Immune System and Epidemics: The Role of African Indigenous Bioactive Substances
Abstract
1. Introduction
2. Literature Search Methodology
3. Results
3.1. African Traditional Fermented Foods
3.1.1. Cereal-Based Fermented Foods
3.1.2. Meat- and Fish-Based Fermented Foods
3.1.3. Dairy Fermented Products
3.2. Herbal Plants for Epidemics and Pandemics
3.2.1. Garcinia kola Heckel (Fam. Clusiaceae) [Bitter Cola]
3.2.2. Artemisia afra Jacq. (Fam. Asteraceae) [African Wormwood]
3.2.3. Piper guineense (Fam. Piperaceae) [African Black Pepper]
3.2.4. Achyranthes Aspera Linn. (Fam. Amaranthaceae)
3.2.5. Allium sativum L. (Fam. Liliaceae) [Garlic]
3.2.6. Moringa oleifera Lam. (Fam. Moringaceae)
3.2.7. Zingiber officinale R. (Fam Zingiberaceae) [Ginger]
3.2.8. Momordica charantia (Fam. Cucurbitaceae) [Bitter Melon]
3.2.9. Curcuma longa L. (Fam. Zingiberaceae) [Tumeric]
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus COVID-19 Dashboard. Available online: https://covid19.who.int (accessed on 8 December 2021).
- WHO. Diseases Cost the African Region $2.4 Trillion A Year, Says Who. 2019. Available online: https://www.afro.who.int/news/diseases-cost-african-region-24-trillion-year-says-who (accessed on 8 December 2021).
- Anaemene, B. Health and Diseases in Africa. In The Development of Africa; Springer: Berlin/Heidelberg, Germany, 2018; pp. 207–226. [Google Scholar]
- WHO. The Environmental Burden of Disease in Africa. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/177155/Synt_R_3.pdf?sequence=3&isAllowed=y (accessed on 17 May 2022).
- Oleribe, O.O.; Suliman, A.A.; Taylor-Robinson, S.D.; Corrah, T. Possible Reasons Why Sub-Saharan Africa Experienced a Less Severe COVID-19 Pandemic in 2020. J. Multidiscip. Healthc. 2021, 14, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Sánchez-Mata, M.; Fernández-Ruiz, V.; Cámara, R.; Cebadera, E.; Domínguez, L. A Review of the Role of Micronutrients and Bioactive Compounds on Immune System Supporting to Fight against the COVID-19 Disease. Foods 2021, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Working Together: An Integration Resource Guide for Immunization Services throughout the Life Course. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/276546/9789241514736-eng.pdf? (accessed on 17 May 2022).
- Achi, O.K.; Asamudo, N.U. Cereal-Based Fermented Foods of Africa as Functional Foods. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 1527–1558. [Google Scholar] [CrossRef]
- Flibert, G.; Abel, T.; Aly, S. African cassava Traditional Fermented Food: The Microorganism’s Contribution to their Nutritional and Safety Values-A Review. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 664–687. [Google Scholar] [CrossRef]
- Phiri, S.; Schoustra, S.E.; Van Den Heuvel, J.; Smid, E.J.; Shindano, J.; Linnemann, A. Fermented Cereal-Based Munkoyo Beverage: Processing Practices, Microbial Diversity and Aroma Compounds. PLoS ONE 2019, 14, e0223501. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Laurent-Babot, C.; Guyot, J.-P. Should Research on the Nutritional Potential and Health Benefits of Fermented Cereals Focus More on the General Health Status of Populations in Developing Countries? Microorganisms 2017, 5, 40. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B12, Folic Acid, and the Immune System. In Nutrition and Immunity; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–114. [Google Scholar]
- Saubade, F.; Hemery, Y.M.; Rochette, I.; Guyot, J.-P.; Humblot, C. Influence of fermentation and other processing steps on the folate content of a traditional African cereal-based fermented food. Int. J. Food Microbiol. 2018, 266, 79–86. [Google Scholar] [CrossRef]
- Bationo, F.; Humblot, C.; Songré-Ouattara, L.T.; Hama-Ba, F.; Le Merrer, M.; Chapron, M.; Kariluoto, S.; Hemery, Y.M. Total folate in West African cereal-based fermented foods: Bioaccessibility and influence of processing. J. Food Compos. Anal. 2019, 85, 103309. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.M.; Guyot, J.-P.; Humblot, C. Lactic acid fermentation as a tool for increasing the folate content of foods. Crit. Rev. Food Sci. Nutr. 2016, 57, 3894–3910. [Google Scholar] [CrossRef]
- Omemu, A.; Oyewole, O.; Bankole, M. Significance of yeasts in the fermentation of maize for ogi production. Food Microbiol. 2007, 24, 571–576. [Google Scholar] [CrossRef]
- Adebo, O.A. African Sorghum-Based Fermented Foods: Past, Current and Future Prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Owusu-Kwarteng, J.; Thorsen, L.; Jespersen, L. Biodiversity and probiotic potential of yeasts isolated from Fura, a West African spontaneously fermented cereal. Int. J. Food Microbiol. 2012, 159, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Kwarteng, J.; Tano-Debrah, K.; Akabanda, F.; Jespersen, L. Technological properties and probiotic potential of Lactobacillus fermentum strains isolated from West African fermented millet dough. BMC Microbiol. 2015, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Greppi, A.; Saubade, F.; Botta, C.; Humblot, C.; Guyot, J.-P.; Cocolin, L. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 2017, 62, 169–177. [Google Scholar] [CrossRef]
- Motey, G.A.; Johansen, P.G.; Owusu-Kwarteng, J.; Ofori, L.A.; Obiri-Danso, K.; Siegumfeldt, H.; Larsen, N.; Jespersen, L. Probiotic potential of Saccharomyces cerevisiae and Kluyveromyces marxianus isolated from West African spontaneously fermented cereal and milk products. Yeast 2020, 37, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Imade, E.E.; Omonigho, S.E.; Babalola, O.O.; Enagbonma, B.J. Lactic acid bacterial bacteriocins and their bioactive properties against food-associated antibiotic-resistant bacteria. Ann. Microbiol. 2021, 71, 1–14. [Google Scholar] [CrossRef]
- Kumar, V.; Sheoran, P.; Gupta, A.; Yadav, J.; Tiwari, S.K. Antibacterial property of bacteriocin produced by Lactobacillus plantarum LD4 isolated from a fermented food. Ann. Microbiol. 2016, 66, 1431–1440. [Google Scholar] [CrossRef]
- Liburdi, K.; Bernini, R.; Esti, M. Fermented Beverages: Geographical Distribution and Bioactive Compounds with Health Benefits. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–151. [Google Scholar]
- Hotessa, N.; Robe, J. Ethiopian Indigenous Traditional Fermented Beverage: The Role of the Microorganisms toward Nutritional and Safety Value of Fermented Beverage. Int. J. Microbiol. 2020, 2020, 8891259. [Google Scholar] [CrossRef]
- Lemi, B.W. Microbiology of Ethiopian Traditionally Fermented Beverages and Condiments. Int. J. Microbiol. 2020, 2020, 1478536. [Google Scholar] [CrossRef]
- Hjortmo, S.B.; Hellström, A.M.; Andlid, T.A. Production of Folates by Yeasts in Tanzanian Fermented Togwa. FEMS Yeast Res. 2008, 8, 781–787. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Kayitesi, E.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Food fermentation and mycotoxin detoxification: An African perspective. Food Control 2019, 106, 106731. [Google Scholar] [CrossRef]
