Preeclampsia and Its Impact on Human Milk Activin A Concentration
Abstract
:1. Introduction
2. Methods
2.1. Setting and Population
2.2. Collection of Human Milk Samples
2.3. Activin A Measurements
2.4. Statistical Methods
3. Results
3.1. Characteristics of the Human Milk Samples
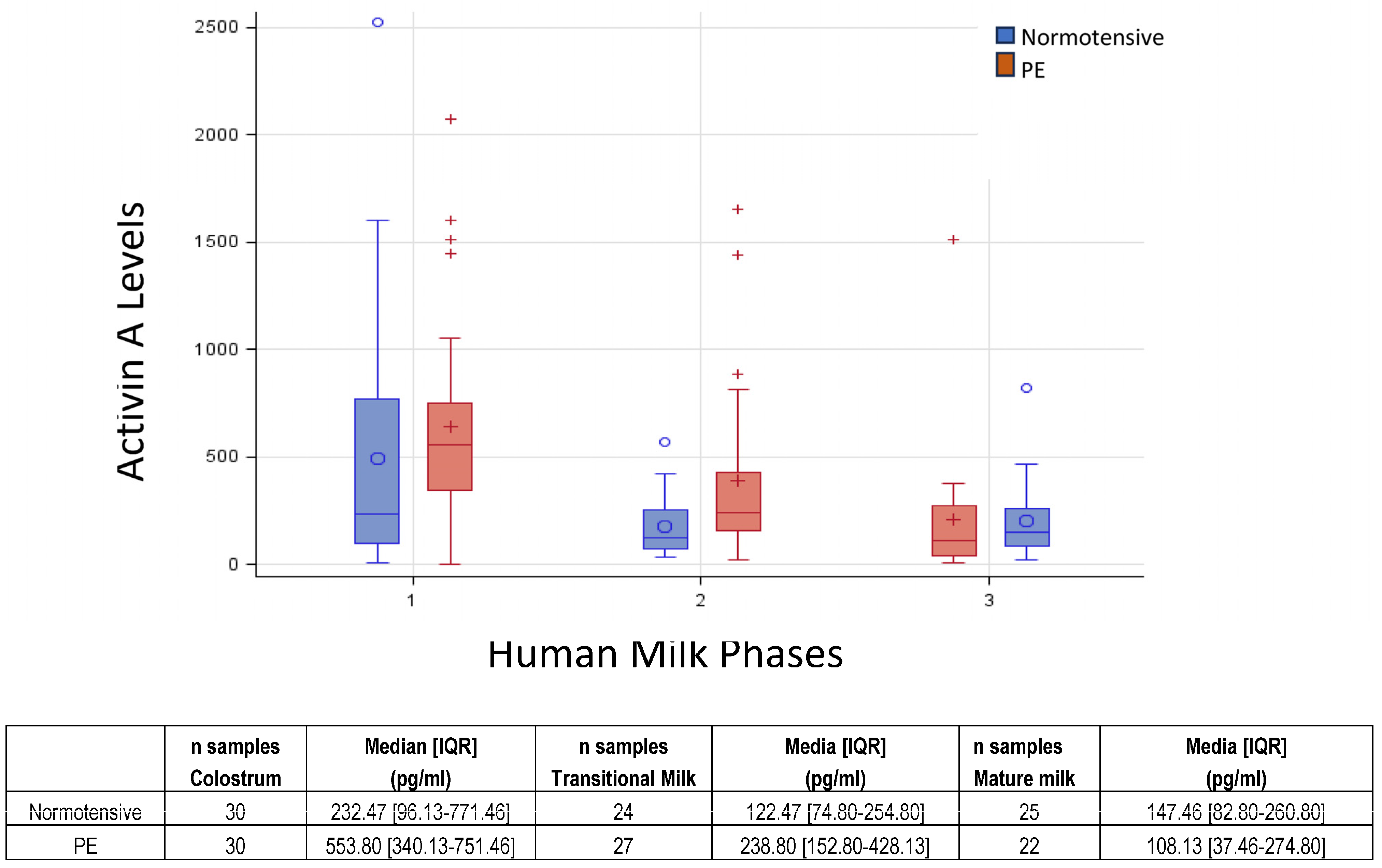
3.2. Activin A Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Uzan, J.; Carbonnel, M.; Piconne, O.; Asmar, R.; Ayoubi, J.M. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc. Health Risk Manag. 2011, 7, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Hod, T.; Cerdeira, A.S.; Karumanchi, S.A. Molecular Mechanisms of Preeclampsia. Cold Spring Harb. Perspect. Med. 2015, 5, a023473. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Markham, K.; Moorehead, P.; Cordero, L.; Nankervis, C.A.; Giannone, P.J. Maternal preeclampsia and neonatal outcomes. J. Pregnancy 2011, 2011, 214365. [Google Scholar] [CrossRef]
- Leitner, Y.; Harel, S.; Geva, R.; Eshel, R.; Yaffo, A.; Many, A. The neurocognitive outcome of IUGR children born to mothers with and without preeclampsia. J. Matern. Fetal Neonatal Med. 2012, 25, 2206–2208. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [CrossRef]
- Horta, B.L.; Victora, C.G. Long-Term Health Effects of Breastfeeding: A Systematic Review; World Health Organization: Geneva, Switzerland, 2013.
