Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine
Abstract
:1. Introduction
2. Migraine as a Diet-Sensitive Disease
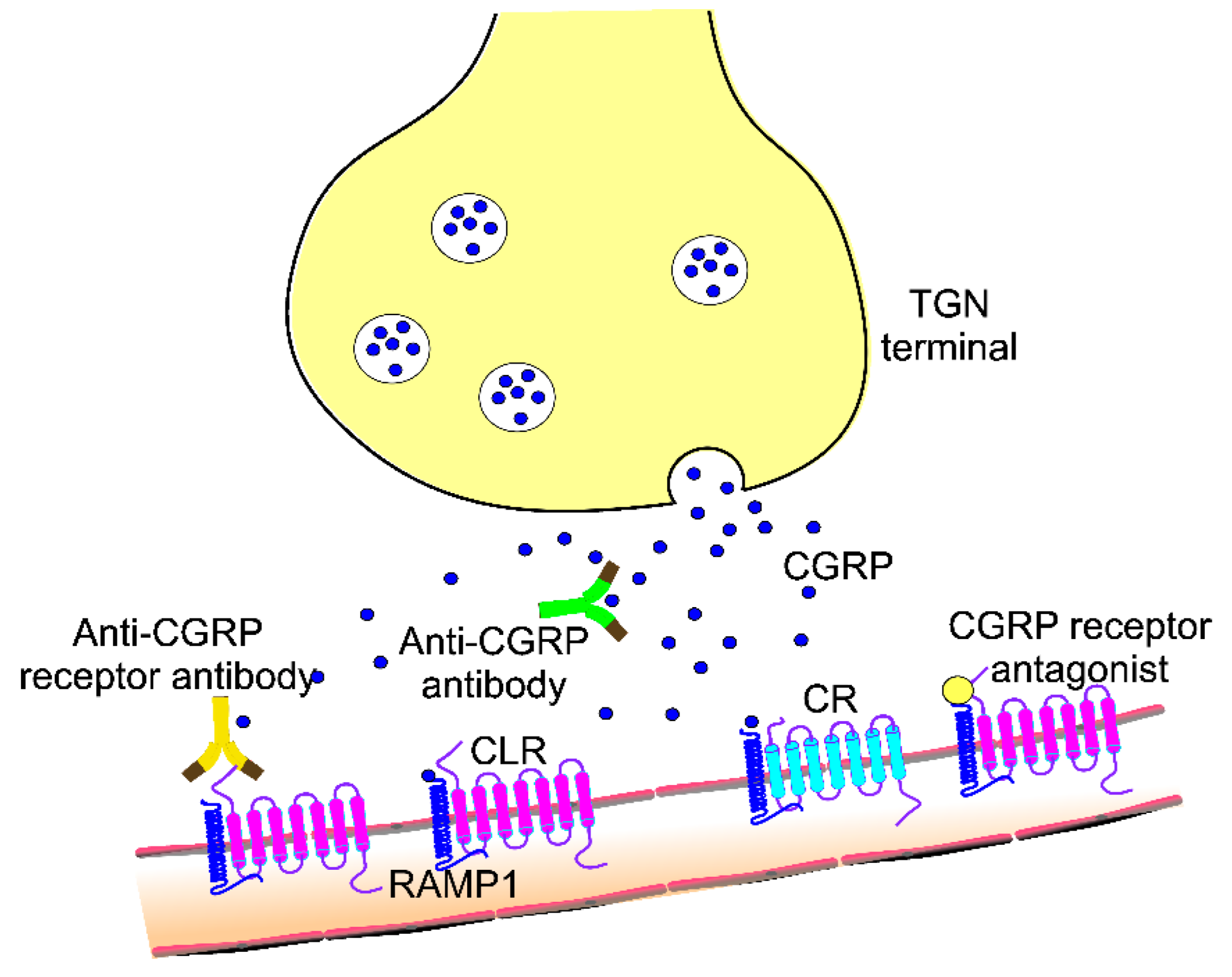
3. CGRP and Its Role in Migraine Pathogenesis and Treatment
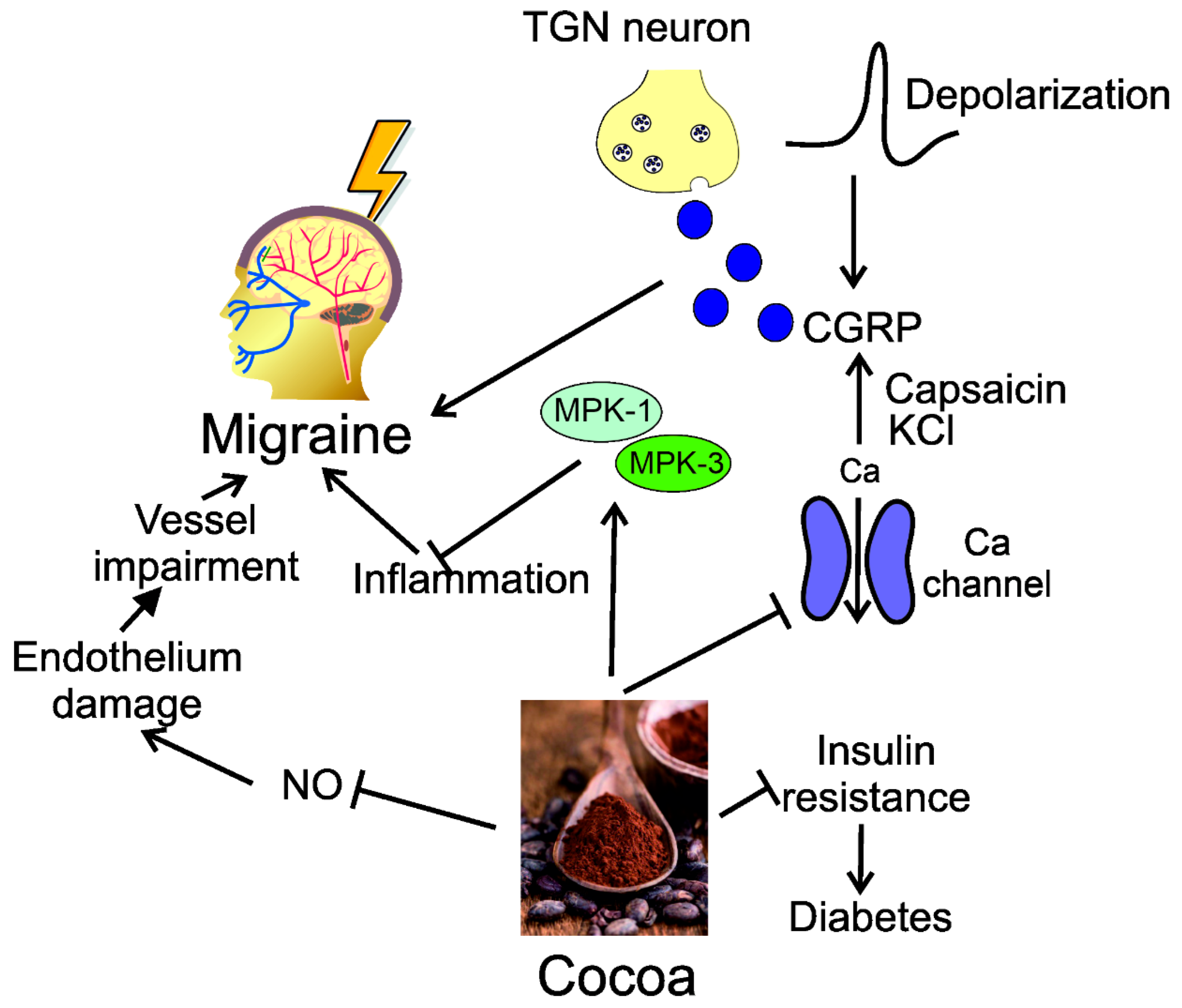
4. Modulation of CGRP by Dietary Nutrients
5. Anorexigenic Potential of CGRP and Its Involvement in the Appetite and Satiety Control
6. CGRP and Metabolic Disorders
7. Dietary Nutrients May Reduce Gastrointestinal Functional Disorders as Unwanted Side Effects of Anti-CGRP Treatment
8. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F. CGRP-based Migraine Therapeutics: How Might They Work, Why So Safe, and What Next? ACS Pharmacol. Transl. Sci. 2019, 2, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Milliron, B.J.; Packel, L.; Dychtwald, D.; Klobodu, C.; Pontiggia, L.; Ogbogu, O.; Barksdale, B.; Deutsch, J. When Eating Becomes Torturous: Understanding Nutrition-Related Cancer Treatment Side Effects among Individuals with Cancer and Their Caregivers. Nutrients 2022, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Fiedler-Kelly, J.B.; Cohen-Barak, O.; Morris, D.N.; Ludwig, E.; Rasamoelisolo, M.; Shen, H.; Levi, M. Population pharmacokinetic modelling and simulation of fremanezumab in healthy subjects and patients with migraine. Br. J. Clin. Pharmacol. 2019, 85, 2721–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krahn, D.D.; Gosnell, B.A.; Levine, A.S.; Morley, J.E. Effects of calcitonin gene-related peptide on food intake. Peptides 1984, 5, 861–864. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Calcitonin gene-related peptide and its receptors: Molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 1996, 17, 533–585. [Google Scholar] [CrossRef]
- Campos, C.A.; Bowen, A.J.; Schwartz, M.W.; Palmiter, R.D. Parabrachial CGRP Neurons Control Meal Termination. Cell Metab. 2016, 23, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Jacobs, B.; Dussor, G. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016, 338, 130–144. [Google Scholar] [CrossRef]
- Khan, J.; Asoom, L.I.A.; Sunni, A.A.; Rafique, N.; Latif, R.; Saif, S.A.; Almandil, N.B.; Almohazey, D.; AbdulAzeez, S.; Borgio, J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021, 139, 111557. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.R.; Ghorbani, Z.; Martelletti, P.; Lampl, C.; Togha, M. Association of diet and headache. J. Headache Pain 2019, 20, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Onofrio, F.; Raimo, S.; Spitaleri, D.; Casucci, G.; Bussone, G. Usefulness of nutraceuticals in migraine prophylaxis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Qubty, W. Retrospective Observational Study on Riboflavin Prophylaxis in Child and Adolescent Migraine. Pediatr. Neurol. 2021, 114, 5–8. [Google Scholar] [CrossRef]
- Hernández, A.G. Palmitoylethanolamide-based nutraceutical Calmux® in preventive treatment of migraine. Clin. Neurol. Neurosurg. 2022, 218, 107282. [Google Scholar] [CrossRef]
