Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview
Abstract
1. Introduction
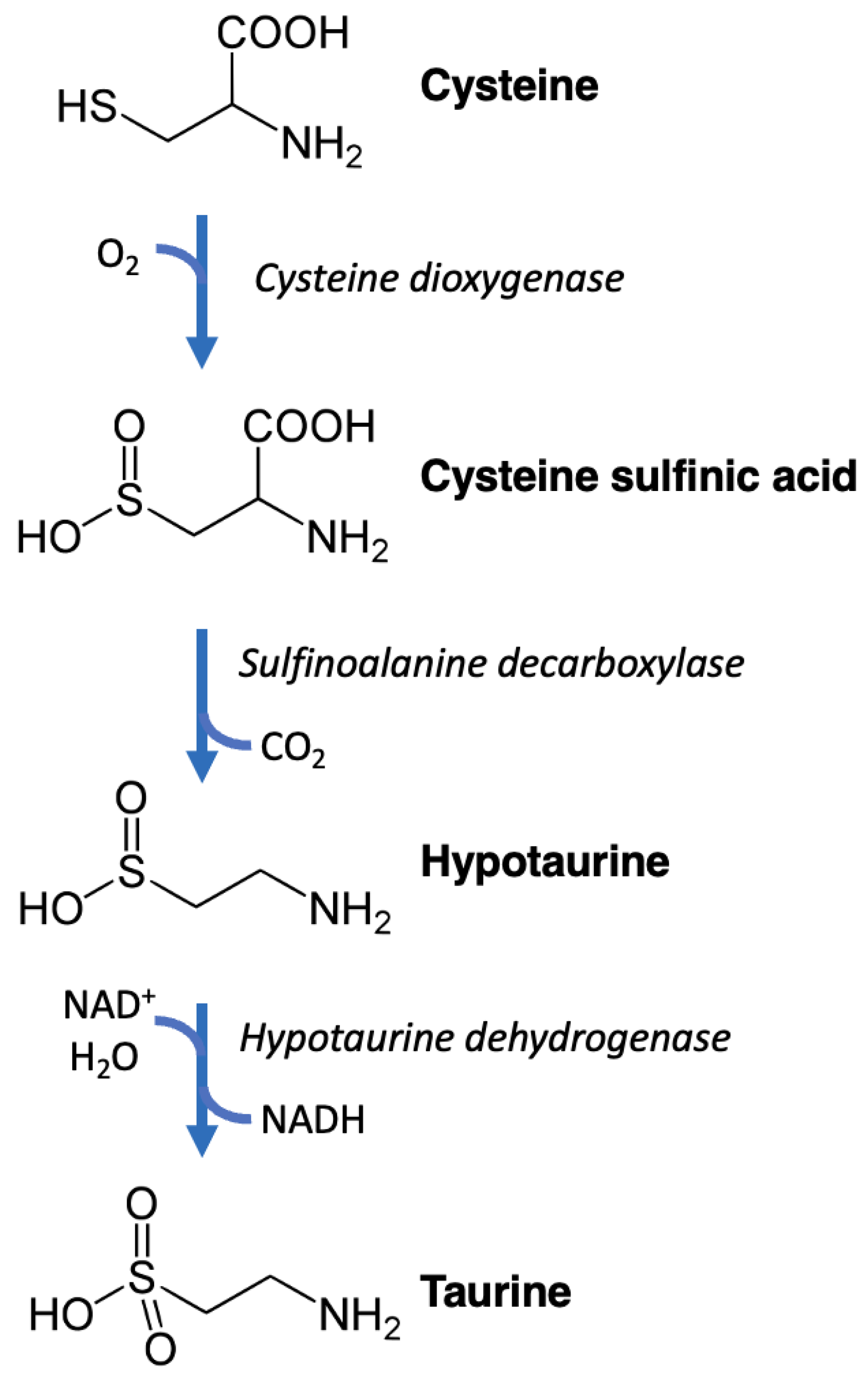
2. Nomenclature, Chemistry, and Biochemistry
3. Taurine and Cardiovascular Health
3.1. Taurine and Cardiac Function
3.2. Taurine and Vascular Function
3.3. Taurine and Athletic Performance
4. Taurine and Aging
4.1. Taurine and Longevity
4.2. Taurine and Cell Senescence
4.3. Taurine and Unfolded Protein Response
4.4. Taurine and Telomere Attrition
4.5. Taurine and Sirtuins
4.6. Taurine and Stem Cells
5. Recommended Intake and Safety Concerns
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Tiedemann, F.; Gmalin, L. Einige neue Bestandtheile der Galle des Ochsen. Ann. Phys. 1827, 85, 326–337. [Google Scholar] [CrossRef]
- Garrod, A. Lectures on the Chemistry of Pathology and Therapeutics: Showing the Application of the Science of Chemistry to the Discovery, Treatment, and Cure of Disease. Lancet 1848, 52, 333–336. [Google Scholar]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.H.; Andersen, M.L.; Cornett, C.; Gradinaru, R.; Grunnet, N. A role for taurine in mitochondrial function. J. Biomed. Sci. 2010, 17 (Suppl. S1), S23. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef]
- Hayes, K.C.; Sturman, J.A. Taurine in metabolism. Annu. Rev. Nutr. 1981, 1, 401–425. [Google Scholar] [CrossRef]
- Sole, M.J.; Jeejeebhoy, K.N. Conditioned nutritional requirements and the pathogenesis and treatment of myocardial failure. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 417–424. [Google Scholar] [CrossRef]
- Hansen, S.H. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab. Res. Rev. 2001, 17, 330–346. [Google Scholar] [CrossRef]
- Wojcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef]
- Laidlaw, S.A.; Shultz, T.D.; Cecchino, J.T.; Kopple, J.D. Plasma and urine taurine levels in vegans. Am. J. Clin. Nutr. 1988, 47, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, C.L.; Haschemeyer, R.H.; Griffith, O.W. In vivo studies of cysteine metabolism. Use of D-cysteinesulfinate, a novel cysteinesulfinate decarboxylase inhibitor, to probe taurine and pyruvate synthesis. J. Biol. Chem. 1988, 263, 16568–16579. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.R.; De La Rosa, J.; Stipanuk, M.H. Metabolism of cysteine in rat hepatocytes. Evidence for cysteinesulphinate-independent pathways. Biochem. J. 1987, 244, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Ding, S.T.; Lee, Y.H.; Wang, Y.C.; Huang, M.F.; Liu, I.H. Taurine homeostasis requires de novo synthesis via cysteine sulfinic acid decarboxylase during zebrafish early embryogenesis. Amino Acids 2013, 44, 615–629. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, J.; Liu, H.; Qiu, G.; Cui, S. Testosterone enhances taurine synthesis by upregulating androgen receptor and cysteine sulfinic acid decarboxylase expressions in male mouse liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G295–G304. [Google Scholar] [CrossRef]
- Magnusson, K.R.; Madl, J.E.; Clements, J.R.; Wu, J.Y.; Larson, A.A.; Beitz, A.J. Colocalization of taurine- and cysteine sulfinic acid decarboxylase-like immunoreactivity in the cerebellum of the rat with monoclonal antibodies against taurine. J. Neurosci. 1988, 8, 4551–4564. [Google Scholar] [CrossRef]
- Sharma, S.; Sahoo, B.M.; Banik, B.K. Biological Effects and Mechanisms of Taurine in Various Therapeutics. Curr. Drug Discov. Technol. 2023, online ahead of print. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Simmons, C.R.; Liu, Q.; Huang, Q.; Hao, Q.; Begley, T.P.; Karplus, P.A.; Stipanuk, M.H. Crystal structure of mammalian cysteine dioxygenase. A novel mononuclear iron center for cysteine thiol oxidation. J. Biol. Chem. 2006, 281, 18723–18733. [Google Scholar] [CrossRef]
- Park, E.; Park, S.Y.; Cho, I.S.; Kim, B.S.; Schuller-Levis, G. A Novel Cysteine Sulfinic Acid Decarboxylase Knock-Out Mouse: Taurine Distribution in Various Tissues with and without Taurine Supplementation. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 461–474. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Q.; Shang, J.; Dong, W.; Wu, S.; Guo, X.; Xie, Z.; Chen, C. The role of taurine in male reproduction: Physiology, pathology and toxicology. Front. Endocrinol. 2023, 14, 1017886. [Google Scholar] [CrossRef]
- Wen, C.; Li, F.; Zhang, L.; Duan, Y.; Guo, Q.; Wang, W.; He, S.; Li, J.; Yin, Y. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol. Nutr. Food Res. 2019, 63, e1800536. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Oja, S.S.; Saransaari, P. Taurine and epilepsy. Epilepsy Res. 2013, 104, 187–194. [Google Scholar] [CrossRef]
- Rosca, A.E.; Vladareanu, A.M.; Mirica, R.; Anghel-Timaru, C.M.; Mititelu, A.; Popescu, B.O.; Caruntu, C.; Voiculescu, S.E.; Gologan, S.; Onisai, M.; et al. Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential. J. Clin. Med. 2022, 11, 666. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Liu, Y.; Gao, J.; Tao, J. The molecular targets of taurine confer anti-hyperlipidemic effects. Life Sci. 2021, 278, 119579. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A.; Pasantes-Morales, H. Role of taurine in neural cell volume regulation. Can. J. Physiol. Pharmacol. 1992, 70, S356–S361. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Li, J.; Yamagata, N.; Xu, B. Taurine Boosts Cellular Uptake of Small D-Peptides for Enzyme-Instructed Intracellular Molecular Self-Assembly. J. Am. Chem. Soc. 2015, 137, 10040–10043. [Google Scholar] [CrossRef] [PubMed]
- Falany, C.N.; Johnson, M.R.; Barnes, S.; Diasio, R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 1994, 269, 19375–19379. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Fujita, M.; Nakamura, M.; Sakono, M.; Nishizono, S.; Sato, M.; Imaizumi, K.; Mori, M.; Fukuda, N. Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin. Exp. Pharmacol. Physiol. 2016, 43, 372–378. [Google Scholar] [CrossRef]
