Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants (Subjects)
2.3. MED-01 and Placebo Capsules
2.4. Outcomes
2.4.1. Nugent Score
2.4.2. Vaginal Conditions and Symptoms
2.4.3. Vaginal Microbiota
2.5. Safety Assessment
2.6. Statistical Analysis
3. Results
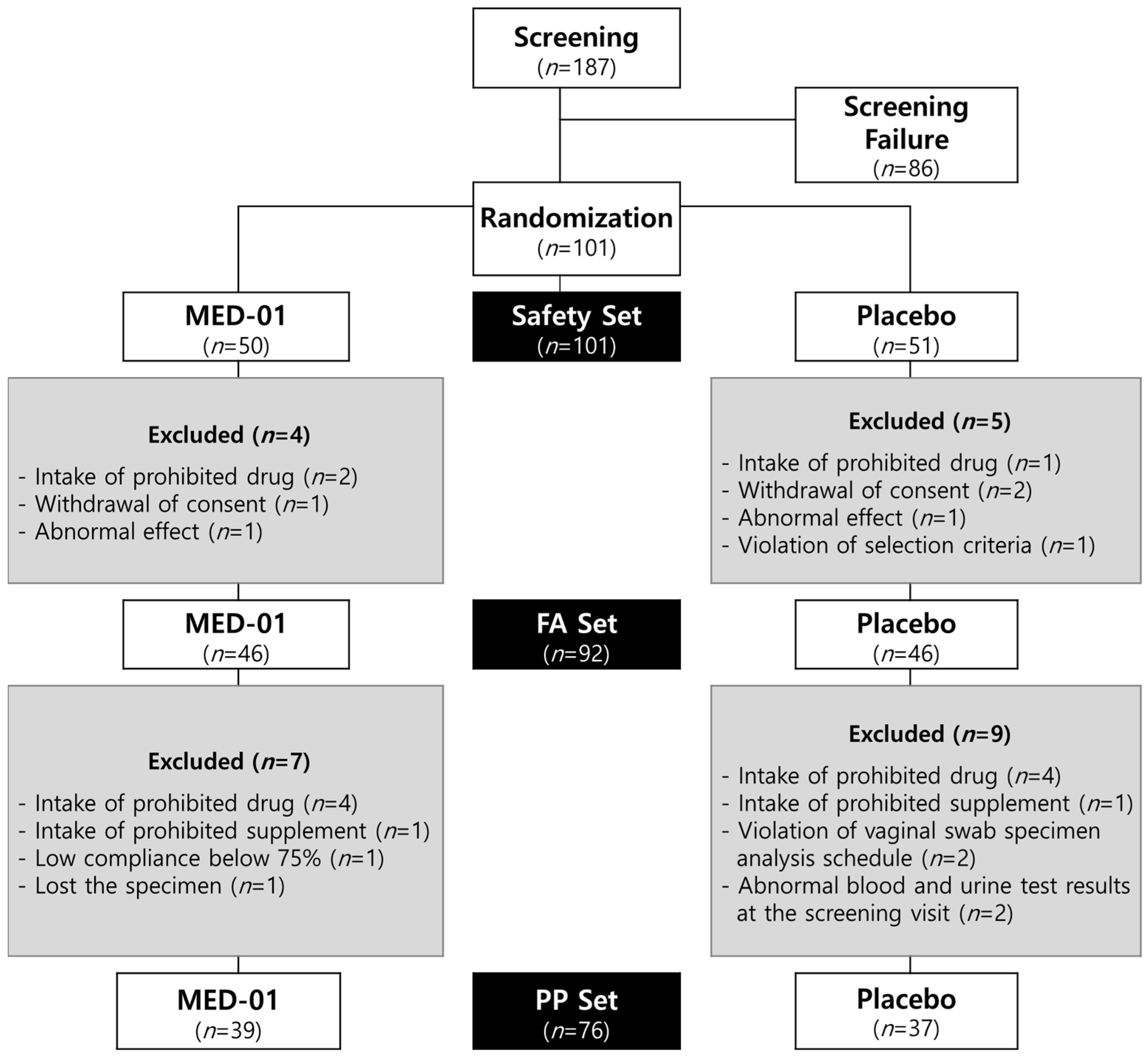
3.1. Participants
3.2. Baseline Characteristics of Participants
3.3. Effect of MED-01 on the Nugent Score and Vaginal pH
3.4. Effect of MED-01 on Vaginal Symptoms
3.5. Effect of MED-01 on Vaginal Microbiota
3.6. Safety of MED-01 and Placebo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagnall, P.; Rizzolo, D. Bacterial vaginosis: A practical review. J. Am. Acad. PAs 2017, 30, 15–21. [Google Scholar] [CrossRef]
- Ness, R.B.; Hillier, S.L.; Kip, K.E.; Soper, D.E.; Stamm, C.A.; McGregor, J.A.; Bass, D.C.; Sweet, R.L.; Rice, P.; Richter, H.E. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet. Gynecol. 2004, 104, 761–769. [Google Scholar] [CrossRef]
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef]
- Atashili, J.; Poole, C.; Ndumbe, P.M.; Adimora, A.A.; Smith, J.S. Bacterial vaginosis and HIV acquisition: A meta-analysis of published studies. Aids 2008, 22, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.S.; Vodstrcil, L.A.; Hocking, J.S.; Law, M.; Pirotta, M.; Garland, S.M.; De Guingand, D.; Morton, A.N.; Fairley, C.K. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin. Infect. Dis. 2013, 56, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Sobel, J.D.; Ferris, D.; Schwebke, J.; Nyirjesy, P.; Wiesenfeld, H.C.; Peipert, J.; Soper, D.; Ohmit, S.E.; Hillier, S.L. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 2006, 194, 1283–1289. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations: London, UK; World Health Organization: Toronto, ON, Canada, 2002. [Google Scholar]
- You, Y.A.; Kwon, E.J.; Choi, S.J.; Hwang, H.S.; Choi, S.K.; Lee, S.M.; Kim, Y.J. Vaginal microbiome profiles of pregnant women in Korea using a 16S metagenomics approach. Am. J. Reprod. Immunol. 2019, 82, e13124. [Google Scholar] [CrossRef]
- Chang, D.-H.; Shin, J.; Rhee, M.-S.; Park, K.-R.; Cho, B.-K.; Lee, S.-K.; Kim, B.-C. Vaginal microbiota profiles of native Korean women and associations with high-risk pregnancy. J. Microbiol. Biotechnol. 2020, 30, 248–258. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Bohbot, J.; Daraï, E.; Bretelle, F.; Brami, G.; Daniel, C.; Cardot, J. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 81–86. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Chetwin, E.; Manhanzva, M.T.; Abrahams, A.G.; Froissart, R.; Gamieldien, H.; Jaspan, H.; Jaumdally, S.Z.; Barnabas, S.L.; Dabee, S.; Happel, A.-U. Antimicrobial and inflammatory properties of South African clinical Lactobacillus isolates and vaginal probiotics. Sci. Rep. 2019, 9, 1917. [Google Scholar] [CrossRef] [Green Version]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol 2013, 36, 229–238. [Google Scholar]
- Reid, G.; Dols, J.; Miller, W. Targeting the vaginal microbiota with probiotics as a means to counteract infections. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 583–587. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Li, Y.; Zhang, T.; Wang, Q.; He, J.; Wang, L.; Li, L.; Yang, N.; Fang, Y. Probiotics for the treatment of women with bacterial vaginosis: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 2019, 864, 172660. [Google Scholar] [CrossRef]
- Choi, S.-I.; Won, G.; Kim, Y.; Kang, C.-H.; Kim, G.-H. Lactobacilli Strain Mixture Alleviates Bacterial Vaginosis through Antibacterial and Antagonistic Activity in Gardnerella vaginalis-Infected C57BL/6 Mice. Microorganisms 2022, 10, 471. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kang, C.-H. In vivo confirmation of the antimicrobial effect of probiotic candidates against Gardnerella vaginalis. Microorganisms 2021, 9, 1690. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Nelson, T.M.; Borgogna, J.; Michalek, R.; Roberts, D.W.; Rath, J.; Glover, E.; Ravel, J.; Shardell, M.; Yeoman, C.J.; Brotman, R.M. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep. 2018, 8, 852. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Kim, J.Y.; Lee, M.Y.; Choi, S.J.; Oh, S.y.; Shim, J.Y.; Roh, C.R. Prospective study of bidet toilet use: Association of abnormal vaginal colonization and preterm birth in high-risk pregnant women. J. Obstet. Gynaecol. Res. 2019, 45, 1134–1142. [Google Scholar] [CrossRef]
- Choi, J.S. Feminine hygiene behaviors and risk factors for bacterial vaginosis in female university students. J. Korean Acad. Soc. Home Health Care Nurs. 2018, 25, 87–95. [Google Scholar]
- Klebanoff, M.A.; Nansel, T.R.; Brotman, R.M.; Zhang, J.; Yu, K.-F.; Schwebke, J.R.; Andrews, W.W. Personal hygienic behaviors and bacterial vaginosis. Sex. Transm. Dis. 2010, 37, 94. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Richey, C.M.; Weiss, H.L. Correlation of behaviors with microbiological changes in vaginal flora. J. Infect. Dis. 1999, 180, 1632–1636. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [Green Version]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Walsh, A.M.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef]