- Quintana, S.; Russo, M.; Torelli, P. Nutraceuticals and migraine: Further strategy for the treatment of specific conditions. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 6565–6567. [Google Scholar] [CrossRef] [PubMed]
- Moskatel, L.S.; Zhang, N. Migraine and Diet: Updates in Understanding. Curr. Neurol. Neurosci. Rep. 2022, 22, 327–334. [Google Scholar] [CrossRef]
- Hajjarzadeh, S.; Shalilahmadi, D.; Nikniaz, Z.; Mahdavi, R.; Hajjarzadeh, S. The comparison of the main dietary and non-dietary trigger factors in women with chronic and episodic migraine. Nutr. Diet. 2022, 79, 616–622. [Google Scholar] [CrossRef]
- Lisicki, M.; Schoenen, J. Old Habits Die Hard: Dietary Habits of Migraine Patients Challenge our Understanding of Dietary Triggers. Front. Neurol. 2021, 12, 748419. [Google Scholar] [CrossRef]
- Martins-Oliveira, M.; Tavares, I.; Goadsby, P.J. Was it something I ate? Understanding the bidirectional interaction of migraine and appetite neural circuits. Brain Res. 2021, 1770, 147629. [Google Scholar] [CrossRef]
- Martin, V.T.; Vij, B. Diet and Headache: Part 2. Headache 2016, 56, 1553–1562. [Google Scholar] [CrossRef]
- Pogoda, J.M.; Gross, N.B.; Arakaki, X.; Fonteh, A.N.; Cowan, R.P.; Harrington, M.G. Severe Headache or Migraine History is Inversely Correlated with Dietary Sodium Intake: NHANES 1999–2004. Headache 2016, 56, 688–698. [Google Scholar] [CrossRef]
- Hajjarzadeh, S.; Nikniaz, Z.; Shalilahmadi, D.; Mahdavi, R.; Behrouz, M. Comparison of Diet Quality Between Women with Chronic and Episodic Migraine. Headache 2019, 59, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Botti, G.; Paolucci, M.; Brunelli, N.; Cecchi, G.; Khazrai, M.; Vernieri, F. Promoting healthy eating can help preventing migraine: A real-life preliminary study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2018, 39, 155–156. [Google Scholar] [CrossRef]
- Altamura, C.; Cecchi, G.; Bravo, M.; Brunelli, N.; Laudisio, A.; Caprio, P.D.; Botti, G.; Paolucci, M.; Khazrai, Y.M.; Vernieri, F. The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study. Nutrients 2020, 12, 1579. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G.; Caprio, M. Ketogenic diet in migraine: Rationale, findings and perspectives. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, D.; Benedetto, C.; Corvisieri, S.; Del Favero, C.; Orlandi, F.; Allais, G.; Sinigaglia, S.; Fadda, M. Effectiveness of ketogenic diet in treatment of patients with refractory chronic migraine. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 3865–3870. [Google Scholar] [CrossRef]
- Lovati, C.; D’Alessandro, C.M.; Ventura, S.D.; Muzio, F.; Pantoni, L. Ketogenic diet in refractory migraine: Possible efficacy and role of ketone bodies—A pilot experience. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 6479–6485. [Google Scholar] [CrossRef]
- Ameghino, L.; Farez, M.F.; Wilken, M.; Goicochea, M.T. Headache in Patients with Celiac Disease and Its Response to the Gluten-Free Diet. J. Oral Facial Pain Headache 2019, 33, 294–300. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef]