- Bar, S.; Milanaik, R.; Adesman, A. Long-term neurodevelopmental benefits of breastfeeding. Curr. Opin. Pediatr. 2016, 28, 559–566. [Google Scholar] [CrossRef]
- Peila, C.; Coscia, A.; Bertino, E.; Li Volti, G.; Galvano, F.; Barbagallo, I.; Visser, G.H.; Gazzolo, D. The Effect of Holder Pasteurization on Activin A Levels in Human Milk. Breastfeed. Med. 2016, 11, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Luisi, S.; Calonaci, G.; Florio, P.; Lombardi, I.; De Felice, C.; Bagnoli, F.; Petraglia, F. Identification of activin A and follistatin in human milk. Growth Factors 2002, 20, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Abella, R.; Frigiola, A.; Giamberti, A.; Tina, G.; Nigro, F.; Florio, P.; Colivicchi, M.; Temporini, F.; Ricotti, A.; et al. Neuromarkers and unconventional biological fluids. J. Matern. Fetal Neonatal Med. 2010, 23 (Suppl. S3), 66–69. [Google Scholar] [CrossRef]
- Tretter, Y.P.; Hertel, M.; Munz, B.; ten Bruggencate, G.; Werner, S.; Alzheimer, C. Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat. Med. 2000, 6, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Serpero, L.D.; Frigiola, A.; Gazzolo, D. Human milk and formulae: Neurotrophic and new biological factors. Early Hum. Dev. 2012, 88 (Suppl. S1), S9–S12. [Google Scholar] [CrossRef] [PubMed]
- Luisi, S.; Florio, P.; Reis, F.M.; Petraglia, F. Expression and secretion of activin A: Possible physiological and clinical implications. Eur. J. Endocrinol. 2001, 145, 225–236. [Google Scholar] [CrossRef]
- Schultz Jel, J.; Witt, S.A.; Glascock, B.J.; Nieman, M.L.; Reiser, P.J.; Nix, S.L.; Kimball, T.R.; Doetschman, T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Investig. 2002, 109, 787–796. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kondo, S.; Sakurai, T.; Etoh, Y.; Shibai, H.; Muramatsu, M. Activin/EDF as an inhibitor of neural differentiation. Biochem. Biophys. Res. Commun. 1990, 173, 193–200. [Google Scholar] [CrossRef]
- Schubert, D.; Kimura, H. Substratum-growth factor collaborations are required for the mitogenic activities of activin and FGF on embryonal carcinoma cells. J. Cell. Biol. 1991, 114, 841–846. [Google Scholar] [CrossRef]
- Iwahori, Y.; Saito, H.; Torii, K.; Nishiyama, N. Activin exerts a neurotrophic effect on cultured hippocampal neurons. Brain Res. 1997, 760, 52–58. [Google Scholar] [CrossRef]
- Wu, D.D.; Lai, M.; Hughes, P.E.; Sirimanne, E.; Gluckman, P.D.; Williams, C.E. Expression of the activin axis and neuronal rescue effects of recombinant activin A following hypoxic-ischemic brain injury in the infant rat. Brain Res. 1999, 835, 369–378. [Google Scholar] [CrossRef]
- Krieglstein, K.; Suter-Crazzolara, C.; Fischer, W.H.; Unsicker, K. TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995, 14, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.E.; Alexi, T.; Williams, C.E.; Clark, R.G.; Gluckman, P.D. Administration of recombinant human Activin-A has powerful neurotrophic effects on select striatal phenotypes in the quinolinic acid lesion model of Huntington’s disease. Neuroscience 1999, 92, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Phillips, D.J.; de Kretser, D.M. Divergent cell-specific effects of activin-A on thymocyte proliferation stimulated by phytohemagglutinin, and interleukin 1beta or interleukin 6 in vitro. Cytokine 2000, 12, 595–602. [Google Scholar] [CrossRef]
- Giguère, Y.; Charland, M.; Bujold, E.; Bernard, N.; Grenier, S.; Rousseau, F.; Lafond, J.; Légaré, F.; Forest, J.C. Combining biochemical and ultrasonographic markers in predicting preeclampsia: A systematic review. Clin. Chem. 2010, 56, 361–375. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef]
- Bertino, E.; Di Nicola, P.; Varalda, A.; Occhi, L.; Giuliani, F.; Coscia, A. Neonatal growth charts. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. S1), 67–69. [Google Scholar] [CrossRef]
- Demir, D.; Demirel Sezer, E.; Turan, V.; Ozturk, S.; Canbay, E.; Yıldırım Sozmen, E.E. How Preeclampsia Affects Oxidant Status and Antiiflammatory Potential of Breast Milk? Free Radic. Biol. Med. 2016, 100, S59. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Dangat, K.; Kilari, A.; Mehendale, S.; Lalwani, S.; Joshi, S. Higher levels of brain derived neurotrophic factor but similar nerve growth factor in human milk in women with preeclampsia. Int. J. Dev. Neurosci. 2013, 31, 209–213. [Google Scholar] [CrossRef]
