Fermented Supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 Protect against Protein Glycation and Inhibit Glycated Protein Ligation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermented Supernatant Samples
2.2. Protein Glycation Assays
2.3. MG-Trapping Assay
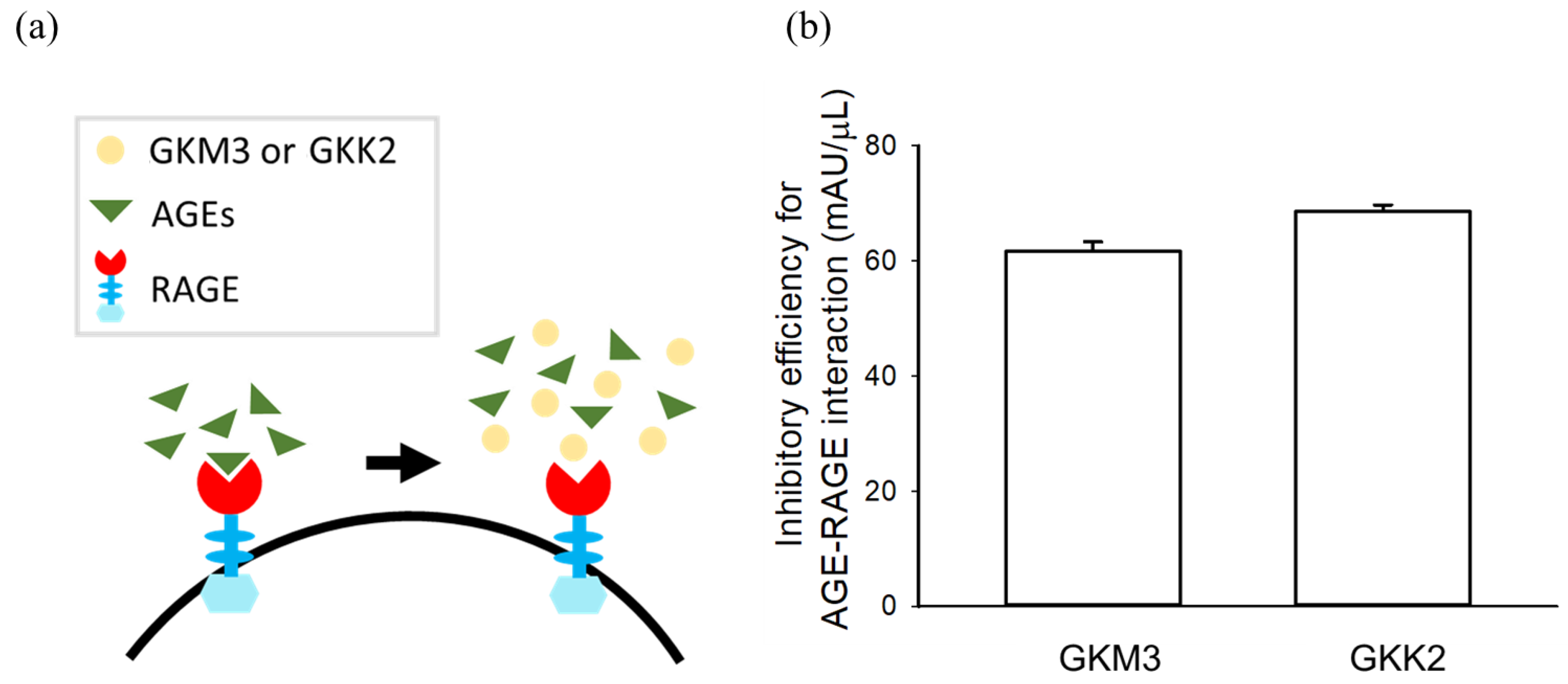
2.4. Glycated Protein–RAGE Binding Assay
2.5. Statistical Analysis
3. Results
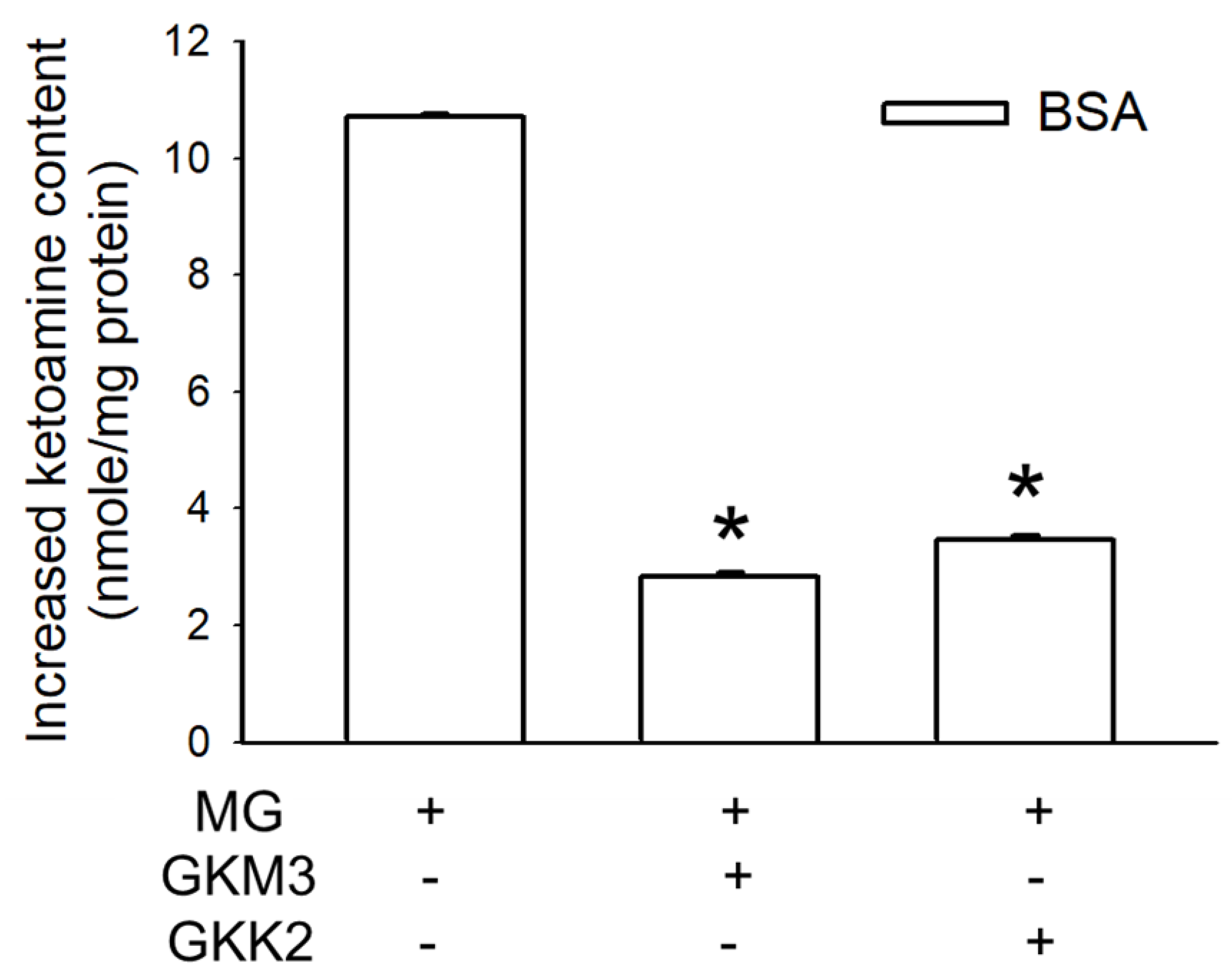
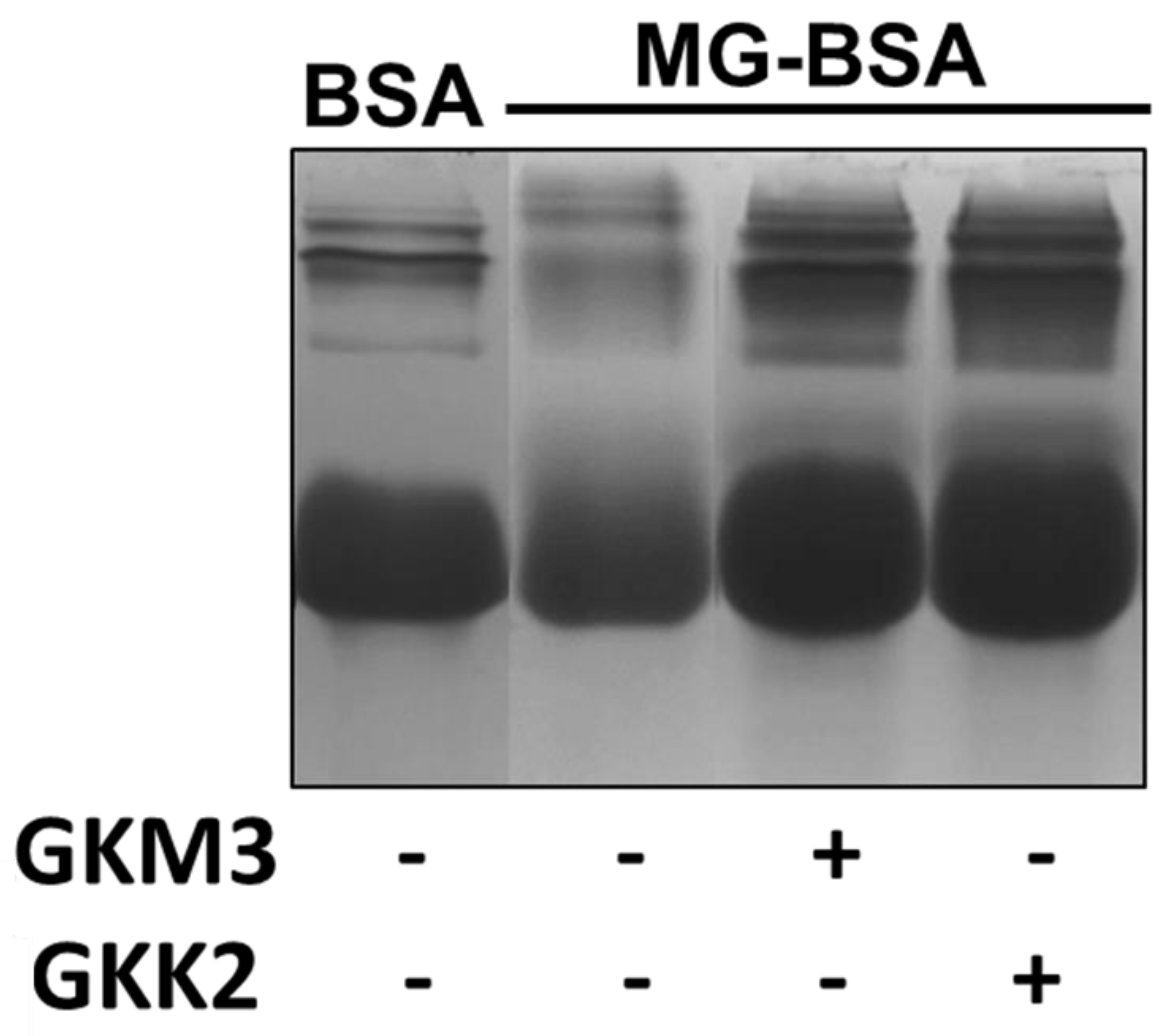
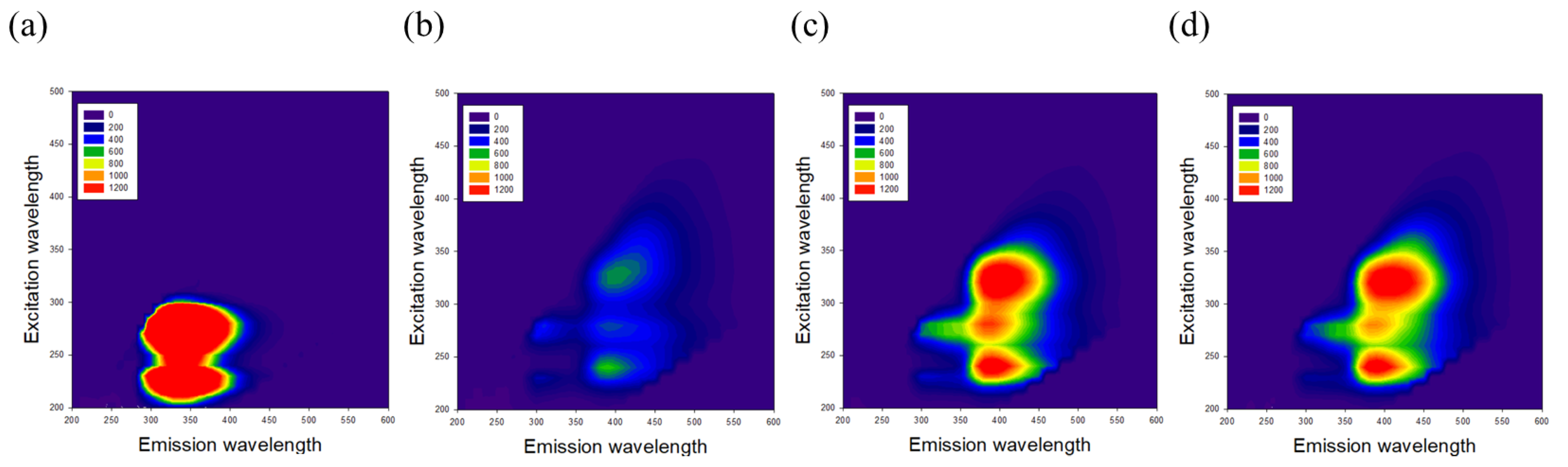
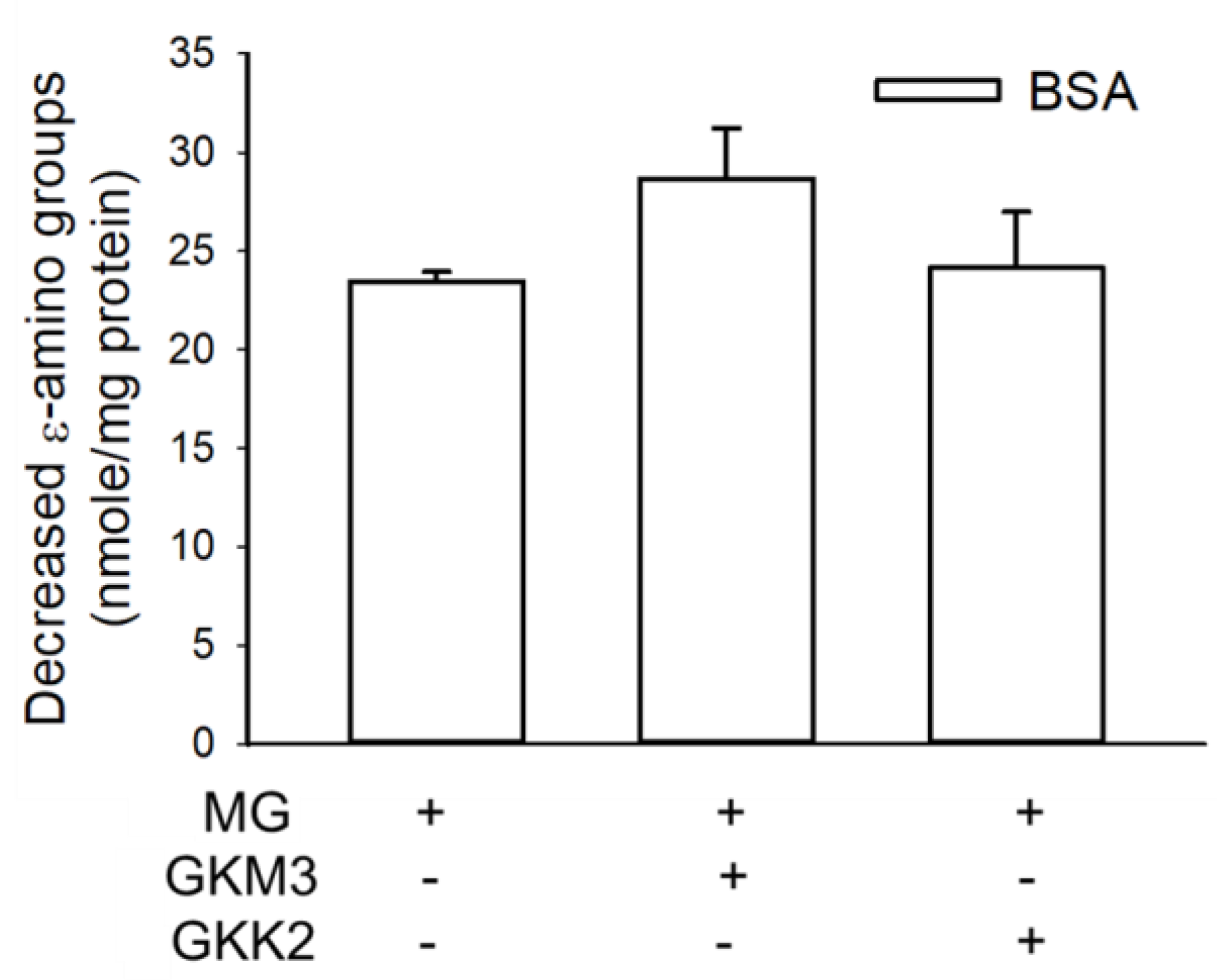
3.1. Effects of GKM3- and GKK2-Fermented Supernatants on Early and Late Stages of Protein Glycation
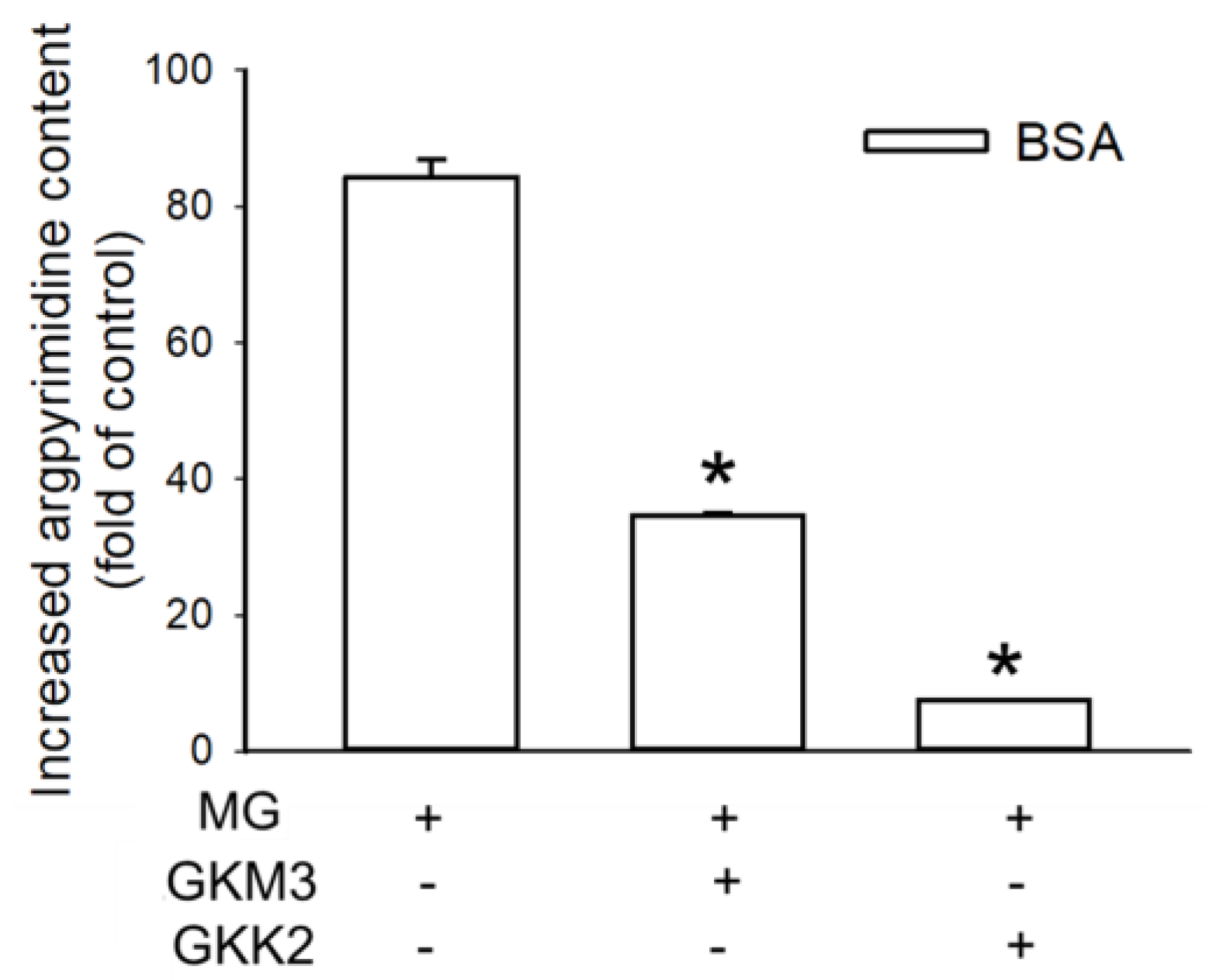
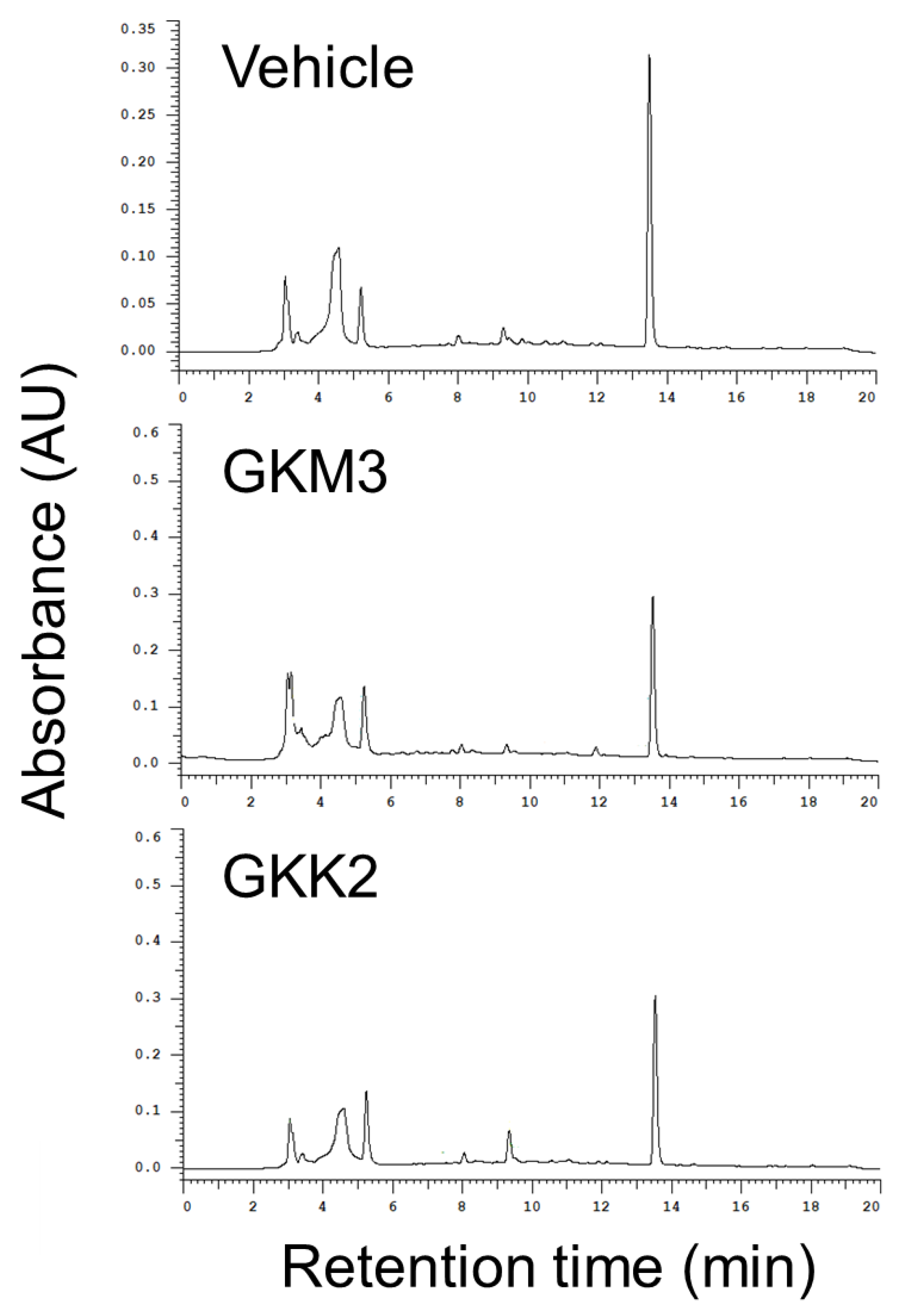
3.2. Effects of GKM3- and GKK2-Fermented Supernatants on Formation of MG-Derived Advanced Glycation end Products and Related RAGE Ligation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hohn, A.; Konig, J.; Grune, T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteom. 2013, 92, 132–159. [Google Scholar] [CrossRef]