- Bellentani, S.; Pecorari, M.; Cordoma, P.; Marchegiano, P.; Manenti, F.; Bosisio, E.; De Fabiani, E.; Galli, G. Taurine increases bile acid pool size and reduces bile saturation index in the hamster. J. Lipid Res. 1987, 28, 1021–1027. [Google Scholar] [CrossRef]
- Batta, A.K.; Salen, G.; Shefer, S.; Tint, G.S.; Dayal, B. The effect of tauroursodeoxycholic acid and taurine supplementation on biliary bile acid composition. Hepatology 1982, 2, 811–816. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Eraqi, M.M.; Alfaiz, F.A. Therapeutic role of taurine as antioxidant in reducing hypertension risks in rats. Heliyon 2020, 6, e03209. [Google Scholar] [CrossRef] [PubMed]
- Degim, Z.; Celebi, N.; Sayan, H.; Babul, A.; Erdogan, D.; Take, G. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids 2002, 22, 187–198. [Google Scholar] [CrossRef]
- Chang, C.Y.; Shen, C.Y.; Kang, C.K.; Sher, Y.P.; Sheu, W.H.; Chang, C.C.; Lee, T.H. Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol. Appl. Pharmacol. 2014, 279, 351–363. [Google Scholar] [CrossRef]
- Wen, C.; Li, F.; Guo, Q.; Zhang, L.; Duan, Y.; Wang, W.; Li, J.; He, S.; Chen, W.; Yin, Y. Protective effects of taurine against muscle damage induced by diquat in 35 days weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, H.; Kwon, D.; Yuk, D.Y.; Jung, Y.S. Taurine Ameliorates Tunicamycin-Induced Liver Injury by Disrupting the Vicious Cycle between Oxidative Stress and Endoplasmic Reticulum Stress. Life 2022, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Niknahad, H.; Mehrabani, P.S.; Arjmand, A.; Alidaee, S.; Mazloomi, S.; Ahmadi, P.; Abdoli, N.; Saeed, M.; Rezaei, M.; Ommati, M.M.; et al. Cirrhosis-induced oxidative stress in erythrocytes: The therapeutic potential of taurine. Clin. Exp. Hepatol. 2023, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, L.; Yin, Y.; Gong, S.; Yang, Y.; Chen, S.; Han, M.; Duan, Y. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants 2022, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.N.; Arif, A.; Ansari, F.A.; Mahmood, R. Cytoprotective effect of taurine against sodium chlorate-induced oxidative damage in human red blood cells: An ex vivo study. Amino Acids 2022, 54, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp. Neurol. 2012, 233, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Faghfouri, A.H.; Seyyed Shoura, S.M.; Fathollahi, P.; Shadbad, M.A.; Papi, S.; Ostadrahimi, A.; Faghfuri, E. Profiling inflammatory and oxidative stress biomarkers following taurine supplementation: A systematic review and dose-response meta-analysis of controlled trials. Eur. J. Clin. Nutr. 2022, 76, 647–658. [Google Scholar] [CrossRef]
- Rafiee, Z.; Garcia-Serrano, A.M.; Duarte, J.M.N. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients 2022, 14, 1292. [Google Scholar] [CrossRef]
- Ochoa-de la Paz, L.D.; Martinez-Davila, I.A.; Miledi, R.; Martinez-Torres, A. Modulation of human GABArho1 receptors by taurine. Neurosci. Res. 2008, 61, 302–308. [Google Scholar] [CrossRef]
- Hilgier, W.; Oja, S.S.; Saransaari, P.; Albrecht, J. Taurine prevents ammonia-induced accumulation of cyclic GMP in rat striatum by interaction with GABAA and glycine receptors. Brain Res. 2005, 1043, 242–246. [Google Scholar] [CrossRef]
- Frosini, M.; Sesti, C.; Dragoni, S.; Valoti, M.; Palmi, M.; Dixon, H.B.; Machetti, F.; Sgaragli, G. Interactions of taurine and structurally related analogues with the GABAergic system and taurine binding sites of rabbit brain. Br. J. Pharmacol. 2003, 138, 1163–1171. [Google Scholar] [CrossRef]
- Hashimoto-Kitsukawa, S.; Okuyama, S.; Aihara, H. Enhancing effect of taurine on the rat caudate spindle. I: Interaction of taurine with the nigro-striatal dopamine system. Pharmacol. Biochem. Behav. 1988, 31, 411–416. [Google Scholar] [CrossRef]
- Kontro, P.; Oja, S.S. Release of taurine, GABA and dopamine from rat striatal slices: Mutual interactions and developmental aspects. Neuroscience 1988, 24, 49–58. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Ramirez-Guerrero, S.; Guardo-Maya, S.; Medina-Rincon, G.J.; Orrego-Gonzalez, E.E.; Cabezas-Perez, R.; Gonzalez-Reyes, R.E. Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship. Front. Mol. Neurosci. 2022, 15, 937789. [Google Scholar] [CrossRef]
- Seol, S.I.; Kim, H.J.; Choi, E.B.; Kang, I.S.; Lee, H.K.; Lee, J.K.; Kim, C. Taurine Protects against Postischemic Brain Injury via the Antioxidant Activity of Taurine Chloramine. Antioxidants 2021, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lee, H.J.; Jeong, Y.J.; Nam, K.R.; Kang, K.J.; Han, S.J.; Lee, K.C.; Lee, Y.J.; Choi, J.Y. Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci. Rep. 2020, 10, 15551. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, R.; Jiang, H.; Li, B.; Geng, Q.; Li, Y.; Qi, J. Taurine inhibits KDM3a production and microglia activation in lipopolysaccharide-treated mice and BV-2 cells. Mol. Cell Neurosci. 2022, 122, 103759. [Google Scholar] [CrossRef]
- Verner, A.; Craig, S.; McGuire, W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, 2007, CD006072. [Google Scholar] [CrossRef] [PubMed]
- Wharton, B.A.; Morley, R.; Isaacs, E.B.; Cole, T.J.; Lucas, A. Low plasma taurine and later neurodevelopment. Arch. Dis. Child. Fetal. Neonatal Ed. 2004, 89, F497–E498. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.L.; Jiang, H.; Niu, S.P.; Wang, X.H.; Du, S. Effects of Taurine Supplementation on Growth in Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Indian J. Pediatr. 2018, 85, 855–860. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Davies, W.E.; Hopkins, P.C.; Rose, S.J. Effects of dietary taurine on auditory function in full-term infants. Adv. Exp. Med. Biol. 1998, 442, 507–514. [Google Scholar] [CrossRef]