- Corbo, M.R.; Campaniello, D.; Speranza, B.; Altieri, C.; Sinigaglia, M.; Bevilacqua, A. Neutralisation of toxins by probiotics during the transit into the gut: Challenges and perspectives. Int. J. Food Sci. Technol. 2018, 53, 1339–1351. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Ayeni, K.; Misihairabgwi, J.M.; Somorin, Y.; Chibuzor-Onyema, I.E.; Oyedele, O.A.; Abia, W.A.; Sulyok, M.; Shephard, G.S.; Krska, R. Traditionally Processed Beverages in Africa: A Review of the Mycotoxin Occurrence Patterns and Exposure Assessment. Compr. Rev. Food Sci. Food Saf. 2018, 17, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Nafuka, S.N.; Misihairabgwi, J.M.; Bock, R.; Ishola, A.; Sulyok, M.; Krska, R. Variation of Fungal Metabolites in Sorghum Malts Used to Prepare Namibian Traditional Fermented Beverages Omalodu and Otombo. Toxins 2019, 11, 165. [Google Scholar] [CrossRef]
- Chiocchetti, G.M.; Jadán-Piedra, C.; Monedero, V.; Zúñiga, M.; Vélez, D.; Devesa, V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit. Rev. Food Sci. Nutr. 2018, 59, 1534–1545. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Mrvčić, J.; Stanzer, D.; Šolić, E.; Stehlik-Tomas, V. Interaction of lactic acid bacteria with metal ions: Opportunities for improving food safety and quality. World J. Microbiol. Biotechnol. 2012, 28, 2771–2782. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Amadi, C.N.; Frazzoli, C.; Dokubo, A. Nigerian foods of probiotics relevance and chronic metal exposure: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 19285–19297. [Google Scholar] [CrossRef]
- Gagaoua, M.; Boudechicha, H.-R. Ethnic meat products of the North African and Mediterranean countries: An overview. J. Ethn. Foods 2018, 5, 83–98. [Google Scholar] [CrossRef]
- Dullius, A.; Rama, G.R.; Giroldi, M.; Goettert, M.I.; Lehn, D.N.; de Souza, C.F.V. Bioactive Peptide Production in Fermented Foods. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–72. [Google Scholar]
- Ben Belgacem, Z.; Abriouel, H.; Ben Omar, N.; Lucas, R.; Martínez-Canamero, M.; Gálvez, A.; Manai, M. Antimicrobial activity, safety aspects, and some technological properties of bacteriocinogenic Enterococcus faecium from artisanal Tunisian fermented meat. Food Control 2010, 21, 462–470. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns—An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Boudechicha, H.-R.; Nasri, I.; Bennaceur, Z.; Sellama, M.; Hafid, K.; Boudjellal, A.; Gagaoua, M. Microbiological changes during the preparation steps of Khliaa Ezir: A traditional cured meat product of Algeria. Integr. Food Nutr. Metab. 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Benlacheheb, R.; Becila, S.; Sentandreu, A.M.; Hafid, K.; Boudechicha, H.-R.; Boudjellal, A. El Gueddid, a traditional Algerian dried salted meat: Physicochemical, microbiological characteristics and proteolysis intensity during its manufacturing process and ripening. Food Sci. Technol. Int. 2019, 25, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Mostefa, N.; Abid, A.; Boumédiène, K.M. Preliminary probiotic potential of selected aerococcus spp., enterococcus spp., and weisella sp. From algerian guedid. J. Microbiol. Biotechnol. Food Sci. 2021, 10, e2937. [Google Scholar] [CrossRef]
- Bader, R.; Becila, S.; Ruiz, P.; Djeghim, F.; Sanah, I.; Boudjellal, A.; Gatellier, P.; Portanguen, S.; Talon, R.; Leroy, S. Physicochemical and microbiological characteristics of El-Guedid from meat of different animal species. Meat Sci. 2021, 171, 108277. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef]
- Pilevar, Z.; Hosseini, H.; Beikzadeh, S.; Khanniri, E.; Alizadeh, A.M. Application of Bacteriocins in Meat and Meat Products: An Update. Curr. Nutr. Food Sci. 2020, 16, 120–133. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef]
- Anihouvi, V.B.; Kindossi, J.M.; Hounhouigan, J.D. Processing and Quality Characteristics of Some Major Fermented Fish Products from Africa: A Critical Review. Int. Res. J. Biol. Sci. 2012, 1, 72–84. [Google Scholar]
- Kindossi, J.M.; Anihouvi, V.B.; Vieira-Dalodé, G.; Akissoé, N.H.; Hounhouigan, D.J. Optimization of the marinating conditions of cassava fish ( Pseudotolithus sp.) fillet for Lanhouin production through application of Doehlert experimental design. Food Sci. Nutr. 2015, 4, 261–268. [Google Scholar] [CrossRef]
- Anihouvi, D.G.H.; Kpoclou, Y.E.; Massih, M.A.; Afé, O.H.I.; Assogba, M.F.; Covo, M.; Scippo, M.; Hounhouigan, D.J.; Anihouvi, V.; Mahillon, J. Microbiological characteristics of smoked and smoked-dried fish processed in Benin. Food Sci. Nutr. 2019, 7, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Fall, M.; Diop, M.B.; Montet, D.; Maiga, A.S.; Guiro, A.T. Fermentation Du Poisson En Afrique de l’Ouest et Défis Sociétaux Pour Une Amélioration Qualitative Des Produits (Adjuevan, Guedj et Lanhouin): Revue de La Littérature. Cah. Agric. 2019, 28, 7. [Google Scholar] [CrossRef]
- Xu, Y.; Zang, J.; Regenstein, J.M.; Xia, W. Technological roles of microorganisms in fish fermentation: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Sanni, A.I.; Asiedu, M.; Ayernor, G.S. Microflora and Chemical Composition of Momoni, a Ghanaian Fermented Fish Condiment. J. Food Compos. Anal. 2002, 15, 577–583. [Google Scholar] [CrossRef]
- Farag, M.A.; Zain, A.E.; Hariri, M.L.; El Aaasar, R.; Khalifa, I.; Elmetwally, F. Potential food safety hazards in fermented and salted fish in Egypt (Feseekh, Renga, Moloha) as case studies and controlling their manufacture using HACCP system. J. Food Saf. 2022, 42, e12973. [Google Scholar] [CrossRef]
- Anihouvi, V.; Sakyi-Dawson, E.; Ayernor, G.; Hounhouigan, J. Microbiological changes in naturally fermented cassava fish (Pseudotolithus sp.) for lanhouin production. Int. J. Food Microbiol. 2007, 116, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Koffi-Nevr, R.; Ouina, T.; Koussemon, M.; Brou, K. Chemical Composition and Lactic Microflora of Adjuevan, A Traditional Ivorian Fermented Fish Condiment. Pak. J. Nutr. 2011, 10, 332–337. [Google Scholar] [CrossRef]
- Kouakou, A.C.; N’Guessan, K.F.; Durand, N.; Thomas, D.A.; Montet, D.; Djè, M.K. Molecular bacterial characterization and free amino acids composition in Ivorian traditional fermented fish produced by two methods. Fish. Sci. 2012, 78, 1125–1136. [Google Scholar] [CrossRef]
- Clémentine, K.-K.A.; Florent, N.G.K.; Solange, A.; Didier, M.; Marcellin, D.K. Salt concentration effect on yeast diversity of the Ivorian traditional fermented fish adjuevan. GSC Biol. Pharm. Sci. 2020, 13, 087–094. [Google Scholar] [CrossRef]
- Lara-Hidalgo, C.E.; Hernández-Sánchez, H.; Hernández-Rodríguez, C.; Dorantes-Álvarez, L. Yeasts in Fermented Foods and Their Probiotic Potential. Austin J. Nutr. Metab. 2017, 4, 1045. [Google Scholar]