- Beuthin, J.; Veronesi, M.; Grosberg, B.; Evans, R.W. Gluten-Free Diet and Migraine. Headache 2020, 60, 2526–2529. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Pinto, A.; Ienca, R.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Spagnoli, A.; Vestri, A.; et al. A Randomized Double-Blind, Cross-Over Trial of very Low-Calorie Diet in Overweight Migraine Patients: A Possible Role for Ketones? Nutrients 2019, 11, 1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evcili, G.; Utku, U.; Öğün, M.N.; Özdemir, G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri 2018, 30, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Frank, M. Low-Glycemic-Index Diet Relieving Migraine but Inducing Muscle Cramps. J. Neurosci. Rural Pract. 2019, 10, 552–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arab, A.; Khorvash, F.; Karimi, E.; Hadi, A.; Askari, G. Associations between adherence to Mediterranean dietary pattern and frequency, duration, and severity of migraine headache: A cross-sectional study. Nutr. Neurosci. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bakırhan, H.; Yıldıran, H.; Cankay, T.U. Associations between diet quality, DASH and Mediterranean dietary patterns and migraine characteristics. Nutr. Neurosci. 2022, 25, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Gualtieri, P.; De Lorenzo, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Cianci, R.; Frank, G.; Schifano, G.; Bigioni, G.; et al. Dietary ω-3 intake for the treatment of morning headache: A randomized controlled trial. Front. Neurol. 2022, 13, 987958. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Suchindran, C.M.; MacIntosh, B.A.; Gaylord, S.; Ringel, A.; Hibbeln, J.R.; Feldstein, A.E.; Mori, T.A.; et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain 2013, 154, 2441–2451. [Google Scholar] [CrossRef] [Green Version]
- Ramsden, C.E.; Zamora, D.; Faurot, K.R.; MacIntosh, B.; Horowitz, M.; Keyes, G.S.; Yuan, Z.X.; Miller, V.; Lynch, C.; Honvoh, G.; et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ 2021, 374, n1448. [Google Scholar] [CrossRef]
- Gelaye, B.; Sacco, S.; Brown, W.J.; Nitchie, H.L.; Ornello, R.; Peterlin, B.L. Body composition status and the risk of migraine: A meta-analysis. Neurology 2017, 88, 1795–1804. [Google Scholar] [CrossRef]
- Ornello, R.; Ripa, P.; Pistoia, F.; Degan, D.; Tiseo, C.; Carolei, A.; Sacco, S. Migraine and body mass index categories: A systematic review and meta-analysis of observational studies. J. Headache Pain 2015, 16, 27. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.L.; Martins, L.B.; Dos Santos, L.C.; Henriques, G.S.; Teixeira, A.L.; Dos Santos Rodrigues, A.M.; Matos Ferreira, A.V. Decreased plasma levels and dietary intake of minerals in women with migraine. Nutr. Neurosci. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.G.; Arriza, J.L.; Leff, S.E.; Swanson, L.W.; Evans, R.M.; Rosenfeld, M.G. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 1985, 229, 1094–1097. [Google Scholar] [CrossRef]
- Amara, S.G.; Jonas, V.; Rosenfeld, M.G.; Ong, E.S.; Evans, R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 1982, 298, 240–244. [Google Scholar] [CrossRef]