- Gaull, G.E. Taurine in human milk: Growth modulator or conditionally essential amino acid? J. Pediatr. Gastroenterol. Nutr. 1983, 2 (Suppl. S1), S266–S271. [Google Scholar] [CrossRef]
- Furukawa, T.; Fukuda, A. Maternal taurine as a modulator of Cl− homeostasis as well as of glycine/GABA(A) receptors for neocortical development. Front. Cell Neurosci. 2023, 17, 1221441. [Google Scholar] [CrossRef]
- Yamori, Y.; Sagara, M.; Arai, Y.; Kobayashi, H.; Kishimoto, K.; Matsuno, I.; Mori, H.; Mori, M. Taurine Intake with Magnesium Reduces Cardiometabolic Risks. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Sagara, M.; Murakami, S.; Mizushima, S.; Liu, L.; Mori, M.; Ikeda, K.; Nara, Y.; Yamori, Y. Taurine in 24-h Urine Samples Is Inversely Related to Cardiovascular Risks of Middle Aged Subjects in 50 Populations of the World. Adv. Exp. Med. Biol. 2015, 803, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Lau, E.; Wijaya, B.P.; Jin, X.; Sutarga, K.; Schwartz, G.D.; Learmont, J.; Wookey, P.J.; Zinellu, A.; Carru, C.; et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: Association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension 2009, 53, 1017–1022. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Husain, T.; Wilson, G.J.; Liu, P.; Sole, M.J.; Backx, P.H. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation 2004, 109, 1877–1885. [Google Scholar] [CrossRef]
- Swiderski, J.; Sakkal, S.; Apostolopoulos, V.; Zulli, A.; Gadanec, L.K. Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients 2023, 15, 2562. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Shimazaki, T.; Nakagawa, S.; Otuki, T.; Kurata, S.; Suzuki, T.; Watanabe, K.; Saigo, K. Chemical synthesis of novel taurine-containing uridine derivatives. Nucleic Acids Res. Suppl. 2002, 2, 11–12. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef]
- Fakruddin, M.; Wei, F.Y.; Suzuki, T.; Asano, K.; Kaieda, T.; Omori, A.; Izumi, R.; Fujimura, A.; Kaitsuka, T.; Miyata, K.; et al. Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease. Cell Rep. 2018, 22, 482–496. [Google Scholar] [CrossRef]
- Kirino, Y.; Goto, Y.; Campos, Y.; Arenas, J.; Suzuki, T. Specific correlation between the wobble modification deficiency in mutant tRNAs and the clinical features of a human mitochondrial disease. Proc. Natl. Acad. Sci. USA 2005, 102, 7127–7132. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Celino, F.T.; Shimizu-Yamaguchi, S.; Miura, C.; Miura, T. Taurine plays an important role in the protection of spermatogonia from oxidative stress. Amino Acids 2012, 43, 2359–2369. [Google Scholar] [CrossRef]
- Tabassum, H.; Rehman, H.; Banerjee, B.D.; Raisuddin, S.; Parvez, S. Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin. Chim. Acta 2006, 370, 129–136. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ito, T.; Baseggio Conrado, A.; Murakami, S. Editorial for Special Issue on “Regulation and Effect of Taurine on Metabolism”. Metabolites 2022, 12, 795. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Batitucci, G.; Abud, G.F.; de Freitas, E.C. Taurine and Exercise: Synergistic Effects on Adipose Tissue Metabolism and Inflammatory Process in Obesity. Adv. Exp. Med. Biol. 2022, 1370, 279–289. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.G.; Munoz, V.R.; Brandao, C.F.C.; Simabuco, F.M.; Pavan, I.C.B.; Nakandakari, S.; Pauli, J.R.; De Moura, L.P.; Ropelle, E.R.; Marchini, J.S.; et al. Taurine upregulates insulin signaling and mitochondrial metabolism in vitro but not in adipocytes of obese women. Nutrition 2022, 93, 111430. [Google Scholar] [CrossRef]
- Brons, C.; Spohr, C.; Storgaard, H.; Dyerberg, J.; Vaag, A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur. J. Clin. Nutr. 2004, 58, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Minami, A.; Harada, N.; Sakamoto, S.; Niwa, Y.; Ohnaka, M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am. J. Clin. Nutr. 2000, 71, 54–58. [Google Scholar] [CrossRef]
- Anuradha, C.V.; Balakrishnan, S.D. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can. J. Physiol. Pharmacol. 1999, 77, 749–754. [Google Scholar] [CrossRef]
- Sarnobat, D.; Moffett, R.C.; Ma, J.; Flatt, P.R.; McClenaghan, N.H.; Tarasov, A.I. Taurine rescues pancreatic beta-cell stress by stimulating alpha-cell transdifferentiation. Biofactors 2023, 49, 646–662. [Google Scholar] [CrossRef]
- Tagawa, R.; Kobayashi, M.; Sakurai, M.; Yoshida, M.; Kaneko, H.; Mizunoe, Y.; Nozaki, Y.; Okita, N.; Sudo, Y.; Higami, Y. Long-Term Dietary Taurine Lowers Plasma Levels of Cholesterol and Bile Acids. Int. J. Mol. Sci. 2022, 23, 1793. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; -Gao, Y.; Cao, X.; Zhang, J.; Chen, W. Cholesterollowing effect of taurine in HepG2 cell. Lipids Health Dis. 2017, 16, 56. [Google Scholar] [CrossRef]
- Yokogoshi, H.; Mochizuki, H.; Nanami, K.; Hida, Y.; Miyachi, F.; Oda, H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. J. Nutr. 1999, 129, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Balkan, J.; Kanbagli, O.; Hatipoglu, A.; Kucuk, M.; Cevikbas, U.; Aykac-Toker, G.; Uysal, M. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci. Biotechnol. Biochem. 2002, 66, 1755–1758. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, L.F.; Fang, J.H.; Su, X.L.; Da, G.L.; Kuwamori, T.; Kagamimori, S. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids 2004, 26, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Pansani, M.C.; Azevedo, P.S.; Rafacho, B.P.; Minicucci, M.F.; Chiuso-Minicucci, F.; Zorzella-Pezavento, S.G.; Marchini, J.S.; Padovan, G.J.; Fernandes, A.A.; Matsubara, B.B.; et al. Atrophic cardiac remodeling induced by taurine deficiency in Wistar rats. PLoS ONE 2012, 7, e41439. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Tan, B.H.; Lucia, M.A.; Schaffer, S.W. Effect of drug-induced taurine depletion on cardiac contractility and metabolism. Biochem. Pharmacol. 1986, 35, 985–989. [Google Scholar] [CrossRef]