- Lai, S.W.T.; Lopez Gonzalez, E.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and its adducts: Induction, repair, and association with disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The role of advanced glycation end products in aging and metabolic diseases: Bridging association and causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Laga, M. Linking TNF-Induced Dysfunction and Formation of Methylglyoxal Derived AGEs in Endothelial Cells. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2018. [Google Scholar]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef]
- Fernandes, A.C.F.; Santana, A.L.; Martins, I.M.; Moreira, D.K.T.; Macedo, J.A.; Macedo, G.A. Anti-glycation effect and the α-amylase, lipase, and α-glycosidase inhibition properties of a polyphenolic fraction derived from citrus wastes. Prep. Biochem. Biotechnol. 2020, 5, 794–802. [Google Scholar] [CrossRef]
- Genova, V.M.; Fernandes, A.C.F.; Hiramatsu, E.; Queirós, L.D.; Macedo, J.A.; Macedo, G.A. Biotransformed antioxidant isoflavone extracts present high-capacity to attenuate the in vitro formation of advanced glycation end products. Food Biotechnol. 2021, 35, 50–66. [Google Scholar]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current status of probiotic and related health benefits. Appl. Food Biotechnol. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Lin, S.W.; Tsai, Y.S.; Chen, Y.L.; Wang, M.F.; Chen, C.C.; Lin, W.H.; Fang, T.J. An examination of Lactobacillus paracasei GKS6 and Bifidobacterium lactis GKK2 isolated from infant feces in an aged mouse model. Evid.-Based Complement Alternat. Med. 2021, 2021, 6692363. [Google Scholar] [CrossRef]
- Lin, S.W.; Tsai, Y.S.; Chen, Y.L.; Wang, M.F.; Chen, C.C.; Lin, W.H.; Fang, T.J. Lactobacillus plantarum GKM3 promotes longevity, memory retention, and reduces brain oxidation stress in SAMP8 mice. Nutrients 2021, 13, 2860. [Google Scholar] [CrossRef]
- Hou, Y.H.; Lin, S.W.; Chang, Z.; Lu, H.C.; Chen, Y.L.; Lin, W.H.; Chen, C.C. Effect of Bifidobacterium lactis GKK2 on OVA-induced asthmatic mice. Hans J. Biomed. 2019, 9, 70–80. [Google Scholar] [CrossRef]
- Hsu, C.L.; Hou, Y.H.; Wang, C.S.; Lin, S.W.; Jhou, B.Y.; Chen, C.C.; Chen, Y.L. Antiobesity and uric acid-lowering effect of Lactobacillus plantarum GKM3 in high-fat-diet-induced obese rats. J. Am. Coll. Nutr. 2019, 38, 623–632. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C. Effect of probiotics Lactobacillus paracasei GKS6, L. plantarum GKM3, and L. rhamnosus GKLC1 on alleviating alcohol-induced alcoholic liver disease in a mouse model. Nutr. Res. Pract. 2020, 14, 299–308. [Google Scholar] [CrossRef]