- Kaiser, E.A.; Russo, A.F. CGRP and migraine: Could PACAP play a role too? Neuropeptides 2013, 47, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Yarwood, R.E.; Imlach, W.L.; Lieu, T.; Veldhuis, N.A.; Jensen, D.D.; Herenbrink, C.K.; Aurelio, L.; Cai, Z.; Christie, M.J.; Poole, D.P.; et al. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc. Natl. Acad. Sci. USA 2017, 114, 12309–12314. [Google Scholar] [CrossRef] [Green Version]
- Hay, D.L.; Walker, C.S. CGRP and its receptors. Headache 2017, 57, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, G.S.; Roosterman, D.; Marvizon, J.C.; Song, B.; Wick, E.; Pikios, S.; Wong, H.; Berthelier, C.; Tang, Y.; Sternini, C.; et al. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J. Comp. Neurol. 2005, 490, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Russo, A.F. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia Int. J. Headache 2019, 39, 1661–1674. [Google Scholar] [CrossRef]
- Hargreaves, R. New migraine and pain research. Headache 2007, 47 (Suppl. S1), S26–S43. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szok, D.; Csáti, A.; Tajti, J. CGRP antagonists and antibodies for the treatment of migraine. Expert Opin. Investig. Drugs 2015, 24, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Gingell, J.J.; Rees, T.A.; Hendrikse, E.R.; Siow, A.; Rennison, D.; Scotter, J.; Harris, P.W.R.; Brimble, M.A.; Walker, C.S.; Hay, D.L. Distinct Patterns of Internalization of Different Calcitonin Gene-Related Peptide Receptors. ACS Pharmacol. Transl. Sci. 2020, 3, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassany, L.; Goadsby, P.J.; Danser, A.H.J.; MaassenVanDenBrink, A. Calcitonin gene-related peptide-targeting drugs for migraine: How pharmacology might inform treatment decisions. Lancet Neurol. 2022, 21, 284–294. [Google Scholar] [CrossRef]
- Bhakta, M.; Vuong, T.; Taura, T.; Wilson, D.S.; Stratton, J.R.; Mackenzie, K.D. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia Int. J. Headache 2021, 41, 499–514. [Google Scholar] [CrossRef]
- Garelja, M.L.; Walker, C.S.; Hay, D.L. CGRP receptor antagonists for migraine. Are they also AMY1 receptor antagonists? Br. J. Pharmacol. 2022, 179, 454–459. [Google Scholar] [CrossRef]
- Brain, S.D.; Russo, A.F.; Hay, D.L. Editorial: Calcitonin Gene-Related Peptide: Novel Biology and Treatments. Front. Physiol. 2022, 13, 964568. [Google Scholar] [CrossRef]
- Harris, R.Z.; Jang, G.R.; Tsunoda, S. Dietary effects on drug metabolism and transport. Clin. Pharmacokinet. 2003, 42, 1071–1088. [Google Scholar] [CrossRef]
- Gazerani, P. A Bidirectional View of Migraine and Diet Relationship. Neuropsychiatr. Dis. Treat. 2021, 17, 435–451. [Google Scholar] [CrossRef]
- Baillie, L.D.; Ahn, A.H.; Mulligan, S.J. Sumatriptan inhibition of N-type calcium channel mediated signaling in dural CGRP terminal fibres. Neuropharmacology 2012, 63, 362–367. [Google Scholar] [CrossRef]