- Lake, N. Loss of cardiac myofibrils: Mechanism of contractile deficits induced by taurine deficiency. Am. J. Physiol. 1993, 264, H1323–H1326. [Google Scholar] [CrossRef]
- Satoh, H.; Nakatani, T.; Tanaka, T.; Haga, S. Cardiac functions and taurine’s actions at different extracellular calcium concentrations in forced swimming stress-loaded rats. Biol. Trace Elem. Res. 2002, 87, 171–182. [Google Scholar] [CrossRef]
- Franconi, F.; Martini, F.; Stendardi, I.; Matucci, R.; Zilletti, L.; Giotti, A. Effect of taurine on calcium levels and contractility in guinea-pig ventricular strips. Biochem. Pharmacol. 1982, 31, 3181–3185. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Seyed-Mozaffari, M.; Kramer, J.; Tan, B.H. Effect of taurine depletion and treatment on cardiac contractility and metabolism. Prog. Clin. Biol. Res. 1985, 179, 167–175. [Google Scholar]
- Kaplan, J.L.; Stern, J.A.; Fascetti, A.J.; Larsen, J.A.; Skolnik, H.; Peddle, G.D.; Kienle, R.D.; Waxman, A.; Cocchiaro, M.; Gunther-Harrington, C.T.; et al. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS ONE 2018, 13, e0209112. [Google Scholar] [CrossRef]
- Samadi, M.; Haghi-Aminjan, H.; Sattari, M.; Hooshangi Shayesteh, M.R.; Bameri, B.; Armandeh, M.; Naddafi, M.; Eghbal, M.A.; Abdollahi, M. The role of taurine on chemotherapy-induced cardiotoxicity: A systematic review of non-clinical study. Life Sci. 2021, 265, 118813. [Google Scholar] [CrossRef]
- Ahmadian, M.; Dabidi Roshan, V.; Ashourpore, E. Taurine Supplementation Improves Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity in Heart Failure. J. Diet. Suppl. 2017, 14, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Roshan, V.D.; Aslani, E.; Stannard, S.R. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther. Adv. Cardiovasc. Dis. 2017, 11, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Sawamura, A.; Awata, N.; Ohta, H.; Hamaguchi, T.; Harada, H.; Takihara, K.; Hasegawa, H.; Yamagami, T.; Ishiyama, T.; et al. Therapeutic effect of taurine in congestive heart failure: A double-blind crossover trial. Clin. Cardiol. 1985, 8, 276–282. [Google Scholar] [CrossRef]
- Beyranvand, M.R.; Khalafi, M.K.; Roshan, V.D.; Choobineh, S.; Parsa, S.A.; Piranfar, M.A. Effect of taurine supplementation on exercise capacity of patients with heart failure. J. Cardiol. 2011, 57, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Sawamura, A.; Awata, N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn. Circ. J. 1992, 56, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi-Takihara, K.; Azuma, J.; Kishimoto, S.; Onishi, S.; Sperelakis, N. Taurine prevention of calcium paradox-related damage in cardiac muscle. Its regulatory action on intracellular cation contents. Biochem. Pharmacol. 1988, 37, 2651–2658. [Google Scholar] [CrossRef]
- Henry, E.F.; MacCormack, T.J. Taurine protects cardiac contractility in killifish, Fundulus heteroclitus, by enhancing sarcoplasmic reticular Ca2+ cycling. J. Comp. Physiol. B 2018, 188, 89–99. [Google Scholar] [CrossRef]
- Gates, M.A.; Morash, A.J.; Lamarre, S.G.; MacCormack, T.J. Intracellular taurine deficiency impairs cardiac contractility in rainbow trout (Oncorhynchus mykiss) without affecting aerobic performance. J. Comp. Physiol. B 2022, 192, 49–60. [Google Scholar] [CrossRef]
- Satoh, H.; Sperelakis, N. Taurine inhibition of fast Na+ current in embryonic chick ventricular myocytes. Eur. J. Pharmacol. 1992, 218, 83–89. [Google Scholar] [CrossRef]
- Oz, E.; Erbas, D.; Gelir, E.; Aricioglu, A. Taurine and calcium interaction in protection of myocardium exposed to ischemic reperfusion injury. Gen. Pharmacol. 1999, 33, 137–141. [Google Scholar] [CrossRef]
- Wong, A.P.; Niedzwiecki, A.; Rath, M. Myocardial energetics and the role of micronutrients in heart failure: A critical review. Am. J. Cardiovasc. Dis. 2016, 6, 81–92. [Google Scholar] [PubMed]
- Dragan, S.; Buleu, F.; Christodorescu, R.; Cobzariu, F.; Iurciuc, S.; Velimirovici, D.; Xiao, J.; Luca, C.T. Benefits of multiple micronutrient supplementation in heart failure: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, A.; Choobineh, S.; Gaeini, A.; Soori, R. Interaction of exercise training with taurine attenuates infarct size and cardiac dysfunction via Akt-Foxo3a-Caspase-8 signaling pathway. Amino Acids 2023, 55, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Niu, Y.; He, W.; Wang, X.; Zhang, X.; Wu, Y.; Zhang, W.; Zhao, L.; Zheng, H.; et al. Deficiency of Pdk1 drives heart failure by impairing taurine homeostasis through Slc6a6. FASEB J. 2023, 37, e23134. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Zhang, M.; Zhang, C.; Piao, F. Taurine Ameliorates Apoptosis via AKT Pathway in the Kidney of Diabetic Rats. Adv. Exp. Med. Biol. 2022, 1370, 227–233. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Wang, Z.; Wu, B.; Zhang, J.; Xu, Y.; Han, X.; Phouthapane, V.; Miao, J. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front. Immunol. 2022, 13, 927215. [Google Scholar] [CrossRef]
- Liu, C.; He, P.; Guo, Y.; Tian, Q.; Wang, J.; Wang, G.; Zhang, Z.; Li, M. Taurine attenuates neuronal ferroptosis by regulating GABA(B)/AKT/GSK3beta/beta-catenin pathway after subarachnoid hemorrhage. Free Radic. Biol. Med. 2022, 193, 795–807. [Google Scholar] [CrossRef]
- Das, J.; Vasan, V.; Sil, P.C. Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 296–308. [Google Scholar] [CrossRef]
- Wei, C.; Ding, X.; Liu, C.; Pei, Y.; Zhong, Y.; Sun, W. Mechanism of taurine in alleviating myocardial oxidative stress in rats after burn through p38 MAPK signaling pathway. Minerva Med. 2019, 110, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Takahashi, K.; Fukuda, T.; Ohyabu, Y.; Yamamoto, I.; Kim, S.; Iwao, H.; Schaffer, S.W.; Azuma, J. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur. J. Pharmacol. 2000, 403, 181–188. [Google Scholar] [CrossRef]