- Armbruster, D.A. Fructosamine: Structure, analysis, and clinical usefulness. Clin. Chem. 1987, 33, 2153–2163. [Google Scholar] [CrossRef]
- Khan, M.A.; Arif, Z.; Khan, M.A.; Moinuddin; Alam, K. Methylglyoxal produces more changes in biochemical and biophysical properties of human IgG under high glucose compared to normal glucose level. PLoS ONE 2018, 13, e0191014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, T.A.; AlRabiah, H.; Bakheit, A.H.; Kalam, M.A.; Zargar, S. Study of binding interaction of rivaroxaban with bovine serum albumin using multi-spectroscopic and molecular docking approach. Chem. Cent. J. 2017, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Huang, H.W.; Lin, J.A.; Huang, S.M.; Yen, G.C. The proglycation effect of caffeic acid leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells. J. Nutr. Biochem. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Hashimoto, C.; Iwaihara, Y.; Chen, S.J.; Tanaka, M.; Watanabe, T.; Matsui, T. Highly-sensitive detection of free advanced glycation end-products by liquid chromatography-electrospray ionization-tandem mass spectrometry with 2,4,6-trinitrobenzene sulfonate derivatization. Anal. Chem. 2013, 85, 4289–4295. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Sero, L.; Sanguinet, L.; Blanchard, P.; Dang, B.T.; Morel, S.; Richomme, P.; Seraphin, D.; Derbre, S. Tuning a 96-well microtiter plate fluorescence-based assay to identify AGE inhibitors in crude plant extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.Y.; Hsiao, W.T.; Chen, X.Y. Efficiency of trapping methylglyoxal by phenols and phenolic acids. J. Food Sci. 2011, 76, H90–H96. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, F.; Saleem, M.; Syed Khaja, A.S.; Zafar, M.; Alharbi, M.S.; Al Hagbani, T.; Khan, M.Y.; Ahmad, W.; Ahmad, S. Antiglycation potential of plant based TiO2 nanoparticle in D-ribose glycated BSA in vitro. Cell Biochem. Funct. 2022, 40, 784–796. [Google Scholar] [CrossRef]
- Banerjee, D.; Mandal, A.; Mukherjee, S. Excited state complex formation between methyl glyoxal and some aromatic bio-molecules: A fluorescence quenching study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 