.A.; Morris, D.; Acs, P.; Akright, B.; Barish, C.; Bhaskar, B.S.; Smith-Nguyen, G.N.; Butcher, A.; et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion 2013, 54, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Barish, C.; He, A.; Bregman, D. Comparative review of the safety and efficacy of ferric carboxymaltose versus standard medical care for the treatment of iron deficiency anemia in bariatric and gastric surgery patients. Obes. Surg. 2013, 23, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Adkinson, N.F.; Strauss, W.E.; Macdougall, I.C.; Bernard, K.E.; Auerbach, M.; Kaper, R.F.; Chertow, G.M.; Krop, J.S. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: A randomized trial. Am. J. Hematol. 2018, 93, 683–690. [Google Scholar] [CrossRef]
- Chu, Z.; Cushway, T.; Wong, M.; Lim, K.; Peh, W.; Ng, C.; Lim, W.; Ong, S.G.K.; Tey, T.; Foo, F.; et al. Incidence and predictors of hypophosphataemia after ferric carboxymaltose use—A 3-year experience from a single institution in Singapore. Br. J. Haematol. 2023, 202, 1199–1204. [Google Scholar] [CrossRef]
- Bircher, A.J.; Auerbach, M. Hypersensitivity from Intravenous Iron Products. Immunol. Allergy Clin. N. Am. 2014, 34, 707–723. [Google Scholar] [CrossRef]
- Ganzoni, A.M. Eisen-Dextran intravenös: Therapeutische und experimentelle Möglichkeiten [Intravenous iron-dextran: Therapeutic and experimental possibilities]. Schweiz. Med. Wochenschr. 1970, 100, 301–303. [Google Scholar]
- Salvadori, U.; Vittadello, F.; Al-Khaffaf, A.; Maier, A.; Cappelletto, P.C.; Daves, M.; Raffeiner, B. Intravenous ferric carboxymaltose is effective and safe in patients with inflammatory rheumatic diseases. Blood Transfus. 2020, 18, 176. [Google Scholar]
- Lahner, E.; Dilaghi, E.; Cingolani, S.; Pivetta, G.; Dottori, L.; Esposito, G.; Marzinotto, I.; Lampasona, V.; Buzzetti, R.; Annibale, B. Gender-sex differences in autoimmune atrophic gastritis. Transl. Res. 2022, 248, 1–10. [Google Scholar] [CrossRef] [PubMed]
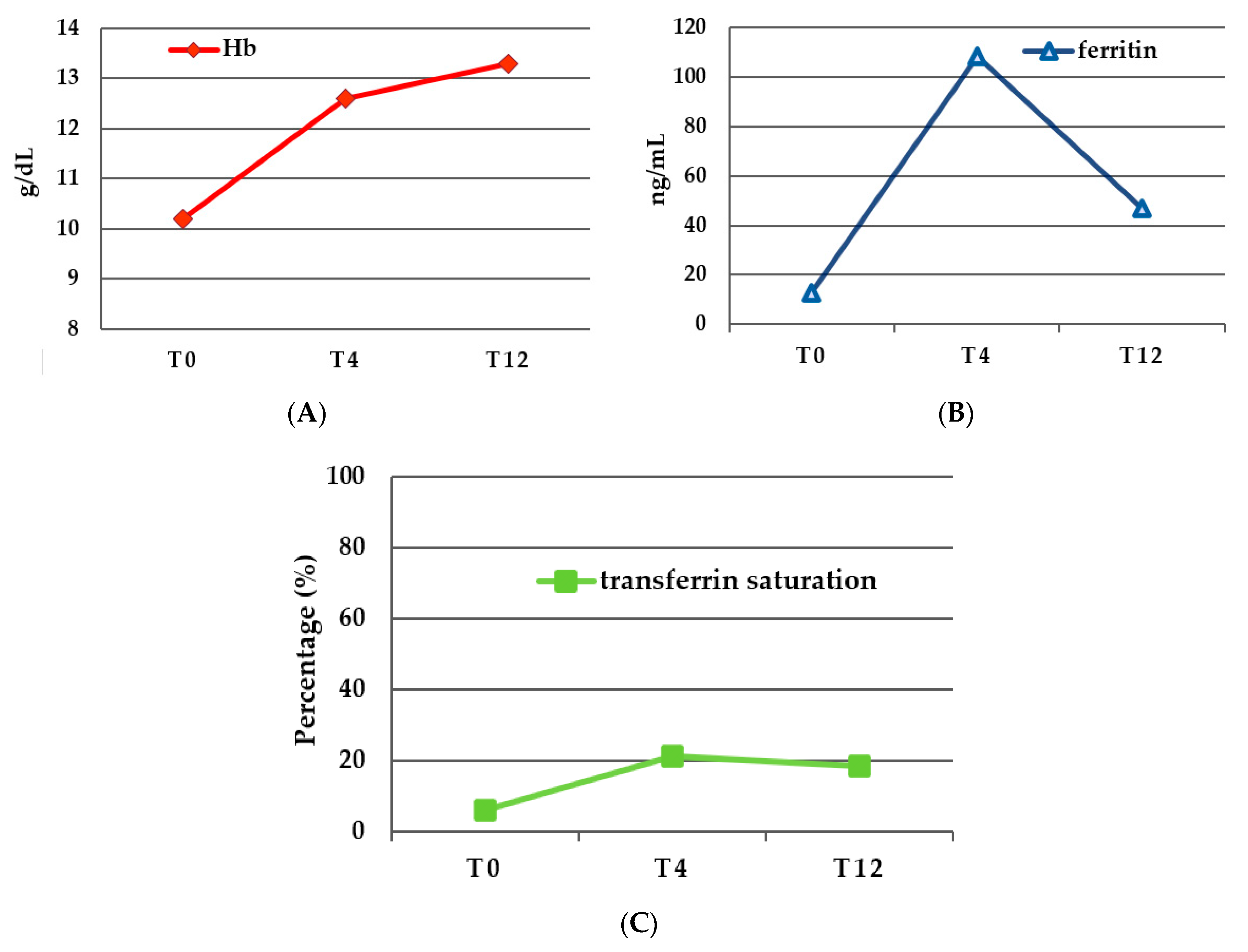
| T0(Tf) | T4 | p-Value T0–T4 | T12 | p-Value T4–T12 | p-Value T0–T12 | |
|---|---|---|---|---|---|---|
| Hb (g/dL ± SD) | 10.23 ± 1.7 | 12.62 ± 1.42 | <0.0001 | 13.28 ± 1.3 | 0.0547 | <0.0001 |