- Takatani, T.; Takahashi, K.; Uozumi, Y.; Matsuda, T.; Ito, T.; Schaffer, S.W.; Fujio, Y.; Azuma, J. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem. Biophys. Res. Commun. 2004, 316, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Choobineh, S.; Ravasi, A.A. Taurine with combined aerobic and resistance exercise training alleviates myocardium apoptosis in STZ-induced diabetes rats via Akt signaling pathway. Life Sci. 2020, 258, 118225. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: Role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol. Appl. Pharmacol. 2009, 240, 73–87. [Google Scholar] [CrossRef]
- Yousuf, M.; Shamsi, A.; Mohammad, T.; Azum, N.; Alfaifi, S.Y.M.; Asiri, A.M.; Mohamed Elasbali, A.; Islam, A.; Hassan, M.I.; Haque, Q.M.R. Inhibiting Cyclin-Dependent Kinase 6 by Taurine: Implications in Anticancer Therapeutics. ACS Omega 2022, 7, 25844–25852. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hu, W.; Hong, Y.; Ruan, L.; Hu, Y.; Liu, D. Taurine Ameliorates Iron Overload-Induced Hepatocyte Injury via the Bcl-2/VDAC1-Mediated Mitochondrial Apoptosis Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 4135752. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Feng, Y.; Bian, Y.; Fu, Z.; Wu, Y.; Ma, Y.; Li, C.; Wang, J.; Dai, J.; et al. Taurine Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR-4/NF-kappaB Pathway. Adv. Exp. Med. Biol. 2022, 1370, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; San, J.; Pang, H.; Du, Y.; Li, W.; Zhou, X.; Yang, X.; Hu, J.; Yang, J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon 2022, 215, 17–27. [Google Scholar] [CrossRef]
- Kp, A.D.; Shimoga Janakirama, A.R.; Martin, A. SIRT1 activation by Taurine: In vitro evaluation, molecular docking and molecular dynamics simulation studies. J. Nutr. Biochem. 2022, 102, 108948. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.S.; Patel, C.; Abdelsayed, R.; Schaffer, S.W. Accelerated NaCl-induced hypertension in taurine-deficient rat: Role of renal function. Kidney Int. 2006, 70, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Lyu, Q.; Wu, G.; Lin, S.; Yang, Q.; Hu, J. Taurine attenuates isoproterenol-induced H9c2 cardiomyocytes hypertrophy by improving antioxidative ability and inhibiting calpain-1-mediated apoptosis. Mol. Cell. Biochem. 2020, 469, 119–132. [Google Scholar] [CrossRef]
- Gentile, S.; Bologna, E.; Terracina, D.; Angelico, M. Taurine-induced diuresis and natriuresis in cirrhotic patients with ascites. Life Sci. 1994, 54, 1585–1593. [Google Scholar] [CrossRef]
- Dlouha, H.; McBroom, M.J. Atrial natriuretic factor in taurine-treated normal and cardiomyopathic hamsters. Proc. Soc. Exp. Biol. Med. 1986, 181, 411–415. [Google Scholar] [CrossRef]
- Guizoni, D.M.; Vettorazzi, J.F.; Carneiro, E.M.; Davel, A.P. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 2020, 94, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Su, J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015, 7, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Fennessy, F.M.; Moneley, D.S.; Wang, J.H.; Kelly, C.J.; Bouchier-Hayes, D.J. Taurine and vitamin C modify monocyte and endothelial dysfunction in young smokers. Circulation 2003, 107, 410–415. [Google Scholar] [CrossRef]
- El Idrissi, A.; Okeke, E.; Yan, X.; Sidime, F.; Neuwirth, L.S. Taurine regulation of blood pressure and vasoactivity. Adv. Exp. Med. Biol. 2013, 775, 407–425. [Google Scholar] [CrossRef]
- Yildiz, O.; Ulusoy, K.G. Effects of taurine on vascular tone. Amino Acids 2022, 54, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Kuroki, G.; Yuan, P.X.; Suzuki, T.; Murakami, M.; Hano, T.; Sasano, H.; Yanagisawa, T. The effect of taurine on the salt-dependent blood pressure increase in the voltage-dependent calcium channel beta 3-subunit-deficient mouse. J. Cardiovasc. Pharmacol. 2003, 41 (Suppl. S1), S127–S131. [Google Scholar] [PubMed]
- Meldrum, M.J.; Tu, R.; Patterson, T.; Dawson, R., Jr.; Petty, T. The effect of taurine on blood pressure, and urinary sodium, potassium and calcium excretion. Adv. Exp. Med. Biol. 1994, 359, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Maruta, H.; Ma, Y.; Yamashita, H. Taurine Stimulates AMP-Activated Protein Kinase and Modulates the Skeletal Muscle Functions in Rats via the Induction of Intracellular Calcium Influx. Int. J. Mol. Sci. 2023, 24, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Kawanaka, K.; Ohmori, H.; Maeda, S. Effects of Taurine Supplementation on Vascular Endothelial Function at Rest and After Resistance Exercise. Adv. Exp. Med. Biol. 2019, 1155, 407–414. [Google Scholar] [CrossRef]
- Katakawa, M.; Fukuda, N.; Tsunemi, A.; Mori, M.; Maruyama, T.; Matsumoto, T.; Abe, M.; Yamori, Y. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens. Res. 2016, 39, 848–856. [Google Scholar] [CrossRef]
- Guizoni, D.M.; Freitas, I.N.; Victorio, J.A.; Possebom, I.R.; Araujo, T.R.; Carneiro, E.M.; Davel, A.P. Taurine treatment reverses protein malnutrition-induced endothelial dysfunction of the pancreatic vasculature: The role of hydrogen sulfide. Metabolism 2021, 116, 154701. [Google Scholar] [CrossRef]
- Casey, R.G.; Gang, C.; Joyce, M.; Bouchier-Hayes, D.J. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J. Vasc. Res. 2007, 44, 31–39. [Google Scholar] [CrossRef]
- Moloney, M.A.; Casey, R.G.; O’Donnell, D.H.; Fitzgerald, P.; Thompson, C.; Bouchier-Hayes, D.J. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diabetes Vasc. Dis. Res. 2010, 7, 300–310. [Google Scholar] [CrossRef]