- De Alberti, D.; Russo, R.; Terruzzi, F.; Nobile, V.; Ouwehand, A.C. Lactobacilli vaginal colonisation after oral consumption of Respecta® complex: A randomised controlled pilot study. Arch. Gynecol. Obstet. 2015, 292, 861–867. [Google Scholar] [CrossRef]
- Coleman, J.S.; Gaydos, C.A. Molecular diagnosis of bacterial vaginosis: An update. J. Clin. Microbiol. 2018, 56, e00342-18. [Google Scholar] [CrossRef] [Green Version]
- van den Munckhof, E.H.; van Sitter, R.L.; Boers, K.E.; Lamont, R.F.; Te Witt, R.; le Cessie, S.; Knetsch, C.W.; van Doorn, L.-J.; Quint, W.G.; Molijn, A. Comparison of Amsel criteria, Nugent score, culture and two CE-IVD marked quantitative real-time PCRs with microbiota analysis for the diagnosis of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 959–966. [Google Scholar] [CrossRef]
- Sharpe, M.; Shah, V.; Freire-Lizama, T.; Cates, E.C.; McGrath, K.; David, I.; Cowan, S.; Letkeman, J.; Stewart-Wilson, E. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: A midwifery-led double-blind randomized controlled pilot trial. J. Matern. Fetal Neonatal Med. 2021, 34, 1814–1821. [Google Scholar] [CrossRef]
- Russo, R.; Edu, A.; De Seta, F. Study on the effects of an oral lactobacilli and lactoferrin complex in women with intermediate vaginal microbiota. Arch. Gynecol. Obstet. 2018, 298, 139–145. [Google Scholar] [CrossRef]
- Dols, J.A.; Molenaar, D.; van der Helm, J.J.; Caspers, M.P.; de Kat Angelino-Bart, A.; Schuren, F.H.; Speksnijder, A.G.; Westerhoff, H.V.; Richardus, J.H.; Boon, M.E. Molecular assessment of bacterial vaginosis by Lactobacillus abundance and species diversity. BMC Infect. Dis. 2016, 16, 180. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Oh, K.Y.; Hong, H.; Jin, C.H.; Shim, E.; Kim, S.H.; Kim, B.-Y. Community state types of vaginal microbiota and four types of abnormal vaginal microbiota in pregnant Korean women. Front. Public Health 2020, 8, 507024. [Google Scholar] [CrossRef]
- Srinivasan, S.; Liu, C.; Mitchell, C.M.; Fiedler, T.L.; Thomas, K.K.; Agnew, K.J.; Marrazzo, J.M.; Fredricks, D.N. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE 2010, 5, e10197. [Google Scholar] [CrossRef] [Green Version]
- Morrill, S.; Gilbert, N.M.; Lewis, A.L. Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence from in vivo Models. Front. Cell. Infect. Microbiol. 2020, 10, 168. [Google Scholar] [CrossRef]