- Cheng, R.; Mantovani, A.; Frazzoli, C. Analysis of Food Safety and Security Challenges in Emerging African Food Producing Areas through a One Health Lens: The Dairy Chains in Mali. J. Food Prot. 2016, 80, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Frazzoli, C. Field anthropological research for context-effective risk analysis science in traditional cultures: The case of Senegal. J. Glob. Health Rep. 2020, 4, e2020043. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. In vitro and in vivo antioxidant potential of milks, yoghurts, fermented milks and cheeses: A narrative review of evidence. Nutr. Res. Rev. 2017, 31, 52–70. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef]
- Liu, Y.; Van Bennekom, E.O.; Zhang, Y.; Abee, T.; Smid, E.J. Long-chain vitamin K2 production in Lactococcus lactis is influenced by temperature, carbon source, aeration and mode of energy metabolism. Microb. Cell Factories 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Walther, B.; Karl, J.P.; Booth, S.L.; Boyaval, P. Menaquinones, Bacteria, and the Food Supply: The Relevance of Dairy and Fermented Food Products to Vitamin K Requirements. Adv. Nutr. Int. Rev. J. 2013, 4, 463–473. [Google Scholar] [CrossRef]
- Mattiello, S.; Caroprese, M.; Matteo, C.G.; Fortina, R.; Martini, A.; Martini, M.; Parisi, G.; Russo, C.; Zecchini, M. ASPA Commission “Animal Productions in Development Cooperation Projects” Typical dairy products in Africa from local animal resources. Ital. J. Anim. Sci. 2017, 17, 740–754. [Google Scholar] [CrossRef]
- Jans, C.; Meile, L.; Kaindi, D.W.M.; Kogi-Makau, W.; Lamuka, P.; Renault, P.; Kreikemeyer, B.; Lacroix, C.; Hattendorf, J.; Zinsstag, J.; et al. African fermented dairy products—Overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 2017, 250, 27–36. [Google Scholar] [CrossRef]
- Agyei, D.; Owusu-Kwarteng, J.; Akabanda, F.; Akomea-Frempong, S. Indigenous African fermented dairy products: Processing technology, microbiology and health benefits. Crit. Rev. Food Sci. Nutr. 2019, 60, 991–1006. [Google Scholar] [CrossRef]
- Uzeh, R.E.; Ohenhen, R.E.; Rojugbokan, A.K. Microbiological and Nutritional Qualities of Dairy Products: Nono and Wara. Nat. Sci. 2006, 4, 37–40. [Google Scholar]
- Awad, S. Microbial safety criteria and quality of traditional Egyptian Karish cheese. Afr. J. Microbiol. Res. 2016, 10, 804–812. [Google Scholar] [CrossRef]
- Nduko, J.M.; Matofari, J.W.; Nandi, Z.O.; Sichangi, M.B. Spontaneously Fermented Kenyan Milk Products: A Review of the Current State and Future Perspectives. Afr. J. Food Sci. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Maleke, M.S.; Adefisoye, M.A.; Doorsamy, W.; Adebo, O.A. Processing, nutritional composition and microbiology of amasi: A Southern African fermented milk product. Sci. Afr. 2021, 12, e00795. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Ayeni, K.I.; Ogunremi, O.R.; Adeleke, R.A.; Oguntoyinbo, F.A.; Warth, B.; Ezekiel, C.N. A review of microbes and chemical contaminants in dairy products in sub-Saharan Africa. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1188–1220. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D. Bacteriocin production by Lactobacillus plantarum AMA-K isolated from Amasi, a Zimbabwean fermented milk product and study of the adsorption of bacteriocin AMA-K to Listeria sp. Braz. J. Microbiol. 2008, 39, 178–187. [Google Scholar] [CrossRef]
- Mosbah, S.; Boudjenah-Haroun, S.; Eddoud, A.; Boual, Z. Therapeutic Aptitude of Fermented Camel Milk: Case of Antimicrobial Activity of Whey Proteins. Int. J. Agric. Biol. Eng. 2018, 1, 19–30. [Google Scholar]
- Muthukumaran, M.S.; Mudgil, P.; Baba, W.N.; Ayoub, M.A.; Maqsood, S. A comprehensive review on health benefits, nutritional composition and processed products of camel milk. Food Rev. Int. 2022, 1–37. [Google Scholar] [CrossRef]
- Behrouz, S.; Saadat, S.; Memarzia, A.; Sarir, H.; Folkerts, G.; Boskabady, M.H. The Antioxidant, Anti-Inflammatory and Immunomodulatory Effects of Camel Milk. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Nyamakwere, F.; Esposito, G.; Dzama, K.; Raffrenato, E. A review of artisanal cheese making: An African perspective. S. Afr. J. Anim. Sci. 2021, 51, 296–309. [Google Scholar] [CrossRef]
- Abdelfatah, E.N. Identification of Lactic Acid Bacteria in Raw Milk and Kariesh Cheese with Special Reference to Lactococcus garvieae. J. Food Nutr. Sci. 2015, 3, 203. [Google Scholar] [CrossRef]
- Olajugbagbe, T.E.; Elugbadebo, O.E.; Omafuvbe, B.O. Probiotic potentials of Pediococuss acidilactici isolated from wara; A Nigerian unripened soft cheese. Heliyon 2020, 6, e04889. [Google Scholar] [CrossRef] [PubMed]
- Seini, S.H.; Keita, A.; Sabiou, M.S.M.; Nafiou, A.I.M.; Maazou, B.A.; Sadou, H.; Ibrahim, A.; Issa, O.; Alma, M.M.; Sidikou, R.S.; et al. Microbiological Characteristics and Nutritional Quality of Traditional Tchoukou Cheese from Niger. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 317–328. [Google Scholar] [CrossRef]
- Doumbouya, I.; Owino, O.; Omolo, K. Probiotic properties of lactic acid bacteria isolated from “tchoukou” traditional milk cheeses produced in selected region of niger. Int. J. Food Sci. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Guetouache, M.; Guessas, B. Characterization and identification of lactic acid bacteria isolated from traditional cheese (Klila) prepared from cows milk. Afr. J. Microbiol. Res. 2015, 9, 71–77. [Google Scholar] [CrossRef]
- Marino, V.M.; Belbeldi, A.; La Terra, S.; Manenti, M.; Licitra, G.; Carpino, S. Survey of Fat Soluble Antioxidants, Linolenic Acid and Conjugated Linoleic Acid Content of Traditional Algerian Bouhezza Cheese. J. Food Agric. Environ. 2012, 10, 186–190. [Google Scholar]
- Ekene, E.N. Garcinia Kola: A Review of Its Ethnomedicinal, Chemical and Pharmacological Properties. Int. J. Curr. Res. Rev. 2014, 6, 1. [Google Scholar]
- Maňourová, A.; Leuner, O.; Tchoundjeu, Z.; Van Damme, P.; Verner, V.; Přibyl, O.; Lojka, B. Medicinal Potential, Utilization and Domestication Status of Bitter Kola (Garcinia kola Heckel) in West and Central Africa. Forests 2019, 10, 124. [Google Scholar] [CrossRef]
- Seanego, C.T.; Ndip, R.N. Identification and Antibacterial Evaluation of Bioactive Compounds from Garcinia kola (Heckel) Seeds. Molecules 2012, 17, 6569–6584. [Google Scholar] [CrossRef]
- Konziase, B. Protective activity of biflavanones from Garcinia kola against Plasmodium infection. J. Ethnopharmacol. 2015, 172, 214–218. [Google Scholar] [CrossRef]
- Kalu, W.; Okafor, P.; Ijeh, I.; Eleazu, C. Effect of kolaviron, a biflavanoid complex from Garcinia kola on some biochemical parameters in experimentally induced benign prostatic hyperplasic rats. Biomed. Pharmacother. 2016, 83, 1436–1443. [Google Scholar] [CrossRef]
- BC, I.C.; Maduka Tochukwu, O.D.; Enyoh, C.E.; JM, I.U. Potential Plants for Treatment and Management of COVID-19 in Nigeria. Acad. J. Chem. 2020, 5, 69–80. [Google Scholar]