- Ferreira Abud, G.; Giolo De Carvalho, F.; Batitucci, G.; Travieso, S.G.; Bueno Junior, C.R.; Barbosa Junior, F.; Marchini, J.S.; de Freitas, E.C. Taurine as a possible antiaging therapy: A controlled clinical trial on taurine antioxidant activity in women ages 55 to 70. Nutrition 2022, 101, 111706. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef]
- Kang, Y.J.; Choi, M.J. Liver Antioxidant Enzyme Activities Increase After Taurine in Ovariectomized Rats. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1071–1080. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.R.; Batistas, T.M.; Victorio, J.A.; Clerici, S.P.; Delbin, M.A.; Carneiro, E.M.; Davel, A.P. Taurine upplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS ONE 2014, 9, e105851. [Google Scholar] [CrossRef]
- Trachtman, H.; Del Pizzo, R.; Rao, P.; Rujikarn, N.; Sturman, J.A. Taurine lowers blood pressure in the spontaneously hypertensive rat by a catecholamine independent mechanism. Am. J. Hypertens. 1989, 2, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Scabora, J.E.; de Lima, M.C.; Lopes, A.; de Lima, I.P.; Mesquita, F.F.; Torres, D.B.; Boer, P.A.; Gontijo, J.A. Impact of taurine supplementation on blood pressure in gestational protein-restricted offspring: Effect on the medial solitary tract nucleus cell numbers, angiotensin receptors, and renal sodium handling. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Mozaffari, M.S. Effects of chronic taurine treatment on reactivity of the rat aorta. Amino Acids 2000, 19, 615–623. [Google Scholar] [CrossRef]
- Sener, G.; Ozer Sehirli, A.; Ipci, Y.; Cetinel, S.; Cikler, E.; Gedik, N.; Alican, I. Taurine treatment protects against chronic nicotine-induced oxidative changes. Fundam. Clin. Pharmacol. 2005, 19, 155–164. [Google Scholar] [CrossRef]
- Liang, W.; Yang, Q.; Wu, G.; Lin, S.; Yang, J.; Feng, Y.; Hu, J. Effects of Taurine and L-Arginine on the Apoptosis of Vascular Smooth Muscle Cells in Insulin Resistance Hypertensive Rats. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 813–819. [Google Scholar] [CrossRef]
- Forzano, I.; Avvisato, R.; Varzideh, F.; Jankauskas, S.S.; Cioppa, A.; Mone, P.; Salemme, L.; Kansakar, U.; Tesorio, T.; Trimarco, V.; et al. L-Arginine in diabetes: Clinical and preclinical evidence. Cardiovasc. Diabetol. 2023, 22, 89. [Google Scholar] [CrossRef]
- Trimarco, V.; Izzo, R.; Lombardi, A.; Coppola, A.; Fiorentino, G.; Santulli, G. Beneficial effects of L-Arginine in patients hospitalized for COVID-19: New insights from a randomized clinical trial. Pharmacol. Res. 2023, 191, 106702. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Fiordelisi, A.; Spigno, L.; Boldrini, L.; Lungonelli, G.; Di Vaia, E.; Santulli, G.; Sorriento, D.; Cerasuolo, F.A.; Trimarco, V.; et al. Effects of Chronic Supplementation of L-Arginine on Physical Fitness in Water Polo Players. Oxid. Med. Cell. Longev. 2021, 2021, 6684568. [Google Scholar] [CrossRef]
- Moludi, J.; Qaisar, S.A.; Kadhim, M.M.; Ahmadi, Y.; Davari, M. Protective and therapeutic effectiveness of taurine supplementation plus low calorie diet on metabolic parameters and endothelial markers in patients with diabetes mellitus: A randomized, clinical trial. Nutr. Metab. 2022, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.; Patterson, S.D.; Tallent, J.; Jeffries, O. The Effects of Oral Taurine on Resting Blood Pressure in Humans: A Meta-Analysis. Curr. Hypertens. Rep. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Hellin, J.; Varillas-Delgado, D. Energy Drinks and Sports Performance, Cardiovascular Risk, and Genetic Associations; Future Prospects. Nutrients 2021, 13, 715. [Google Scholar] [CrossRef]
- Ozan, M.; Buzdagli, Y.; Eyipinar, C.D.; Baygutalp, N.K.; Yuce, N.; Oget, F.; Kan, E.; Baygutalp, F. Does Single or Combined Caffeine and Taurine Supplementation Improve Athletic and Cognitive Performance without Affecting Fatigue Level in Elite Boxers? A Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 4399. [Google Scholar] [CrossRef]
- Kurtz, J.A.; VanDusseldorp, T.A.; Doyle, J.A.; Otis, J.S. Taurine in sports and exercise. J. Int. Soc. Sports Nutr. 2021, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.M.; McStay, C.L.; Meng, X. Public Concern about the Sale of High-Caffeine Drinks to Children 12 Years or Younger: An Australian Regulatory Perspective. Biomed Res. Int. 2015, 2015, 707149. [Google Scholar] [CrossRef]
- Dawodu, A.; Cleaver, K. Behavioural correlates of energy drink consumption among adolescents: A review of the literature. J. Child Health Care 2017, 21, 446–462. [Google Scholar] [CrossRef]
- Kaur, A.; Yousuf, H.; Ramgobin-Marshall, D.; Jain, R.; Jain, R. Energy drink consumption: A rising public health issue. Rev. Cardiovasc. Med. 2022, 23, 83. [Google Scholar] [CrossRef]
- Erdmann, J.; Wicinski, M.; Wodkiewicz, E.; Nowaczewska, M.; Slupski, M.; Otto, S.W.; Kubiak, K.; Huk-Wieliczuk, E.; Malinowski, B. Effects of Energy Drink Consumption on Physical Performance and Potential Danger of Inordinate Usage. Nutrients 2021, 13, 2506. [Google Scholar] [CrossRef] [PubMed]
- Nuss, T.; Morley, B.; Scully, M.; Wakefield, M. Energy drink consumption among Australian adolescents associated with a cluster of unhealthy dietary behaviours and short sleep duration. Nutr. J. 2021, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Kriebs, A. Taurine levels modulate aging. Nat. Aging 2023, 3, 758–759. [Google Scholar] [CrossRef]
- Ferreira, J. Systemic taurine decline drives aging. Lab Anim. 2023, 52, 175. [Google Scholar] [CrossRef]
- Izquierdo, J.M. Taurine as a possible therapy for immunosenescence and inflammaging. Cell. Mol. Immunol. 2023, online ahead of print. [Google Scholar] [CrossRef]
- Barbiera, A.; Sorrentino, S.; Fard, D.; Lepore, E.; Sica, G.; Dobrowolny, G.; Tamagnone, L.; Scicchitano, B.M. Taurine Administration Counteracts Aging-Associated Impingement of Skeletal Muscle Regeneration by Reducing Inflammation and Oxidative Stress. Antioxidants 2022, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef] [PubMed]