103–109. [Google Scholar] [CrossRef]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B. The effects of fermentation with lactic acid bacteria on the antioxidant and anti-glycation properties of edible cyanobacteria and microalgae. LWT 2021, 135, 110029. [Google Scholar] [CrossRef]
- Elhassan, G.O.M.; Adhikari, A.; Yousuf, S.; Rahman, M.H.; Khalid, A.; Omer, H.; Fun, H.K.; Jahan, H.; Choudhary, M.I.; Yagi, S. Phytochemistry and antiglycation activity of Aloe sinkatana Reynolds. Phytochem. Lett. 2012, 5, 725–728. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Khodadadi, I.; Tavilani, H.; Goodarzi, M.T. Aqueous extract of Anethum Graveolens L. has potential antioxidant and antiglycation effects. Iran. J. Basic Med. Sci. 2016, 41, 328–333. [Google Scholar]
- Muniz, A.; Garcia, A.H.; Pérez, R.M.; García, E.V.; González, D.E. In vitro inhibitory activity of Acca sellowiana fruit extract on end products of advanced glycation. Diabetes Ther. 2018, 9, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Gomes, R.; Sousa Silva, M.; Quintas, A.; Cordeiro, C.; Freire, A.; Pereira, P.; Martins, A.; Monteiro, E.; Barroso, E.; Ponces Freire, A. Argpyrimidine, a methylglyoxal-derived