- Oladele, J.O.; Ajayi, E.I.; Oyeleke, O.M.; Oladele, O.T.; Olowookere, B.D.; Adeniyi, B.M.; Oyewole, O.I.; Oladiji, A.T. A systematic review on COVID-19 pandemic with special emphasis on curative potentials of Nigeria based medicinal plants. Heliyon 2020, 6, e04897. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, O.A.; Femi-Oyewo, M.N.; Bamiro, O.A.; Bakre, L.G.; Alabi, A.; Ashidi, J.S.; Balogun-Agbaje, O.A.; Hassan, O.M.; Fakoya, G. Ethnomedicinal herbs in African traditional medicine with potential activity for the prevention, treatment, and management of coronavirus disease 2019. Futur. J. Pharm. Sci. 2021, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Awogbindin, I.O.; Olaleye, D.O.; Farombi, E.O. Kolaviron Improves Morbidity and Suppresses Mortality by Mitigating Oxido-Inflammation in BALB/c Mice Infected with Influenza Virus. Viral Immunol. 2015, 28, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Obi, R.; Olayinka, A.; Adesegun, S. The antiviral activities of Garcinia kola (Heckel.) and Azadirachta indica (A. Juss.) on viruses of public health importance in Nigeria. Int. J. Infect. Dis. 2020, 101, 119. [Google Scholar] [CrossRef]
- Nworu, C.S.; Akah, P.A.; Esimone, C.O.; Okoli, C.O.; Okoye, F.B.C. Immunomodulatory Activities of Kolaviron, a Mixture of Three Related Biflavonoids of Garcinia Kola Heckel. Immunopharmacol. Immunotoxicol. 2008, 30, 317–332. [Google Scholar] [CrossRef]
- Ukaoma, A.A.; Okechukwu, R.I.; Ukaoma, V.O.; Iwuagwu, M. Phytochemical screening and antibacterial properties of Garcinia kola. J. Phytopharm. 2013, 2, 34–38. [Google Scholar] [CrossRef]
- Farombi, E.O. African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr. J. Biotechnol. 2003, 2, 662–671. [Google Scholar] [CrossRef]
- Adaramoye, O.; Awogbindin, I.; Okusaga, J. Effect of Kolaviron, a Biflavonoid Complex from Garcinia kola Seeds, on Ethanol-Induced Oxidative Stress in Liver of Adult Wistar Rats. J. Med. Food 2009, 12, 584–590. [Google Scholar] [CrossRef]
- Farombi, E.O.; Shrotriya, S.; Surh, Y.-J. Kolaviron Inhibits Dimethyl Nitrosamine-Induced Liver Injury by Suppressing COX-2 and INOS Expression via NF-ΚB and AP-1. Life Sci. 2009, 84, 149–155. [Google Scholar] [CrossRef]
- Farombi, E.O.; Owoeye, O. Antioxidative and Chemopreventive Properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Health 2011, 8, 2533–2555. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Kumar, N.V.A.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef] [PubMed]
- Okoye, T.C.; Uzor, P.F.; Onyeto, C.A.; Okereke, E.K. Safe African Medicinal Plants for Clinical Studies. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 535–555. [Google Scholar]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial Constituents of Artemisia Afra Jacq. Ex Willd. against Periodontal Pathogens. Evid. Based Complement Alternat. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef] [PubMed]
- Moyo, P.; Kunyane, P.; Selepe, M.A.; Eloff, J.N.; Niemand, J.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.-M. Bioassay-guided isolation and identification of gametocytocidal compounds from Artemisia afra (Asteraceae). Malar. J. 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.; Rao, R. Antiviral and Immunomodulation Effects of Artemisia. Medicina 2021, 57, 217. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, J.; Xing, Y.-Q. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed. Pharmacother. 2017, 85, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. Ebiomedicine 2014, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer Activity of Artemisinin and its Derivatives. Anticancer. Res. 2017, 37, 5995–6003. [Google Scholar] [CrossRef]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The Antiviral Activities of Artemisinin and Artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Uzun, T.; Toptas, O. Artesunate: Could Be an Alternative Drug to Chloroquine in COVID-19 Treatment? Chin. Med. 2020, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. A potential treatment of COVID-19 with TGF-β blockade. Int. J. Biol. Sci. 2020, 16, 1954–1955. [Google Scholar] [CrossRef] [PubMed]
- Ene-Obong, H.; Onuoha, N.; Aburime, L.; Mbah, O. Chemical composition and antioxidant activities of some indigenous spices consumed in Nigeria. Food Chem. 2018, 238, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ajibesin, K.; Umoh, U.; Bala, D. The use of medicinal plants to treat sexually transmitted diseases in Nigeria: Ethnomedicinal survey of Niger Delta Region. Int. J. Green Pharm. 2011, 5, 181. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Oben, J.E.; Ngogang, J.Y. In Vitro Antioxidant Activity of Three Piper Species. J. Herb. Pharmacother. 2008, 7, 49–64. [Google Scholar] [CrossRef]
- Umadevi, P.; Deepti, K.; Venugopal, D.V.R. Synthesis, anticancer and antibacterial activities of piperine analogs. Med. Chem. Res. 2013, 22, 5466–5471. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Fyhrquist, P.; Vuorela, H.; Julkunen-Tiitto, R.; Holm, Y. Alkaloid-Rich Crude Extracts, Fractions and Piperamide Alkaloids of Piper guineense Possess Promising Antibacterial Effects. Antibiotics 2018, 7, 98. [Google Scholar] [CrossRef]
- Philipova, I.; Valcheva, V.; Mihaylova, R.; Mateeva, M.; Doytchinova, I.; Stavrakov, G. Synthetic piperine amide analogs with antimycobacterial activity. Chem. Biol. Drug Des. 2017, 91, 763–768. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials. Antibiotics 2019, 8, 55. [Google Scholar] [CrossRef]
- Kim, N.; Do, J.; Bae, J.; Jin, H.K.; Kim, J.-H.; Inn, K.-S.; Oh, M.S.; Lee, J.K. Piperlongumine Inhibits Neuroinflammation via Regulating NF-ΚB Signaling Pathways in Lipopolysaccharide-Stimulated BV2 Microglia Cells. J. Pharmacol. Sci. 2018, 137, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Singh, V. A Census of P. Longum’s Phytochemicals and Their Network Pharmacological Evaluation for Identifying Novel Drug-like Molecules against Various Diseases, with a Special Focus on Neurological Disorders. PLoS ONE 2018, 13, e0191006. [Google Scholar] [CrossRef] [PubMed]
- Osho, I.B.; Adebayo, I.A.; Ajayi, O.I. Immunological Evaluation of Antiviral Activity of Methanolic Extract of Piper Guineense against Newcastle Disease in Experimentally Infected Broiler Chickens. Int. J. Mol. Vet. Res. 2016, 6, 2. [Google Scholar] [CrossRef]
- Sharma, V.; Chaudhary, U. An Overview on Indigenous Knowledge of Achyranthes Aspera. J. Crit. Rev. 2015, 2, 7–19. [Google Scholar]
- Mukherjee, H.; Ojha, D.; Bag, P.; Chandel, H.S.; Bhattacharyya, S.; Chatterjee, T.K.; Mukherjee, P.K.; Chakraborti, S.; Chattopadhyay, D. Anti-herpes virus activities of Achyranthes aspera: An Indian ethnomedicine, and its triterpene acid. Microbiol. Res. 2013, 168, 238–244. [Google Scholar] [CrossRef]
- Ajibesin, K.; René, N.; Bala, D.; Essiett, U. Antimicrobial Activities of the Extracts and Fractions of Allanblackia floribunda. Biotechnol. 2008, 7, 129–133. [Google Scholar] [CrossRef][Green Version]