- Vidal Valero, M. Taurine supplement makes animals live longer—What it means for people is unclear. Nature 2023, online ahead of print. [Google Scholar] [CrossRef]
- McGaunn, J.; Baur, J.A. Taurine linked with healthy aging. Science 2023, 380, 1010–1011. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Li, M.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis. Biomedicines 2023, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Graham, F. Daily briefing: Taurine makes animals live longer—But don’t binge on Red Bulls yet. Nature 2023, online ahead of print. [Google Scholar] [CrossRef]
- Jun, H.; Choi, M.J. Relationship Between Taurine Intake and Cardiometabolic Risk Markers in Korean Elderly. Adv. Exp. Med. Biol. 2019, 1155, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef]
- Kowald, A.; Passos, J.F.; Kirkwood, T.B.L. On the evolution of cellular senescence. Aging Cell 2020, 19, e13270. [Google Scholar] [CrossRef]
- Yi, S.; Lin, K.; Jiang, T.; Shao, W.; Huang, C.; Jiang, B.; Li, Q.; Lin, D. NMR-based metabonomic analysis of HUVEC cells during replicative senescence. Aging 2020, 12, 3626–3646. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, G.; Luo, J.; Zhao, X.; Zhang, M. Taurine postponed the replicative senescence of rat bone marrow-derived multipotent stromal cells in vitro. Mol. Cell. Biochem. 2012, 366, 259–267. [Google Scholar] [CrossRef]
- Ito, T.; Yoshikawa, N.; Inui, T.; Miyazaki, N.; Schaffer, S.W.; Azuma, J. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS ONE 2014, 9, e107409. [Google Scholar] [CrossRef]
- Ito, T.; Yamamoto, N.; Nakajima, S.; Schaffer, S.W. Beta-Catenin and SMAD3 Are Associated with Skeletal Muscle Aging in the Taurine Transpoeter Knockout Mouse. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 497–502. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Du, G.; Liu, Z.; Yu, Z.; Zhuo, Z.; Zhu, Y.; Zhou, J.; Li, Y.; Chen, H. Taurine represses age-associated gut hyperplasia in Drosophila via counteracting endoplasmic reticulum stress. Aging Cell 2021, 20, e13319. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Liu, X.; Zuo, J.; Wang, K.; Liu, W.; Ge, J. Exogenous taurine attenuates mitochondrial oxidative stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta Biochim. Biophys. Sin. 2013, 45, 359–367. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sinha, K.; Banerjee, S.; Sil, P.C. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors 2016, 42, 647–664. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, G.; Dong, C.; Li, Z.; Zhou, D.; Huang, L.; Li, W.; Huang, G.; Yan, J. Parental Folate Deficiency Inhibits Proliferation and Increases Apoptosis of Neural Stem Cells in Rat Offspring: Aggravating Telomere Attrition as a Potential Mechanism. Nutrients 2023, 15, 2843. [Google Scholar] [CrossRef]
- Gao, Z.; Daquinag, A.C.; Fussell, C.; Zhao, Z.; Dai, Y.; Rivera, A.; Snyder, B.E.; Eckel-Mahan, K.L.; Kolonin, M.G. Age-associated telomere attrition in adipocyte progenitors predisposes to metabolic disease. Nat. Metab. 2020, 2, 1482–1497. [Google Scholar] [CrossRef]
- Varzideh, F.; Gambardella, J.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Molecular Mechanisms Underlying Pluripotency and Self-Renewal of Embryonic Stem Cells. Int. J. Mol. Sci. 2023, 24, 8386. [Google Scholar] [CrossRef]
- Mashyakhy, M.; Alkahtani, A.; Abumelha, A.S.; Sharroufna, R.J.; Alkahtany, M.F.; Jamal, M.; Robaian, A.; Binalrimal, S.; Chohan, H.; Patil, V.R.; et al. Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 491. [Google Scholar] [CrossRef]
- Gokarn, R.; Solon-Biet, S.; Youngson, N.A.; Wahl, D.; Cogger, V.C.; McMahon, A.C.; Cooney, G.J.; Ballard, J.W.O.; Raubenheimer, D.; Morris, M.J.; et al. The Relationship Between Dietary Macronutrients and Hepatic Telomere Length in Aging Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 446–449. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.Y.; Li, L.S.; Liu, Y.S. Sirtuins at the Crossroads between Mitochondrial Quality Control and Neurodegenerative Diseases: Structure, Regulation, Modifications, and Modulators. Aging Dis. 2023, 14, 794–824. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Watroba, M.; Dudek, I.; Skoda, M.; Stangret, A.; Rzodkiewicz, P.; Szukiewicz, D. Sirtuins, epigenetics and longevity. Ageing Res. Rev. 2017, 40, 11–19. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Abd Elwahab, A.H.; Ramadan, B.K.; Schaalan, M.F.; Tolba, A.M. A Novel Role of SIRT1/ FGF-21 in Taurine Protection Against Cafeteria Diet-Induced Steatohepatitis in Rats. Cell. Physiol. Biochem. 2017, 43, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ai, Y.; Niu, X.; Shang, F.; Li, Z.; Liu, H.; Li, W.; Ma, W.; Chen, R.; Wei, T.; et al. Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem. Biol. Interact. 2020, 317, 108972. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, H.; Wang, W.; Jin, H.; Feng, G.; Jia, N. Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem. Biophys. Res. Commun. 2014, 447, 485–489. [Google Scholar] [CrossRef]
- Chou, C.T.; Lin, W.F.; Kong, Z.L.; Chen, S.Y.; Hwang, D.F. Taurine prevented cell cycle arrest and restored neurotrophic gene expression in arsenite-treated SH-SY5Y cells. Amino Acids 2013, 45, 811–819. [Google Scholar] [CrossRef]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Li, X.W.; Gao, H.Y.; Liu, J. The role of taurine in improving neural stem cells proliferation and differentiation. Nutr. Neurosci. 2017, 20, 409–415. [Google Scholar] [CrossRef]