- Slavin, M.; Bourguignon, J.; Jackson, K.; Orciga, M.A. Impact of Food Components on in vitro Calcitonin Gene-Related Peptide Secretion—A Potential Mechanism for Dietary Influence on Migraine. Nutrients 2016, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol. Pain 2010, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Ramos, S.; Martín, M.A.; Goya, L. Effects of Cocoa Antioxidants in Type 2 Diabetes Mellitus. Antioxidants 2017, 6, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbey, M.J.; Patil, V.V.; Vause, C.V.; Durham, P.L. Repression of calcitonin gene-related peptide expression in trigeminal neurons by a Theobroma cacao extract. J. Ethnopharmacol. 2008, 115, 238–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cady, R.J.; Durham, P.L. Cocoa-enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res. 2010, 1323, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, Z.; Rafiee, P.; Fotouhi, A.; Haghighi, S.; Magham, R.R.; Ahmadi, Z.S.; Djalali, M.; Zareei, M.; Jahromi, S.R.; Shahemi, S.; et al. The effects of vitamin D supplementation on interictal serum levels of calcitonin gene-related peptide (CGRP) in episodic migraine patients: Post hoc analysis of a randomized double-blind placebo-controlled trial. J. Headache Pain 2020, 21, 22. [Google Scholar] [CrossRef] [Green Version]
- Tauchen, J. Natural Products and their (Semi-)Synthetic Forms in the Treatment of Migraine: History and Current Status. Curr. Med. Chem. 2020, 27, 3784–3808. [Google Scholar] [CrossRef]
- Rezaie, S.; Askari, G.; Khorvash, F.; Tarrahi, M.J.; Amani, R. Effects of Curcumin Supplementation on Clinical Features and Inflammation, in Migraine Patients: A Double-Blind Controlled, Placebo Randomized Clinical Trial. Int. J. Prev. Med. 2021, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Almohaimeed, H.M.; Mohammedsaleh, Z.M.; Batawi, A.H.; Balgoon, M.J.; Ramadan, O.I.; Baz, H.A.; Al Jaouni, S.; Ayuob, N.N. Synergistic Anti-inflammatory and Neuroprotective Effects of Cinnamomum cassia and Zingiber officinale Alleviate Diabetes-Induced Hippocampal Changes in Male Albino Rats: Structural and Molecular Evidence. Front. Cell Dev. Biol. 2021, 9, 727049. [Google Scholar] [CrossRef] [PubMed]
- Zareie, A.; Sahebkar, A.; Khorvash, F.; Bagherniya, M.; Hasanzadeh, A.; Askari, G. Effect of cinnamon on migraine attacks and inflammatory markers: A randomized double-blind placebo-controlled trial. Phytother. Res. 2020, 34, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tomé, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 2012, 25, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, C.; Lasbleiz, A.; Guye, M.; Dutour, A.; Gaborit, B.; Ranjeva, J.P. The Role of the Human Hypothalamus in Food Intake Networks: An MRI Perspective. Front. Nutr. 2022, 8, 760914. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, G.S.; Alemi, F.; Kirkland, J.G.; Grady, E.F.; Corvera, C.U.; Bhargava, A. Localization of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) in human gastrointestinal tract. Peptides 2012, 35, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Kamiyoshi, A.; Sakurai, T.; Ichikawa-Shindo, Y.; Kawate, H.; Yang, L.; Tanaka, M.; Xian, X.; Imai, A.; Zhai, L.; et al. Endogenous Calcitonin Gene-Related Peptide Regulates Lipid Metabolism and Energy Homeostasis in Male Mice. Endocrinology 2017, 158, 1194–1206. [Google Scholar] [CrossRef] [Green Version]