- Edwin, S.; Jarald, E.E.; Deb, L.; Jain, A.; Kinger, H.; Dutt, K.; Raj, A.A. Wound Healing and Antioxidant Activity of Achyranthes aspera. Pharm. Biol. 2008, 46, 824–828. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Srivastava, P.K.; Verma, N.; Sharma, J. Effect of seeds of Achyranthes aspera on the immune responses and expression of some immune-related genes in carp Catla catla. Fish Shellfish. Immunol. 2014, 41, 64–69. [Google Scholar] [CrossRef]
- Rao, A.; Balachandran, B. Role of Oxidative Stress and Antioxidants in Neurodegenerative Diseases. Nutr. Neurosci. 2002, 5, 291–309. [Google Scholar] [CrossRef]
- Abi Beaulah, G.; Mohamed Sadiq, A.; Jaya Santhi, R. Antioxidant and Antibacterial Activity of Achyranthes Aspera: An in Vitro Study. Ann. Biol. Res. 2011, 2, 662–670. [Google Scholar]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Brantner, A.; Mukainaka, T.; Nobukuni, Y.; Kuchide, M.; Konoshima, T.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein–Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2002, 177, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Tandon, S. Achyranthes aspera Root Extracts Induce Human Colon Cancer Cell (COLO-205) Death by Triggering the Mitochondrial Apoptosis Pathway and S Phase Cell Cycle Arrest. Sci. World J. 2014, 2014, 129697. [Google Scholar] [CrossRef]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S. Pandemics and Traditional Plant-Based Remedies. A Historical-Botanical Review in the Era of COVID19. Front. Plant Sci. 2020, 11, 571042. [Google Scholar] [CrossRef]
- Otunola, A.G.; Asowata-Ayodele, A.M.; Afolayan, A.J. Assessment of the polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of acetone and aqueous extracts of Lippia javanica (Burm.F.) spreng. Pharmacogn. Mag. 2016, 12, 353–362. [Google Scholar] [CrossRef]
- Tijani, K.B.; Alfa, A.A.; Sezor, A.A. Studies on Phytochemical, Nutraceutical Profiles and Potential Medicinal Values of Allium sativum Linn (Lilliaceae) on Bacterial Meningitis. Int. Neuropsychiatr. Dis. J. 2019, 13, 1–15. [Google Scholar] [CrossRef]
- Sulaiman, F.A.; Kazeem, M.O.; Waheed, A.M.; Temowo, S.O.; Azeez, I.O.; Zubair, F.I.; Adeyemi, T.A.; Nyang, A.; Adeyemi, O.S. Antimicrobial and Toxic Potential of Aqueous Extracts of Allium Sativum, Hibiscus Sabdariffa and Zingiber Officinale in Wistar Rats. J. Taibah Univ. Sci. 2014, 8, 315–322. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Mphuthi, D.D.; Husaini, D.C. Traditional medicinal plants used by hypertensive patients in Belize: A qualitative evaluation of beliefs and practices. Bull. Natl. Res. Cent. 2022, 46, 107. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The Antioxidant Mechanisms Underlying the Aged Garlic Extract- and S-Allylcysteine-Induced Protection. Oxidative Med. Cell. Longev. 2012, 2012, 907162. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Abenavoli, L.; Borrelli, F.; Capasso, R.; Izzo, A.A.; Lembo, F.; Romano, B.; Capasso, F. Garlic: Empiricism or Science? Nat. Prod. Commun. 2009, 4, 1934578X0900401231. [Google Scholar] [CrossRef]
- Badal, D.S.; Dwivedi, A.K.; Kumar, V.; Singh, S.; Prakash, A.; Verma, S.; Kumar, J. Effect of Organic Manures and Inorganic Fertilizers on Growth, Yield and Its Attributing Traits in Garlic (Allium Sativum L.). J. Pharmacogn. Phytochem. 2019, 8, 587–590. [Google Scholar]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological Effects of Allium Species Grown in Iraq. An Overview. Int. J. Pharm. Health Care Res. 2013, 1, 132–147. [Google Scholar]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evidence-Based Complement. Altern. Med. 2017, 2017, 7821095. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, G.L.; Elewa, Y.H.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium Sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Inamdar, M.N. Pharmacodynamic and Pharmacokinetic Interactions of Propranolol with Garlic (Allium sativum) in Rats. Evidence-Based Complement. Altern. Med. 2011, 2011, 824042. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Ebadi, A.G. Antibacterial Effect of Garlic (Allium Sativum L.) on Staphylococcus Aureus. Pak. J. Biol. Sci. 2006, 9, 1577–1579. [Google Scholar] [CrossRef][Green Version]
- Jang, H.-J.; Lee, H.-J.; Yoon, D.-K.; Ji, D.-S.; Kim, J.-H.; Lee, C.-H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2017, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Shaheen, H.M.; Abushouk, A.I.; Toraih, E.A.; Fawzy, M.S.; Alansari, W.S.; Aleya, L.; Bungau, S. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ. Sci. Pollut. Res. 2018, 25, 23909–23916. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Dou, C.; Li, N.; Kang, F.; Wang, Y.; Cao, Z.; Yang, X.; Dong, S. Alliin Attenuated RANKL-Induced Osteoclastogenesis by Scavenging Reactive Oxygen Species through Inhibiting Nox1. Int. J. Mol. Sci. 2016, 17, 1516. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Nicco, C.; Batteux, F.; Slusarenko, A.J. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria: Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.R.; Wilson, P. Antibacterial Activity of a New, Stable, Aqueous Extract of Allicin against Methicillin-Resistant Staphylococcus Aureus. Br. J. Biomed. Sci. 2004, 61, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Iwai, A.; Yano, T. Effect of red pepper Capsicum annuum var. conoides and garlic Allium sativum on plasma lipid levels and cecal microflora in mice fed beef tallow. Food Chem. Toxicol. 2004, 42, 1695–1700. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, J.; Jain, M.; Yadav, P.; Goel, A.; Yadava, P.K. Bactericidal Efficacy of Allium sativum (garlic) Against Multidrug Resistant Vibrio cholerae O1 Epidemic Strains. Def. Sci. J. 2016, 66, 479–484. [Google Scholar] [CrossRef][Green Version]
- Razis, A.F.A.; Ibrahim, M.D.; Kntayya, S.B. Health Benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, Nutritional, and Therapeutic Significance of Moringa Oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef] [PubMed]
- Mashamaite, C.V.; Pieterse, P.J.; Mothapo, P.N.; Phiri, E.E. Moringa oleifera in South Africa: A review on its production, growing conditions and consumption as a food source. S. Afr. J. Sci. 2021, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nouman, W.; Siddiqui, M.T.; BASRA, S.; AHMED, M.; FAROOQ, H.; ZUBAIR, M.; GULL, T. Biomass Production and Nutritional Quality of Moringa Oleifera as a Field Crop. Turk. J. Agric. For. 2013, 37, 410–419. [Google Scholar] [CrossRef]
- El-Hak, H.N.; Moustafa, A.R.A.; Mansour, S.R. Toxic effect of Moringa peregrina seeds on histological and biochemical analyses of adult male Albino rats. Toxicol. Rep. 2017, 5, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwal-Okeng, J.W. Phytochemicals and Uses of Moringa Oleifera Leaves in Ugandan Rural Communities. J. Med. Plant Res. 2010, 4, 753–757. [Google Scholar] [CrossRef]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef]