- Mendling, W.; Palmeira-de-Oliveira, A.; Biber, S.; Prasauskas, V. An update on the role of Atopobium vaginae in bacterial vaginosis: What to consider when choosing a treatment? A mini review. Arch. Gynecol. Obstet. 2019, 300, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, M.C.; Desmond, R.A.; Schwebke, J.R. Association of Mobiluncus curtisii with recurrence of bacterial vaginosis. Sex. Transm. Dis. 2008, 35, 611. [Google Scholar] [CrossRef] [Green Version]
- Africa, C.W.; Nel, J.; Stemmet, M. Anaerobes and bacterial vaginosis in pregnancy: Virulence factors contributing to vaginal colonisation. Int. J. Environ. Res. Public Health 2014, 11, 6979–7000. [Google Scholar] [CrossRef]
- Amegashie, C.P.; Gilbert, N.M.; Peipert, J.F.; Allsworth, J.E.; Lewis, W.G.; Lewis, A.L. Relationship between nugent score and vaginal epithelial exfoliation. PLoS ONE 2017, 12, e0177797. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.M.A.; Diniz, C.G.; Fernandes, A.A.S.; Souza-Sotte, D.M.K.; Freitas, M.C.R.; Machado, A.B.F.; Silva, V.L. Assessment of vaginal microbiota in Brazilian women with and without bacterial vaginosis and comparison with Nugent score. J. Infect. Dev. Ctries. 2018, 12, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Malaguti, N.; Bahls, L.D.; Uchimura, N.S.; Gimenes, F.; Consolaro, M.E.L. Sensitive detection of thirteen bacterial vaginosis-associated agents using multiplex polymerase chain reaction. BioMed Res. Int. 2015, 2015, 645853. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-H.; Wu, S.-R.; Pang, J.-C.; Ramireddy, L.; Chiang, Y.-C.; Lin, C.-K.; Tsen, H.-Y. Designing primers and evaluation of the efficiency of propidium monoazide–Quantitative polymerase chain reaction for counting the viable cells of Lactobacillus gasseri and Lactobacillus salivarius. J. Food Drug Anal. 2017, 25, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendimann, L.; Kauf, P.; Fieseler, L.; Gantenbein-Demarchi, C.; Schwenninger, S.M. Development of a quantitative PCR assay for rapid detection of Lactobacillus plantarum and Lactobacillus fermentum in cocoa bean fermentation. J. Microbiol. Methods 2015, 115, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Daroczy, K.; Xiao, B.; Yu, L.; Chen, R.; Liao, Q. Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J. Med. Microbiol. 2012, 61, 729–739. [Google Scholar] [CrossRef] [PubMed]




| Organism (Morphotype) | Number/Oil Immersion Field | Score |
|---|---|---|
| Lactobacillus-like (parallel sided, Gram-positive rods) | >30 | 0 |
| 5–30 | 1 | |
| 1–4 | 2 | |
| <1 | 3 | |
| 0 | 4 | |
| Mobiluncus-like (curved, Gram-negative rods) | >5 | 2 |