- Sanford, D.; Luong, L.; Gabalski, A.; Oh, S.; Vu, J.P.; Pisegna, J.R.; Germano, P. An Intraperitoneal Treatment with Calcitonin Gene-Related Peptide (CGRP) Regulates Appetite, Energy Intake/Expenditure, and Metabolism. J. Mol. Neurosci. 2019, 67, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.A.; Calchary, W.A.; Nandar, W. Effect of calcitonin gene-related peptide (CGRP) on avian appetite-related processes. Behav. Brain Res. 2009, 196, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Jing, M.Y.; Wang, J.F.; Weng, X.Y. The approach to the mechanism of calcitonin gene-related peptide-inducing inhibition of food intake. J. Anim. Physiol. Anim. Nutr. 2010, 94, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, R.M.; Morse, J.R.; Norgren, R. The nucleus of the solitary tract in the monkey: Projections to the thalamus and brain stem nuclei. J. Comp. Neurol. 1980, 190, 259–282. [Google Scholar] [CrossRef]
- Herbert, H.; Moga, M.M.; Saper, C.B. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990, 293, 540–580. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.E.; Han, S.; Palmiter, R.D. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 4582–4586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luquet, S.H.; Vaudry, H.; Granata, R. Editorial: Neuroendocrine Control of Feeding Behavior. Front. Endocrinol. 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Thomas, D.M.; Tudor-Locke, C.; Katzmarzyk, P.T.; Earnest, C.P.; Rodarte, R.Q.; Martin, C.K.; Blair, S.N.; Bouchard, C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS ONE 2011, 6, e19657. [Google Scholar] [CrossRef] [Green Version]
- Bond, D.S.; Roth, J.; Nash, J.M.; Wing, R.R. Migraine and obesity: Epidemiology, possible mechanisms and the potential role of weight loss treatment. Obes. Rev. 2011, 12, e362–e371. [Google Scholar] [CrossRef] [Green Version]
- Zelissen, P.M.; Koppeschaar, H.P.; Lips, C.J.; Hackeng, W.H. Calcitonin gene-related peptide in human obesity. Peptides 1991, 12, 861–863. [Google Scholar] [CrossRef]
- Gram, D.X.; Hansen, A.J.; Wilken, M.; Elm, T.; Svendsen, O.; Carr, R.D.; Ahrén, B.; Brand, C.L. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur. J. Endocrinol. 2005, 153, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.S.; Li, X.; Whiting, L.; Glyn-Jones, S.; Zhang, S.; Hickey, A.J.; Sewell, M.A.; Ruggiero, K.; Phillips, A.R.; Kraegen, E.W.; et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 2010, 151, 4257–4269. [Google Scholar] [CrossRef] [Green Version]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Fagherazzi, G.; El Fatouhi, D.; Fournier, A.; Gusto, G.; Mancini, F.R.; Balkau, B.; Boutron-Ruault, M.C.; Kurth, T.; Bonnet, F. Associations Between Migraine and Type 2 Diabetes in Women: Findings from the E3N Cohort Study. JAMA Neurol. 2019, 76, 257–263. [Google Scholar] [CrossRef]