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.; Dey, A. Moringa oleifera Lam. and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2019, 129, 272–282. [Google Scholar] [CrossRef]
- Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Zhang, M.; Liang, N.; Li, C.; He, Z. Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 2021, 95, 107561. [Google Scholar] [CrossRef]
- Otunola, G.A.; Afolayan, A.J. Antidiabetic Effect of Combined Spices of Allium Sativum, Zingiber Officinale and Capsicum Frutescens in Alloxan-Induced Diabetic Rats. Front. Life Sci. 2015, 8, 314–323. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Babu, K.N. Ginger: The Genus Zingiber; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Sowley, E.N.K.; Kankam, F. Harnessing the Therapeutic Properties of Ginger (Zingiber officinale Roscoe) for the Management of Plant Diseases. In Ginger Cultivation and Its Antimicrobial and Pharmacological Potentials; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Dhanik, J.; Arya, N.; Nand, V. A Review on Zingiber Officinale. J. Pharmacogn. Phytochem. 2017, 6, 174–184. [Google Scholar]
- Taoheed, A.A.; Tolulope, A.A.; Saidu, A.B.; Odewumi, O.G.; Sunday, R.M.; Usman, M. Phytochemical Properties, Proximate and Mineral Composition of Curcuma longa Linn. and Zingiber officinale Rosc.: A Comparative Study. J. Sci. Res. Rep. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Sombie, E.N.; Tibiri, A.; N’Do, J.Y.-P.; Traore, T.K.; Ouedraogo, N.; Hilou, A.; Guissou, P.I.; Nacoulma, O.G. Ethnobotanical study and antioxidant activity of anti-hepatitis plants extracts of the COMOE province, Burkina Faso. Int. J. Biol. Chem. Sci. 2018, 12, 1308. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.V.; Murthy, P.S.; Manjunatha, J.; Bettadaiah, B. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014, 159, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Suekawa, M.; Ishige, A.; Yuasa, K.; Sudo, K.; Aburada, M.; Hosoya, E. Pharmacological studies on Ginger. I. Pharmacological actions of pungent constituents, (6)-gingerol and (6)-shogaol. J. Pharmacobio-Dyn. 1984, 7, 836–848. [Google Scholar] [CrossRef]
- Mbadiko, C.M.; Inkoto, C.L.; Gbolo, B.Z.; Lengbiye, E.M.; Kilembe, J.T.; Matondo, A.; Mwanangombo, D.T.; Ngoyi, E.M.; Bongo, G.N.; Falanga, C.M.; et al. A Mini Review on the Phytochemistry, Toxicology and Antiviral Activity of Some Medically Interesting Zingiberaceae Species. J. Complement. Altern. Med Res. 2020, 9, 44–56. [Google Scholar] [CrossRef]
- Chang, J.S.; Wang, K.C.; Yeh, C.F.; Shieh, D.E.; Chiang, L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 145, 146–151. [Google Scholar] [CrossRef]
- Rathinavel, T.; Palanisamy, M.; Srinivasan, P.; Subramanian, A.; Thangaswamy, S. Phytochemical 6-Gingerol -A promising Drug of choice for COVID-19. Int. J. Adv. Sci. Eng. 2020, 6, 1482–1489. [Google Scholar] [CrossRef]
- Kabuto, H.; Nishizawa, M.; Tada, M.; Higashio, C.; Shishibori, T.; Kohno, M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] Prevents 6-Hydroxydopamine-induced Dopamine Depression in Mouse Striatum and Increases Superoxide Scavenging Activity in Serum. Neurochem. Res. 2005, 30, 325–332. [Google Scholar] [CrossRef]
- Asnani, V.; Verma, R.J. Antioxidative Effect of Rhizome of Zingiber Officinale on Paraben Induced Lipid Peroxidation: An in Vitro Study. Acta Pol. Pharm. 2007, 64, 35–37. [Google Scholar] [PubMed]
- Kim, J.-K.; Kim, Y.; Na, K.-M.; Surh, Y.-J.; Kim, T.-Y. (6)-Gingerol Prevents UVB-Induced ROS Production and COX-2 Expression in Vitro and in Vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whelan, R.; Pattnaik, B.; Ludwig, K.; Subudhi, E.; Rowland, H.; Claussen, N.; Zucker, N.; Uppal, S.; Kushner, D.M.; et al. Terpenoids from Zingiber officinale (Ginger) Induce Apoptosis in Endometrial Cancer Cells through the Activation of p53. PLoS ONE 2012, 7, e53178. [Google Scholar] [CrossRef]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, A.S.; Aneja, R. Ginger Phytochemicals Exhibit Synergy to Inhibit Prostate Cancer Cell Proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Turgeon, D.K.; Wright, B.D.; Sidahmed, E.; Ruffin, M.T.; Brenner, D.E.; Sen, A.; Zick, S.M. Effect of ginger root on cyclooxygenase-1 and 15-hydroxyprostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur. J. Cancer Prev. 2013, 22, 455–460. [Google Scholar] [CrossRef]
- Ling, H.; Yang, H.; Tan, S.-H.; Chui, W.-K.; Chew, E.-H. 6-Shogaol, an Active Constituent of Ginger, Inhibits Breast Cancer Cell Invasion by Reducing Matrix Metalloproteinase-9 Expression via Blockade of Nuclear Factor-ΚB Activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef]
- Kim, E.-C.; Min, J.-K.; Kim, T.-Y.; Lee, S.-J.; Yang, H.-O.; Han, S.; Kim, Y.-M.; Kwon, Y.-G. (6)-Gingerol, a Pungent Ingredient of Ginger, Inhibits Angiogenesis in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308. [Google Scholar] [CrossRef]
- Flynn, D.L.; Rafferty, M.F.; Boctor, A.M. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: Inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot. Med. 1986, 22, 357–360. [Google Scholar] [CrossRef]
- Kiuchi, F.; Iwakami, S.; Shibuya, M.; Hanaoka, F.; Sankawa, U. Inhibition of Prostaglandin and Leukotriene Biosynthesis by Gingerols and Diarylheptanoids. Chem. Pharm. Bull. 1992, 40, 387–391. [Google Scholar] [CrossRef]
- Beloin, N.; Gbeassor, M.; Akpagana, K.; Hudson, J.; de Soussa, K.; Koumaglo, K.; Arnason, J.T. Ethnomedicinal Uses of Momordica Charantia (Cucurbitaceae) in Togo and Relation to Its Phytochemistry and Biological Activity. J. Ethnopharmacol. 2005, 96, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bakare, R.I.; Magbagbeola, O.A.; Akinwande, A.I.; Okunowo, O.W. Nutritional and Chemical Evaluation of Momordica Charantia. J. Med. Plants Res. 2010, 4, 2189–2193. [Google Scholar]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef]
- Bourinbaiar, A.S.; Lee-Huang, S. The Activity of Plant-Derived Antiretroviral Proteins MAP30 and GAP31 against Herpes Simplex Virus Infectionin Vitro. Biochem. Biophys. Res. Commun. 1996, 219, 923–929. [Google Scholar] [CrossRef]
- Rebultan, S.P. Bitter melon therapy: An experimental treatment of HIV infection. Aids Asia Voice Asian Solidar. Against AIDS 1995, 2, 6–7. [Google Scholar]
- Raina, K.; Kumar, D.; Agarwal, R. Promise of bitter melon ( Momordica charantia ) bioactives in cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 116–129. [Google Scholar] [CrossRef]
- Ray, R.B.; Raychoudhuri, A.; Steele, R.; Nerurkar, P. Bitter Melon (Momordica Charantia) Extract Inhibits Breast Cancer Cell Proliferation by Modulating Cell Cycle Regulatory Genes and Promotes ApoptosisBitter Melon Extract Inhibits Breast Cancer Cell Growth. Cancer Res. 2010, 70, 1925–1931. [Google Scholar]