advanced glycation end-product in familial amyloidotic polyneuropathy. Biochem. J. 2005, 385, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, Y.; Wada, R.; Baba, M.; Takeuchi, M.; Hanyu-Itabashi, C.; Yagihashi, S. Neuropathy induced by exogenously administered advanced glycation end-products in rats. J. Diabetes Investig. 2010, 1, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senatus, L.M.; Schmidt, A.M. The AGE-RAGE Axis: Implications for age-associated arterial diseases. Front. Genet. 2017, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Isermann, B.; Bierhaus, A.; Humpert, P.M.; Rudofsky, G.; Chavakis, T.; Ritzel, R.; Wendt, T.; Morcos, M.; Kasperk, C.; Hamann, A.; et al. AGE-RAGE: A hypothesis or a mechanism? Herz 2004, 29, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.S.; Chen, Y.P.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Huang, G.J. Lactobacillus rhamnosus GKLC1 ameliorates cisplatin-induced chronic nephrotoxicity by inhibiting cell inflammation and apoptosis. Biomed. Pharmacother. 2022, 147, 112701. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-W.; Wu, C.-H.; Jao, Y.-C.; Tsai, Y.-S.; Chen, Y.-L.; Chen, C.-C.; Fang, T.J.; Chau, C.-F. Fermented Supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 Protect against Protein Glycation and Inhibit Glycated Protein Ligation. Nutrients 2023, 15, 277. https://doi.org/10.3390/nu15020277
Lin S-W, Wu C-H, Jao Y-C, Tsai Y-S, Chen Y-L, Chen C-C, Fang TJ, Chau C-F. Fermented Supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 Protect against Protein Glycation and Inhibit Glycated Protein Ligation. Nutrients. 2023; 15(2):277. https://doi.org/10.3390/nu15020277
Chicago/Turabian StyleLin, Shih-Wei, Chi-Hao Wu, Ya-Chien Jao, You-Shan Tsai, Yen-Lien Chen, Chin-Chu Chen, Tony J. Fang, and Chi-Fai Chau. 2023. "Fermented Supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 Protect against Protein Glycation and Inhibit Glycated Protein Ligation" Nutrients 15, no. 2: 277. https://doi.org/10.3390/nu15020277
APA StyleLin, S. -W., Wu, C. -H., Jao, Y. -C., Tsai, Y. -S., Chen, Y. -L., Chen, C. -C., Fang, T. J., & Chau, C. -F. (2023). Fermented Supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 Protect against Protein Glycation and Inhibit Glycated Protein Ligation. Nutrients, 15(2), 277. https://doi.org/10.3390/nu15020277