| <1–4 | 1 | |
| 0 | 0 | |
| Gardnerella/bacteroides-like (tiny, gram variable coccobacilli and pleomorphic rods with vacuoles) | >30 | 4 |
| 5–30 | 3 | |
| 1–4 | 2 | |
| <1 | 1 | |
| 0 | 0 |
| Variable | MED-01 (n = 39) | Placebo (n = 37) | p-Value | |
|---|---|---|---|---|
| Sex (female, %) | 100 | 100 | ||
| Age (years) | 39.56 ± 6.58 | 37.03 ± 7.15 | 0.111 T | |
| Drinking | (Y/N) | 20/19 | 20/17 | 1.000 F |
| Smoking | (Y/N) | 4/35 | 1/36 | 0.163 F |
| Frequency of exercising | ||||
| 0–2 times/week | 32 (82.05) | 27 (72.97) | 0.429 F | |
| 3–7 times/week | 7 (17.95) | 10 (27.02) | ||
| Frequency of showering | ||||
| 1–3 times/week | 4 (10.26) | 3 (8.11) | 0.668 F | |
| 4–7 times/week | 34 (87.18) | 31 (83.78) | ||
| 2 or more times/week | 1 (2.56) | 3 (8.11) | ||
| Usage of swimming pool | (Y/N) | 1/38 | 0/37 | 1.000 F |
| Usage of public baths | (Y/N) | 1/38 | 3/34 | 0.418 F |
| Usage of bidet | (Y/N) | 11/28 | 6/37 | 0.210 C |
| Types of sanitary products | ||||
| disposable sanitary pad | (Y/N) | 36/3 | 34/3 | 1.000 F |
| cotton sanitary napkins | (Y/N) | 2/37 | 3/34 | 0.671 F |
| menstrual cup | (Y/N) | 2/37 | 1/36 | 1.000 F |
| tampon | (Y/N) | 3/36 | 7/30 | 0.186 F |
| Frequency of intercourse | ||||
| none | 12 (30.77) | 13 (35.14) | 0.251 F | |
| 1 time/month | 20 (51.28) | 12 (32.43) | ||
| 1 time/week | 4 (10.26) | 9 (24.32) | ||
| 2–3 times/week | 2 (5.13) | 3 (8.11) | ||
| 4 or more times/week | 1 (2.56) | 0 (0.00) | ||
| Variable | MED-01 (n = 39) | Placebo (n = 37) | |||
|---|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | p-Value | |
| Nugent score | |||||
| 0–3 (Normal) | - | 10 (25.6%) | - | 5 (13.5%) | 0.382 |
| 4–6 (Intermediate) | 39 (100%) | 23 (59.0%) | 37 (100%) | 24 (64.9%) | |
| 7–9 (BV) | - | 6 (15.4%) | - | 8 (21.6%) | |
| Variable | MED-01 (n = 50) | Placebo (n = 51) | p-Value | ||
|---|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | ||
| Hematology | |||||
| RBC (1012/L) | 4.26 ± 0.29 | 4.30 ± 0.28 | 4.30 ± 0.31 | 4.25 ± 0.25 | 0.134 T |
| Hb (g/dL) | 12.64 ± 1.10 | 12.80 ± 1.09 | 12.83 ± 1.18 | 12.74 ± 1.32 | 0.075 W |
| Hct (%) | 38.15 ± 2.73 | 38.46 ± 2.63 | 38.66 ± 3.06 | 38.43 ± 3.30 | 0.229 T |
| WBC (109/L) | 5.69 ± 1.54 | 5.83 ± 1.81 | 5.98 ± 1.75 | 5.89 ± 1.88 | 0.809 W |
| Platelet (103/µL) | 242.92 ± 50.67 | 251.71 ± 54.35 | 269.63 ± 66.38 | 269.48 ± 62.27 | 0.215 T |
| Neutrophil (%) | 56.23 ± 7.44 | 56.35 ± 8.65 | 58.49 ± 9.86 | 56.54 ± 8.97 | 0.787 W |
| Lymphocyte (%) | 33.46 ± 6.89 | 33.52 ± 7.80 | 31.48 ± 8.69 | 32.84 ± 8.60 | 0.490 T |