- Cekmen, M.B.; Balat, A.; Balat, O.; Aksoy, F.; Yurekli, M.; Erbagci, A.B.; Sahinoz, S. Decreased adrenomedullin and total nitrite levels in breast milk of preeclamptic women. Clin. Biochem. 2004, 37, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Erbağci, A.B.; Cekmen, M.B.; Balat, O.; Balat, A.; Aksoy, F.; Tarakçioğlu, M. Persistency of high proinflammatory cytokine levels from colostrum to mature milk in preeclampsia. Clin. Biochem. 2005, 38, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Freitas, N.A.; Santiago, L.T.C.; Kurokawa, C.S.; Meira Junior, J.D.; Corrente, J.E.; Rugolo, L.M.S.S. Effect of preeclampsia on human milk cytokine levels. J. Matern. Fetal Neonatal Med. 2019, 32, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Dangat, K.; Upadhyay, D.; Kilari, A.; Sharma, U.; Kemse, N.; Mehendale, S.; Lalwani, S.; Wagh, G.; Joshi, S.; Jagannathan, N.R. Altered breast milk components in preeclampsia; An in-vitro proton NMR spectroscopy study. Clin. Chim. Acta 2016, 463, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dangat, K.D.; Mehendale, S.S.; Yadav, H.R.; Kilari, A.S.; Kulkarni, A.V.; Taralekar, V.S.; Joshi, S.R. Long-chain polyunsaturated fatty acid composition of breast milk in pre-eclamptic mothers. Neonatology 2010, 97, 190–194. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, X.; Jin, L.; Stringfield, T.; Lin, L.; Chen, Y. Expression profiles of adiponectin receptors in mouse embryos. Gene Expr. Patterns 2005, 5, 711–715. [Google Scholar] [CrossRef]
- Saito, S.; Sakai, M.; Sasaki, Y.; Tanebe, K.; Tsuda, H.; Michimata, T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin. Exp. Immunol. 1999, 117, 550–555. [Google Scholar] [CrossRef]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J. Reprod. Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef]
- Dangat, K.; Kilari, A.; Mehendale, S.; Lalwani, S.; Joshi, S. Preeclampsia alters milk neurotrophins and long chain polyunsaturated fatty acids. Int. J. Dev. Neurosci. 2014, 33, 115–121. [Google Scholar] [CrossRef]
- Peila, C.; Bertino, E.; Cresi, F.; Coscia, A. Interactions between preeclampsia and composition of the human milk: What do we know? J. Matern. Fetal Neonatal Med. 2022, 35, 6219–6225. [Google Scholar] [CrossRef]
| Normotensive N = 46 | Preeclamptic N = 39 | ||
|---|---|---|---|
| Maternal characteristics | |||
| Age (years) | median [IQR] | 33.5 [31–37] | 35 [31–38] |
| Italian | n (%) | 35 (76.1) | 31 (79.5) |
| Caesarian Section | n (%) | 25 (54.4) | 28 (71.8) |
| Weight gain (kg) | mean (SD) | 10.9 (4.75) | 10.4 (5.65) |
| Primigravida | n (%) | 29 (63.0) | 25 (64.0) |
| Smoker | n (%) | 6 (13.0) | 2 (5.1) |
| Newborn characteristics | |||
| Singleton | n (%) | 38 (82.6) | 36 (92.3) |
| IUGR | n (%) | 2 (4.4) | 16 (41.0) |
| GA (weeks) | median [IQR] | 37 [31;39] | 32 [29–35] |
| Girls | n (%) | 19 (41.3) | 19 (48.7) |
| Birth weight (g) | mean (SD) | 2345 (1028) | 1542 (720) |
| Birth weight (z-score) | mean (SD | −0.21 (0.934) | −1.16 (0.810) |
| SGA | n (%) | 6 (13.0) | 19 (50.0) |
| LGA | n (%) | 2 (4.4) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coscia, A.; Riboldi, L.; Spada, E.; Bertino, E.; Sottemano, S.; Barbagallo, I.; Livolti, G.; Galvano, F.; Gazzolo, D.; Peila, C. Preeclampsia and Its Impact on Human Milk Activin A Concentration. Nutrients 2023, 15, 4296. https://doi.org/10.3390/nu15194296
Coscia A, Riboldi L, Spada E, Bertino E, Sottemano S, Barbagallo I, Livolti G, Galvano F, Gazzolo D, Peila C. Preeclampsia and Its Impact on Human Milk Activin A Concentration. Nutrients. 2023; 15(19):4296. https://doi.org/10.3390/nu15194296
Chicago/Turabian StyleCoscia, Alessandra, Lorenzo Riboldi, Elena Spada, Enrico Bertino, Stefano Sottemano, Ignazio Barbagallo, Giovanni Livolti, Fabio Galvano, Diego Gazzolo, and Chiara Peila. 2023. "Preeclampsia and Its Impact on Human Milk Activin A Concentration" Nutrients 15, no. 19: 4296. https://doi.org/10.3390/nu15194296