- Rivera-Mancilla, E.; Al-Hassany, L.; Villalón, C.M.; MaassenVanDenBrink, A. Metabolic Aspects of Migraine: Association with Obesity and Diabetes Mellitus. Front. Neurol. 2021, 12, 686398. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Ahrén, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, S.A.; Walia, M.; Drury, M.; Petrov, M.S. Calcitonin gene-related peptide: Neuroendocrine communication between the pancreas, gut, and brain in regulation of blood glucose. Ann. Transl. Med. 2017, 5, 419. [Google Scholar] [CrossRef] [Green Version]
- Halloran, J.; Lalande, A.; Zang, M.; Chodavarapu, H.; Riera, C.E. Monoclonal therapy against calcitonin gene-related peptide lowers hyperglycemia and adiposity in type 2 diabetes mouse models. Metab. Open 2020, 8, 100060. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.; Schaeffler, B.; Hirman, J.; Hompesch, M.; Pederson, S.; Smith, J. Tolerability of eptinezumab in overweight, obese or type 1 diabetes patients. Endocrinol. Diabetes Metab. 2021, 4, e00217. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Rhew, K. Association between Gastrointestinal Diseases and Migraine. Int. J. Environ. Res. Public Health 2022, 19, 4018. [Google Scholar] [CrossRef]
- Ailani, J.; Kaiser, E.A.; Mathew, P.G.; McAllister, P.; Russo, A.F.; Vélez, C.; Ramajo, A.P.; Abdrabboh, A.; Xu, C.; Rasmussen, S.; et al. Role of Calcitonin Gene-Related Peptide on the Gastrointestinal Symptoms of Migraine-Clinical Considerations: A Narrative Review. Neurology 2022, 99, 841–853. [Google Scholar] [CrossRef]
- Ma, W.J.; Yin, Y.C.; Zhang, B.K.; Li, Y.J.; Li, W.Q. Calcitonin gene-related peptide-mediated pharmacological effects in cardiovascular and gastrointestinal diseases (Review). Mol. Med. Rep. 2021, 23, 27. [Google Scholar] [CrossRef]
- Holzer, P.; Holzer-Petsche, U. Constipation Caused by Anti-calcitonin Gene-Related Peptide Migraine Therapeutics Explained by Antagonism of Calcitonin Gene-Related Peptide’s Motor-Stimulating and Prosecretory Function in the Intestine. Front. Physiol. 2021, 12, 820006. [Google Scholar] [CrossRef]
- Falkenberg, K.; Bjerg, H.R.; Olesen, J. Two-Hour CGRP Infusion Causes Gastrointestinal Hyperactivity: Possible Relevance for CGRP Antibody Treatment. Headache J. Head Face Pain 2020, 60, 929–937. [Google Scholar] [CrossRef]
- Vernieri, F.; Altamura, C.; Brunelli, N.; Costa, C.M.; Aurilia, C.; Egeo, G.; Fofi, L.; Favoni, V.; Pierangeli, G.; Lovati, C.; et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: A multicenter prospective cohort study (the GARLIT study). J. Headache Pain 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, F.; Brunelli, N.; Marcosano, M.; Aurilia, C.; Egeo, G.; Lovati, C.; Favoni, V.; Perrotta, A.; Maestrini, I.; Rao, R.; et al. Maintenance of response and predictive factors of 1-year GalcanezumAb treatment in real-life migraine patients in Italy: The multicenter prospective cohort GARLIT study. Eur. J. Neurol. 