- Han, X.; Chesney, R.W. Knockdown of TauT expression impairs human embryonic kidney 293 cell development. Adv. Exp. Med. Biol. 2013, 776, 307–320. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Wu, W.; Hu, P.; Wang, Q. Taurine enhances mouse cochlear neural stem cell transplantation via the cochlear lateral wall for replacement of degenerated spiral ganglion neurons via sonic hedgehog signaling pathway. Cell Tissue Res. 2019, 378, 49–57. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Xu, L.; Wu, T.; Cui, L.; Xu, D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids 2014, 46, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Castaneda, N.E.; Gonzalez-Corona, J.; Griego, E.; Galvan, E.J.; Ochoa-de la Paz, L.D. Taurine Promotes Differentiation and Maturation of Neural Stem/Progenitor Cells from the Subventricular Zone via Activation of GABA(A) Receptors. Neurochem. Res. 2023, 48, 2206–2219. [Google Scholar] [CrossRef]
- Hernandez-Benitez, R.; Ramos-Mandujano, G.; Pasantes-Morales, H. Taurine stimulates proliferation and promotes neurogenesis of mouse adult cultured neural stem/progenitor cells. Stem Cell Res. 2012, 9, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Huang, H.; Li, Z.; Liu, X.; Fan, W.; Wang, X.; Sun, X.; Zhu, J.; Zhou, H.; Wei, H. Taurine Promotes the Cartilaginous Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells In Vitro. Neurochem. Res. 2017, 42, 2344–2353. [Google Scholar] [CrossRef]
- Miyazaki, T.; Honda, A.; Ikegami, T.; Matsuzaki, Y. The role of taurine on skeletal muscle cell differentiation. Adv. Exp. Med. Biol. 2013, 776, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Elango, R. Tolerable Upper Intake Level for Individual Amino Acids in Humans: A Narrative Review of Recent Clinical Studies. Adv. Nutr. 2023, 14, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Rana, S.K.; Sanders, T.A. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986, 56, 17–27. [Google Scholar] [CrossRef]
- Elshorbagy, A.; Jerneren, F.; Basta, M.; Basta, C.; Turner, C.; Khaled, M.; Refsum, H. Amino acid changes during transition to a vegan diet supplemented with fish in healthy humans. Eur. J. Nutr. 2017, 56, 1953–1962. [Google Scholar] [CrossRef]
- Caine, J.J.; Geracioti, T.D. Taurine, energy drinks, and neuroendocrine effects. Clevel. Clin. J. Med. 2016, 83, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.P.; Charles, R.P.; Redmond, H.P.; Bouchier-Hayes, D.J. Taurine and human nutrition. Clin. Nutr. 1997, 16, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Ki, K.N.; Chung, H.Y. Proximate composition, amino Acid, mineral, and heavy metal content of dried laver. Prev Nutr. Food Sci. 2013, 18, 139–144. [Google Scholar] [CrossRef]
- Purchas, R.W.; Rutherfurd, S.M.; Pearce, P.D.; Vather, R.; Wilkinson, B.H. Concentrations in beef and lamb of taurine, carnosine, coenzyme Q10, and creatine. Meat Sci. 2004, 66, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Q.; Meng, Q.; Yue, C.; Xu, C. Identification and expression of cysteine sulfinate decarboxylase, possible regulation of taurine biosynthesis in Crassostrea gigas in response to low salinity. Sci. Rep. 2017, 7, 5505. [Google Scholar] [CrossRef]
- Vidot, H.; Cvejic, E.; Carey, S.; Strasser, S.I.; McCaughan, G.W.; Allman-Farinelli, M.; Shackel, N.A. Randomised clinical trial: Oral taurine supplementation versus placebo reduces muscle cramps in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2018, 48, 704–712. [Google Scholar] [CrossRef]
- Hladun, O.; Papaseit, E.; Martin, S.; Barriocanal, A.M.; Poyatos, L.; Farre, M.; Perez-Mana, C. Interaction of Energy Drinks with Prescription Medication and Drugs of Abuse. Pharmaceutics 2021, 13, 491. [Google Scholar] [CrossRef]
- Rubio, C.; Camara, M.; Giner, R.M.; Gonzalez-Munoz, M.J.; Lopez-Garcia, E.; Morales, F.J.; Moreno-Arribas, M.V.; Portillo, M.P.; Bethencourt, E. Caffeine, D-glucuronolactone and Taurine Content in Energy Drinks: Exposure and Risk Assessment. Nutrients 2022, 14, 5103. [Google Scholar] [CrossRef]
- McBroom, M.J.; Welty, J.D. Comparison of taurine-verapamil interaction in hamsters and rats. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1985, 80, 217–219. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef]

| Tissue | Content in μmol/L (Liquid) or μmol/g (Solid) |
|---|---|
| Bile | ~200 |
| Plasma | 50–100 |
| Leukocytes and platelets | 10–50 |
| Retina | 30–40 |
| Heart | 6–25 |
| Brain | 0.8–20 |
| Skeletal muscle | 2.2–5.4 |
| Kidney | 1.4–1.8 |
| Liver | 0.3–2 |
| Erythrocytes | 0.05–0.08 |
| Food | Average Content in mg/100 g |
|---|---|
| Pyropia tenera (red algae, seaweed, dried) | 979 |
| Scallops (raw) | 827 |
| Mussels (raw) | 655 |
| Porphyra haitanensis (seaweed, dried) | 646 |
| Clams (raw) | 520 |
| Oysters (raw) | 507 |
| Octopus (raw) | 388 |
| Squid (raw) | 356 |
| Turkey (raw), dark meat | 306 |
| Chicken (raw), dark meat | 169 |
| White fish (raw) | 151 |
| Pork (raw) | 61 |
| Salami (cured) | 59 |
| Ham (baked) | 50 |
| Lamb (raw), dark meat | 47 |
| Beef (raw) | 43 |
| Tuna (canned) | 42 |
| Shrimps (raw) | 39 |
| Goat’s milk (pasteurized) | 6.8 |
| Egg yolk | 3.7 |
| Yogurt | 3.3 |
| Cow’s milk (pasteurized) | 2.4 |
| Ice cream | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santulli, G.; Kansakar, U.; Varzideh, F.; Mone, P.; Jankauskas, S.S.; Lombardi, A. Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients 2023, 15, 4236. https://doi.org/10.3390/nu15194236
Santulli G, Kansakar U, Varzideh F, Mone P, Jankauskas SS, Lombardi A. Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients. 2023; 15(19):4236. https://doi.org/10.3390/nu15194236
Chicago/Turabian StyleSantulli, Gaetano, Urna Kansakar, Fahimeh Varzideh, Pasquale Mone, Stanislovas S. Jankauskas, and Angela Lombardi. 2023. "Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview" Nutrients 15, no. 19: 4236. https://doi.org/10.3390/nu15194236
APA StyleSantulli, G., Kansakar, U., Varzideh, F., Mone, P., Jankauskas, S. S., & Lombardi, A. (2023). Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients, 15(19), 4236. https://doi.org/10.3390/nu15194236