| RDW (% ± SD) | 17.6 ± 6 | 21.9 ± 10.9 | 0.0542 | 16.63 ± 4.8 | 0.0013 | 0.0229 |
| Ferritin (ng/mL ± SD) | 12.9 ± 29.3 | 108.2 ± 106.4 | <0.0001 | 46.95 ± 74.9 | <0.0001 | 0.3151 |
| Transferrin saturation (% ± SD) | 6.12 ± 3.9 | 21.25 ± 7.85 | =0.0001 | 18.67 ± 9.95 | 0.7021 | <0.001 |
| Relapse | Non-Relapse | p-Value | |
|---|---|---|---|
| Female | 50.8% | 27.7% | 0.0042 |
| Age > 55 years old | 27.7% | 16.9% | 0.3343 |
| NSAIDs | 10.9% | 14.1% | 0.2482 |
| Cobalamin supplementation | 40.3% | 27.4% | 0.179 |
| Severe atrophy | 16.9% | 21.5% | 0.1475 |
| Severe anaemia | 3.1% | 3.1% | 0.8244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dottori, L.; Corleone Tsar'kov, D.; Dilaghi, E.; Pivetta, G.; Scalamonti, S.; Ligato, I.; Esposito, G.; Annibale, B.; Lahner, E. Efficacy and Safety of Intravenous Ferric Carboxymaltose Treatment of Iron Deficiency Anaemia in Patients with Corpus Atrophic Gastritis: A Retrospective Study. Nutrients 2023, 15, 4199. https://doi.org/10.3390/nu15194199
Dottori L, Corleone Tsar'kov D, Dilaghi E, Pivetta G, Scalamonti S, Ligato I, Esposito G, Annibale B, Lahner E. Efficacy and Safety of Intravenous Ferric Carboxymaltose Treatment of Iron Deficiency Anaemia in Patients with Corpus Atrophic Gastritis: A Retrospective Study. Nutrients. 2023; 15(19):4199. https://doi.org/10.3390/nu15194199
Chicago/Turabian StyleDottori, Ludovica, Daniil Corleone Tsar'kov, Emanuele Dilaghi, Giulia Pivetta, Silvia Scalamonti, Irene Ligato, Gianluca Esposito, Bruno Annibale, and Edith Lahner. 2023. "Efficacy and Safety of Intravenous Ferric Carboxymaltose Treatment of Iron Deficiency Anaemia in Patients with Corpus Atrophic Gastritis: A Retrospective Study" Nutrients 15, no. 19: 4199. https://doi.org/10.3390/nu15194199
APA StyleDottori, L., Corleone Tsar'kov, D., Dilaghi, E., Pivetta, G., Scalamonti, S., Ligato, I., Esposito, G., Annibale, B., & Lahner, E. (2023). Efficacy and Safety of Intravenous Ferric Carboxymaltose Treatment of Iron Deficiency Anaemia in Patients with Corpus Atrophic Gastritis: A Retrospective Study. Nutrients, 15(19), 4199. https://doi.org/10.3390/nu15194199