| Monocyte (%) | 6.60 ± 1.81 | 6.45 ± 1.88 | 6.45 ± 2.09 | 6.75 ± 2.18 | 0.234 T |
| Eosinophil (%) | 2.40 ± 1.61 | 2.37 ± 1.66 | 2.24 ± 1.64 | 2.54 ± 2.20 | 0.204 W |
| Basophil (%) | 0.69 ± 0.34 | 0.59 ± 0.36 | 0.64 ± 0.31 | 0.58 ± 0.29 | 0.934 W |
| MCV (fL) | 89.74 ± 4.83 | 89.44 ± 4.95 | 90.02 ± 5.31 | 90.43 ± 6.19 | 0.819 T |
| Blood chemistry | |||||
| AST (IU/L) | 20.48 ± 8.54 | 20.56 ± 6.21 | 19.10 ± 4.98 | 19.28 ± 5.26 | 0.811 W |
| ALT (IU/L) | 15.48 ± 7.98 | 16.69 ± 9.01 | 14.76 ± 8.84 | 14.61 ± 9.29 | 0.630 W |
| Protein (g/dL) | 7.21 ± 0.34 | 7.23 ± 0.38 | 7.25 ± 0.35 | 7.25 ± 0.43 | 0.936 T |
| Albumin (g/dL) | 4.40 ± 0.26 | 4.43 ± 0.24 | 4.50 ± 0.27 | 4.43 ± 0.28 | 0.132 W |
| Total bilirubin (mg/dL) | 0.61 ± 0.26 | 0.64 ± 0.28 | 0.65 ± 0.31 | 0.60 ± 0.27 | 0.087 W |
| ALP (IU/L) | 54.62 ± 13.93 | 55.98 ± 12.73 | 53.27 ± 13.85 | 54.54 ± 13.17 | 0.371 T |
| Creatinine (mg/dL) | 0.67 ± 0.10 | 0.66 ± 0.10 | 0.64 ± 0.09 | 0.64 ± 0.09 | 0.678 T |
| BUN (mg/dL) | 12.40 ± 2.90 | 12.44 ± 2.77 | 11.16 ± 2.63 | 11.00 ± 2.54 | 0.991 W |
| Uric acid (mg/dL) | 4.20 ± 0.92 | 4.39 ± 1.20 | 4.25 ± 0.94 | 4.20 ± 1.00 | 0.169 W |
| γ-GTP | 15.32 ± 11.89 | 16.02 ± 10.56 | 17.37 ± 12.79 | 16.74 ± 10.95 | 0.767 W |
| Anthropometry | |||||
| Systolic blood pressure (mmHg) | 114.38 ± 13.73 | 118.47 ± 14.93 | 114.43 ± 12.73 | 113.35 ± 12.77 | 0.072 T |
| Diastolic blood pressure (mmHg) | 70.60 ± 11.29 | 71.36 ± 13.70 | 71.08 ± 8.79 | 70.11 ± 8.93 | 0.338 T |
| Pulse (times/min) | 78.10 ± 9.63 | 81.20 ± 10.83 | 81.96 ± 10.78 | 81.37 ± 9.03 | 0.112 T |
| Body weight (kg) | 58.08 ± 8.12 | 58.23 ± 8.49 | 57.20 ± 7.30 | 56.67 ± 7.20 | 0.605 T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Lee, E.S.; Park, S.T.; Jeong, S.Y.; Yun, Y.; Kim, Y.; Jeong, Y.; Kang, C.-H.; Choi, H.J. Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 331. https://doi.org/10.3390/nu15020331
Park S-H, Lee ES, Park ST, Jeong SY, Yun Y, Kim Y, Jeong Y, Kang C-H, Choi HJ. Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(2):331. https://doi.org/10.3390/nu15020331
Chicago/Turabian StylePark, Sung-Ho, Eun Sil Lee, Sung Taek Park, Soo Young Jeong, Yeoul Yun, YongGyeong Kim, Yulah Jeong, Chang-Ho Kang, and Hyun Jin Choi. 2023. "Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 15, no. 2: 331. https://doi.org/10.3390/nu15020331
APA StylePark, S.-H., Lee, E. S., Park, S. T., Jeong, S. Y., Yun, Y., Kim, Y., Jeong, Y., Kang, C.-H., & Choi, H. J. (2023). Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 15(2), 331. https://doi.org/10.3390/nu15020331