- Kwatra, D.; Subramaniam, D.; Ramamoorthy, P.; Standing, D.; Moran, E.; Velayutham, R.; Mitra, A.; Umar, S.; Anant, S. Methanolic Extracts of Bitter Melon Inhibit Colon Cancer Stem Cells by Affecting Energy Homeostasis and Autophagy. Evidence-Based Complement. Altern. Med. 2013, 2013, 702869. [Google Scholar] [CrossRef]
- Fang, E.F.; Froetscher, L.; Scheibye-Knudsen, M.; Bohr, V.A.; Wong, J.H.; Ng, T.B. Emerging Antitumor Activities of the Bitter Melon (Momordica charantia). Curr. Protein Pept. Sci. 2019, 20, 296–301. [Google Scholar] [CrossRef]
- Akinpelu, C.A.; Adebayo, O.S.; Adewale, O.M.; Adebisi-Adelani, O.O. An Analysis of Turmeric Utilisation Pattern in Ekiti State, Nigeria. Niger. J. Horticult. Sci. 2012, 17, 68–72. [Google Scholar]
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid.-Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed]
- Nwaekpe, J.O.; Anyaegbunam, H.N.; Okoye, B.C.; Asumugha, G.N. Promotion of Turmeric for the Food/Pharmaceutical Industry in Nigeria. Am. J. Exp. Agric. 2015, 8, 335–341. [Google Scholar] [CrossRef]
- Omosa, L.K.; Midiwo, J.O.; Kuete, V. Curcuma Longa. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 425–435. [Google Scholar] [CrossRef]
- Iwu, M.M. African Medicinal Plants; CRC Press: College Park, MD, USA, 1993. [Google Scholar]
- Aggarwal, M.L.; Chacko, K.M.; Kuruvilla, B.T. Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: A bioavailable turmeric formulation. Mol. Med. Rep. 2015, 13, 592–604. [Google Scholar] [CrossRef]
- Uchejeso, O.M.; Chinaza, I.R.; Goodluck, O.A.; Rinpan, J.I. Some Igbo Indigenous Plants with Anti-COVID-19 Properties. In Alternative Medicine-Update; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Nwokocha, C.R.; Ozolua, I.R.; Owu, D.U.; Nwokocha, I.M.; Ugwu, A.C. Antihypertensive properties of Allium sativum (garlic) on normotensive and two kidney one clip hypertensive rats. Niger. J. Physiol. Sci. 2011, 26, 213–218. [Google Scholar]
- Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S164–S179. [Google Scholar] [CrossRef]
- Mahooti, M.; Miri, S.M.; Abdolalipour, E.; Ghaemi, A. The Immunomodulatory Effects of Probiotics on Respiratory Viral Infections: A Hint for COVID-19 Treatment? Microb. Pathog. 2020, 148, 104452. [Google Scholar] [CrossRef] [PubMed]
- Mirashrafi, S.; Moravejolahkami, A.R.; Zehi, Z.B.; Kermani, M.A.H.; Bahreini-Esfahani, N.; Haratian, M.; Dashti, M.G.; Pourhossein, M. The efficacy of probiotics on virus titres and antibody production in virus diseases: A systematic review on recent evidence for COVID-19 treatment. Clin. Nutr. ESPEN 2021, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Visser, M.P.J.; Dofferhoff, A.S.M.; Vermeer, C.; Janssens, W.; Walk, J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nutr. 2021, 126, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Kampmann, F.; Israelsen, S.; Andersen, L.; Jørgensen, H.; Sandholt, H.; Jørgensen, N.; Thysen, S.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Chollet, M. Menaquinones, Bacteria, and Foods: Vitamin K2 in the Diet. In Vitamin K2-Vital for Health and Wellbeing; Gordeladze, J.O., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2016, 10, 91–102. [Google Scholar] [CrossRef]
- Sofowora, A. The present status of knowledge of the plants used in traditional medicine in western Africa: A medical approach and a chemical evaluation. J. Ethnopharmacol. 1980, 2, 109–118. [Google Scholar] [CrossRef]
- Husaini, D.C.; Orisakwe, O.E.; Mphuthi, D.D.; Garba, S.M.; Obasi, C.N.; Nwachukwu, I.E. Phytotherapies for COVID-19 in Latin America and the Caribbean (LAC): Implications for present and future pandemics. Arab Gulf J. Sci. Res. 2023. ahead of print. [Google Scholar] [CrossRef]
- Moeti, M.; Cabore, J.; Kasolo, F.; Yoti, Z.; Zawaira, F.; Chibi, F.Z.M.; Rajatonirina, S.; Karamagi, H.; Rees, H.; Mihigo, R.; et al. The COVID-19 pandemic: Research and health development in the World Health Organisation Africa region. Pan Afr. Med. J. 2020, 35 (Supp. 2), 1–3. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Orish, C.N.; Nwanaforo, E.O. Coronavirus disease (COVID-19) and Africa: Acclaimed home remedies. Sci. Afr. 2020, 10, e00620. [Google Scholar] [CrossRef]
- Attah, A.F.; Fagbemi, A.A.; Olubiyi, O.; Dada-Adegbola, H.; Oluwadotun, A.; Elujoba, A.; Babalola, C.P. Therapeutic Potentials of Antiviral Plants Used in Traditional African Medicine with COVID-19 in Focus: A Nigerian Perspective. Front. Pharmacol. 2021, 12, 596855. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Luo, H.; Tang, Q.-L.; Shang, Y.-X.; Liang, S.-B.; Yang, M.; Robinson, N.; Liu, J.-P. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin. J. Integr. Med. 2020, 26, 243–250. [Google Scholar] [CrossRef]
- Im, K.; Kim, J.; Min, H. Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. J. Ginseng Res. 2016, 40, 309–314. [Google Scholar] [CrossRef]
- Kolodziej, H. Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion. Pharmaceuticals 2011, 4, 1295–1314. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef]
- Ang, L.; Lee, H.W.; Choi, J.Y.; Zhang, J.; Lee, M.S. Herbal medicine and pattern identification for treating COVID-19: A rapid review of guidelines. Integr. Med. Res. 2020, 9, 100407. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 3066–3090. [Google Scholar] [CrossRef]
- Greene, M.W.; Roberts, A.P.; Frugé, A.D. Negative Association Between Mediterranean Diet Adherence and COVID-19 Cases and Related Deaths in Spain and 23 OECD Countries: An Ecological Study. Front. Nutr. 2021, 8, 591964. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Singh, A.K.; Gillies, C.L.; Singh, R.; Singh, A.; Chudasama, Y.; Coles, B.; Seidu, S.; Zaccardi, F.; Davies, M.J.; Khunti, K. Prevalence of co-morbidities and their association with mortality in patients with COVID -19: A systematic review and meta-analysis. Diabetes Obes. Metab. 2020, 22, 1915–1924. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Fotsing Yannick Stéphane, F.; Kezetas Jean Jules, B.; El-Saber Batiha, G.; Ali, I.; Ndjakou Bruno, L. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicinal Plants; A. El-Shemy, H., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
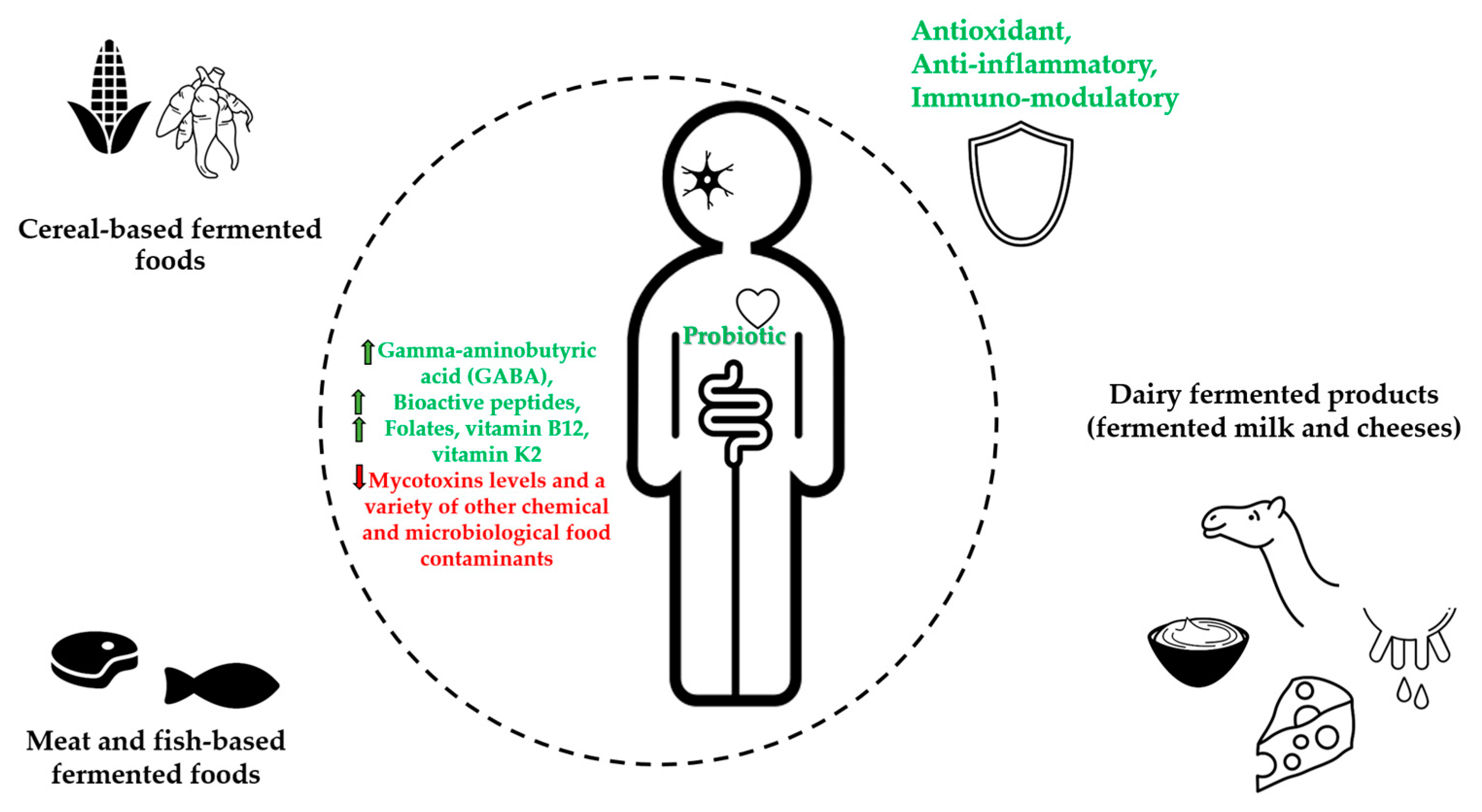
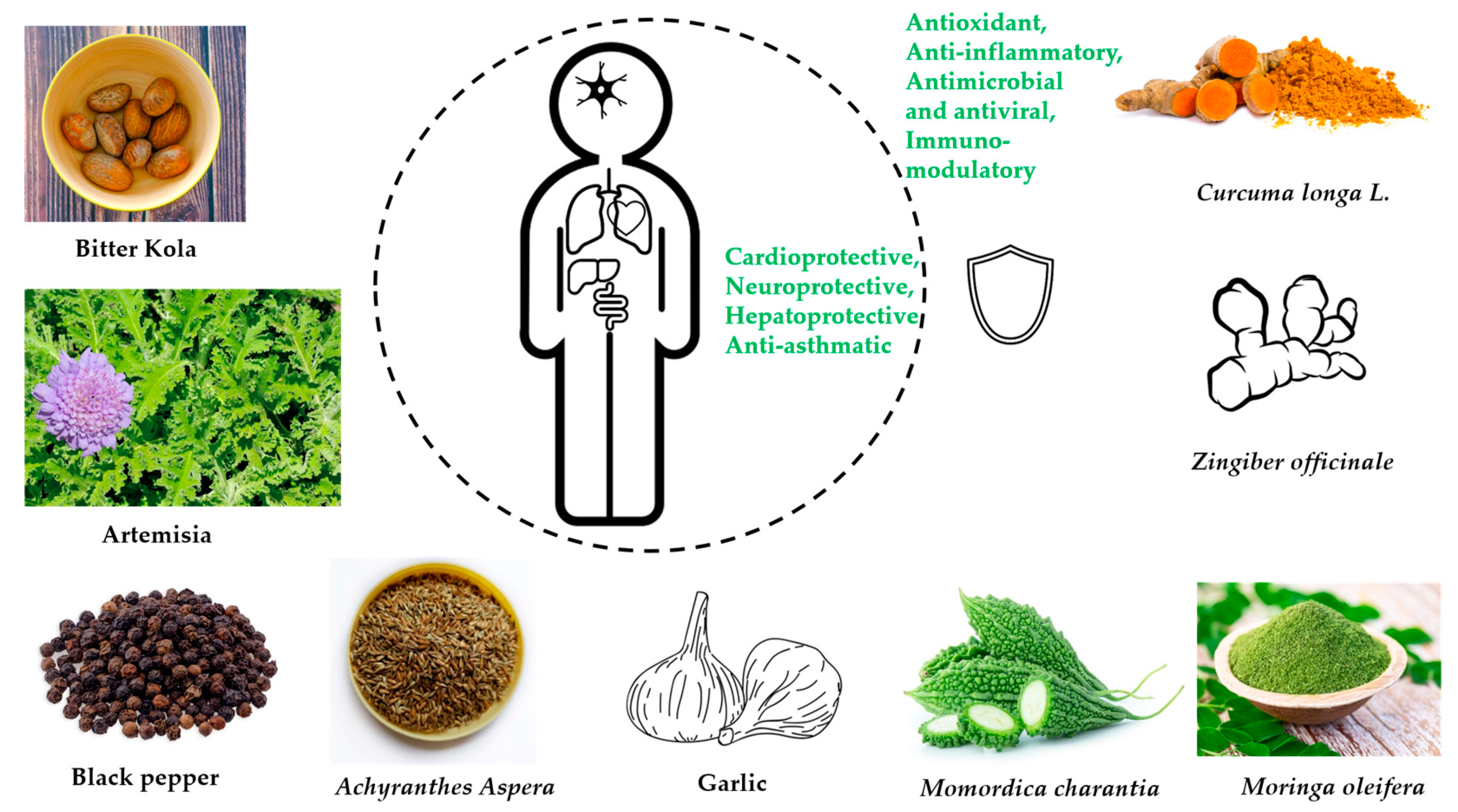
| Fermented Foods | Raw Food Materials | Micro-Organisms | Bioactive Compounds | Potential Health Benefits | References |
|---|---|---|---|---|---|
| Cereal-based fermented foods | Maize (Zea mays), Sorghum (Sorghum bicolor), Millet (Peninsetum americanum), Acha or Fonio (Digitaria exilis), Cassava (Manihot esculenta) | Bacteria (Lactobacillus, Lactococcus, Leuconostoc Pedicoccus genera) Yeasts (Saccharomyces, Rhodotorula, Candida, Kluyveromyces, and Geotrichum genera) Filamentous molds (Aspergillus, Rhizopus, Fusarium, and Penicillium, genera) | Soluble non-starch polysaccharides (e.g., arabinoxylan and β–glucan) Nondigestible carbohydrates (e.g., galacto- and fructo–oligosaccharides) Folates | Promote rich nondigestible carbohydrates (prebiotics), increase in phenolic compounds, gamma-aminobutyric acid (GABA), and bioactive peptides contents. Increase folates, decrease mycotoxins levels, increase health benefits of probiotic consumption, reduce exposure to a variety of other chemical food contaminants and detoxification | [8,17,18,29, 33,35,36] |