2023, 30, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Nagy, A.J.; Janelidze, M.; Giorgadze, G.; Hirman, J.; Cady, R.; Mehta, L.; Buse, D.C. Impact of Baseline Characteristics on the Efficacy and Safety of Eptinezumab in Patients with Migraine: Subgroup Analyses of PROMISE-1 and PROMISE-2. Clin. Ther. 2022, 44, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Cesaris, F.; Ferrari, A.; Benemei, S.; Fattori, D.; Chiarugi, A. Effectiveness of anti-CGRP monoclonal antibodies on central symptoms of migraine. Cephalalgia Int. J. Headache 2022, 42, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, F.; Altamura, C.; Brunelli, N.; Costa, C.M.; Aurilia, C.; Egeo, G.; Fofi, L.; Favoni, V.; Lovati, C.; Bertuzzo, D.; et al. Rapid response to galcanezumab and predictive factors in chronic migraine patients: A 3-month observational, longitudinal, cohort, multicenter, Italian real-life study. Eur. J. Neurol. 2022, 29, 1198–1208. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Nyári, A.; Vécsei, L. CGRP and CGRP-Receptor as Targets of Migraine Therapy: Brain Prize-2021. CNS Neurol. Disord. Drug Targets 2022, 21, 460–478. [Google Scholar] [CrossRef]

| Diet | Feature | Reference |
|---|---|---|
| Healthy Eating Plate | Half of the plate is dedicated to fruits and vegetables, a quarter to whole grains, and a quarter to proteins. | [24,25] |
| Ketogenic diet | A strong restriction of carbohydrates with a higher intake of lipids and proteins. | [26,27,28] |
| Gluten-free diet | Avoids ingestion of wheat, rye, barley, malt, and their derivatives and, instead, encompasses gluten-free alternatives such as rice, quinoa, corn, and potatoes and food groups that are naturally devoid of gluten such as fruits, vegetables, seafood, meat, legumes, nuts, and most dairy products. | [29,30,31] |
| Low-calorie diet | Assumes consuming around 1200 to 1500 calories per day | [32] |
| Low-glycemic index diet | Contains items such as most non-starchy vegetables, beans and legumes, nuts, and most fruits, but not pineapple and watermelon | [33,34] |
| Mediterranean diet | Emphasis on whole grains, vegetables, legumes, fruits, nuts, olive oil, and moderate animal-based protein, excluding meat | [35,36,37] |
| Fatty acid diet | Various adjustments to the composition of fatty acids | [38,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fila, M.; Chojnacki, J.; Sobczuk, P.; Chojnacki, C.; Blasiak, J. Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine. Nutrients 2023, 15, 289. https://doi.org/10.3390/nu15020289
Fila M, Chojnacki J, Sobczuk P, Chojnacki C, Blasiak J. Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine. Nutrients. 2023; 15(2):289. https://doi.org/10.3390/nu15020289
Chicago/Turabian StyleFila, Michal, Jan Chojnacki, Piotr Sobczuk, Cezary Chojnacki, and Janusz Blasiak. 2023. "Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine" Nutrients 15, no. 2: 289. https://doi.org/10.3390/nu15020289
APA StyleFila, M., Chojnacki, J., Sobczuk, P., Chojnacki, C., & Blasiak, J. (2023). Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine. Nutrients, 15(2), 289. https://doi.org/10.3390/nu15020289