| Meat- and fish-based fermented foods | Meat, Fish | Bacteria (Leuconostoc, Lactobacillus, Enterococcus Aerococcus, Bacillus genera) Yeasts (Pichia, Candida, Hanseniaspora, Kluyveromyces Torulaspora, and Kluyveromyces genera) | Bioactive peptides, Bacteriocins | Antioxidant activity, increase health benefits of probiotic consumption, reduction of microbiological hazards | [11,38,45, 52,59] |
| Dairy fermented products (fermented milk and cheeses) | Milk | Bacteria (Lactococcus Leuconostoc Streptococcus, Lactobacillus, Pediococcus genera) Yeasts (Saccharomyces, Candida, Kluyveromyces genera) | Bioactive peptides, Conjugated Linoleic Acid, Vitamin B12, Vitamin K2 Bacteriocins | Antioxidant, immunomodulatory, source of vitamin B12 and vitamin K2, increase health benefits of probiotic consumption, protection against food-spoilage | [11,38,61,65 68] |
| Herbs/ Medicinal Plants | Traditional Uses | Bioactive Substances | Pharmacologic Activity | Immunologic Activity | Antiviral Activity | References |
|---|---|---|---|---|---|---|
| Garcinia kola Heckel (Fam. Clusiaceae) [Bitter cola] | Typhoid fever, bronchitis, bacterial infections, malignant tumors, skin infections, tuberculosis, gastritis, cold, jaundice | Alkaloids, phenols, saponins, sterols, tannins, garciniflavanone, kolanone, garcinoic acid, kolaflavanone, and kolaviron | Antiviral, antiasthma, antioxidant, antidiabetic, antihypertensive, antibacterial, antiasthma, and for hepatoprotective activities | Antioxidant, hepatoprotective, immunomodulatory, metal chelating, potent radical scavenger, modulate oxidative stress | Polioviruses, measles virus, yellow fever virus, influenza, herpes simplex Virus-1, HIV | [85,86,93,94,97,99,201] |
| Artemisia Afra Jacq. (Fam. Asteraceae) | Influenza, respiratory infections, cough, malaria, diabetes, and fever | Dihydroxybishopsolicepolide, scopoletin, acacetin, flavonoids, yomogiartemin | Cytotoxic, anticancer, antiviral | Antioxidant, anti-inflammatory | Influenza virus A, human herpes viruses 1 and 2, Hepatitis B and C, HIV-1 viruses | [103,104,105,106,109,110,113] |
| Piper guineense (Fam. Piperaceae) [African black pepper] | Sexually transmitted diseases | Piperine, piperlongumine, ligans, monoterpenes, terpenoids, sterols, sesquiterpenes, and volatile oils | Antibacterial, anticancer, antiviral, antiproliferative, antifungal, antihelminth | Antioxidant, anti-inflammatory | Newcastle disease virus | [103,114,120,122] |
| Achyranthes Aspera Linn. (Fam. Amaranthaceae) | Dysentery, arthritis, malaria, hemorrhoids, fever, pain, and diarrhea | Triacontanol, eugenol, ecdysterone, betaine, ascorbic acid | Diuretic, anti-inflammatory, anti-asthmatic, and valuable for pneumonia | Antioxidant, immune boosting, chemopreventative | Herpes simplex virus type 1 (HSV-1, oral herpes) and type 2 (HSV-2, genital herpes). | [92,124,125,126] |
| Allium sativum L. (Fam. Liliaceae) [Garlic] | Influenza, typhus, cholera, dysentery, toothaches, snake bites, arthritis, and hypertension | Phenols, flavonoids, saponins, allicin (thiosulfate), diallyl trisulfate, ajoenes, diallyl disulfide | Anticancer, antimicrobial, flu, diabetes, hypertension, arthritis, and for the prevention of cancer | Antioxidants, immune booster | Vesicular stomatitis virus, Human rhinovirus type 2, influenza virus type 3, human cytomegalovirus, influenza B type virus, and herpes simplex 1 and 2 | [134,142,143,147,155,210] |
| Moringa oleifera Lam. (Fam. Moringaceae) | Food, livestock feed, nutrition, medicines | isothiocyanate, phenolic acids, polyphenols, sterols, alkaloids, terpene, flavonoids, and flavanol glycosides | Anti-parasitic, antituberculosis anticancer, antiviral, antidiabetic, sexually transmitted infections, typhoid fever, antihypertensive | Anti-inflammatory, cardio-protective, neuro-protective, hepato-protective | Influenza A virus, new castle disease virus, herpes simplex virus, Epstein-Barr virus, hepatitis B virus, and foot-and-mouth disease virus in cloven-footed animals | [160,161,162,163,168,210] |
| Zingiber officinale R. (Fam Zingiberaceae) [Ginger] | Influenza, cough, sore throats, arthritis, lung diseases, peptic ulcer disease, hypertension, infectious diseases | Steroids, phenols, alkaloids, gingerols, zingerone, zingiberol, paradols, and shogaols | Antiarthritic, anticancer, antioxidant, antirhinoviral, antimicrobial, antiglycemic | Antioxidant, anti-inflammatory | Enterovirus 71, Japanese encephalitis virus, Epstein-Barr virus, herpes simplex virus types 1 and 2, influenza A virus, human immunodeficiency viruses, coronavirus SARS-CoV-1, rhinovirus, chikungunya virus, respiratory syncytial virus | [92,173,174,175,176,177,178,182,186] |
| Momordica charantia (Fam. Cucurbitaceae) [Bitter melon] | Diabetes, treat viral infections, toothache, diarrhea, gastrointestinal infections, ritual purposes | Minerals, vitamins, phenol compounds, triterpene, lipid, protein, glycosides, steroids, saponins, flavonoids | Antiviral, recurrent respiratory tract infections, anthelmintic, anticancer, antidiabetic, abortifacient, contraceptive | Antioxidant | HSV-1 and SINV viruses, HIV-1 | [194,195,196, 198] |
| Curcuma longa L. (Fam. Zingiberaceae) [Tumeric] | Conjunctivitis, smallpox, sinusitis | Curcuminoids, Quercetin, Cuscumin, zingiberine, borneol, alpha phellandrene | Antioxidant, anticancer, immunomodulatory, antimicrobial, antiviral | Antioxidant, immune modulator | HIV-1, H1N1, hepatitis C, parainfluenza virus type-3, H6N1, human papillomavirus, and coxsackievirus B3 | [205,206,207, 209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazzoli, C.; Grasso, G.; Husaini, D.C.; Ajibo, D.N.; Orish, F.C.; Orisakwe, O.E. Immune System and Epidemics: The Role of African Indigenous Bioactive Substances. Nutrients 2023, 15, 273. https://doi.org/10.3390/nu15020273
Frazzoli C, Grasso G, Husaini DC, Ajibo DN, Orish FC, Orisakwe OE. Immune System and Epidemics: The Role of African Indigenous Bioactive Substances. Nutrients. 2023; 15(2):273. https://doi.org/10.3390/nu15020273
Chicago/Turabian StyleFrazzoli, Chiara, Gerardo Grasso, Danladi Chiroma Husaini, Doris Nnenna Ajibo, Fortune Chiemelie Orish, and Orish E. Orisakwe. 2023. "Immune System and Epidemics: The Role of African Indigenous Bioactive Substances" Nutrients 15, no. 2: 273. https://doi.org/10.3390/nu15020273
APA StyleFrazzoli, C., Grasso, G., Husaini, D. C., Ajibo, D. N., Orish, F. C., & Orisakwe, O. E. (2023). Immune System and Epidemics: The Role of African Indigenous Bioactive Substances. Nutrients, 15(2), 273. https://doi.org/10.3390/nu15020273









