Analysis of Fecal Short-Chain Fatty Acids (SCFAs) in Healthy Children during the First Two Years of Life: An Observational Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Brief Description of the Conducted Measurements
2.3. Ethical Information
2.4. Stool Sampling Scheme
2.5. Isolation of SCFA
2.6. Gas Chromatography
2.7. Statistical Analysis
3. Results
3.1. SCFA Concentrations over Time
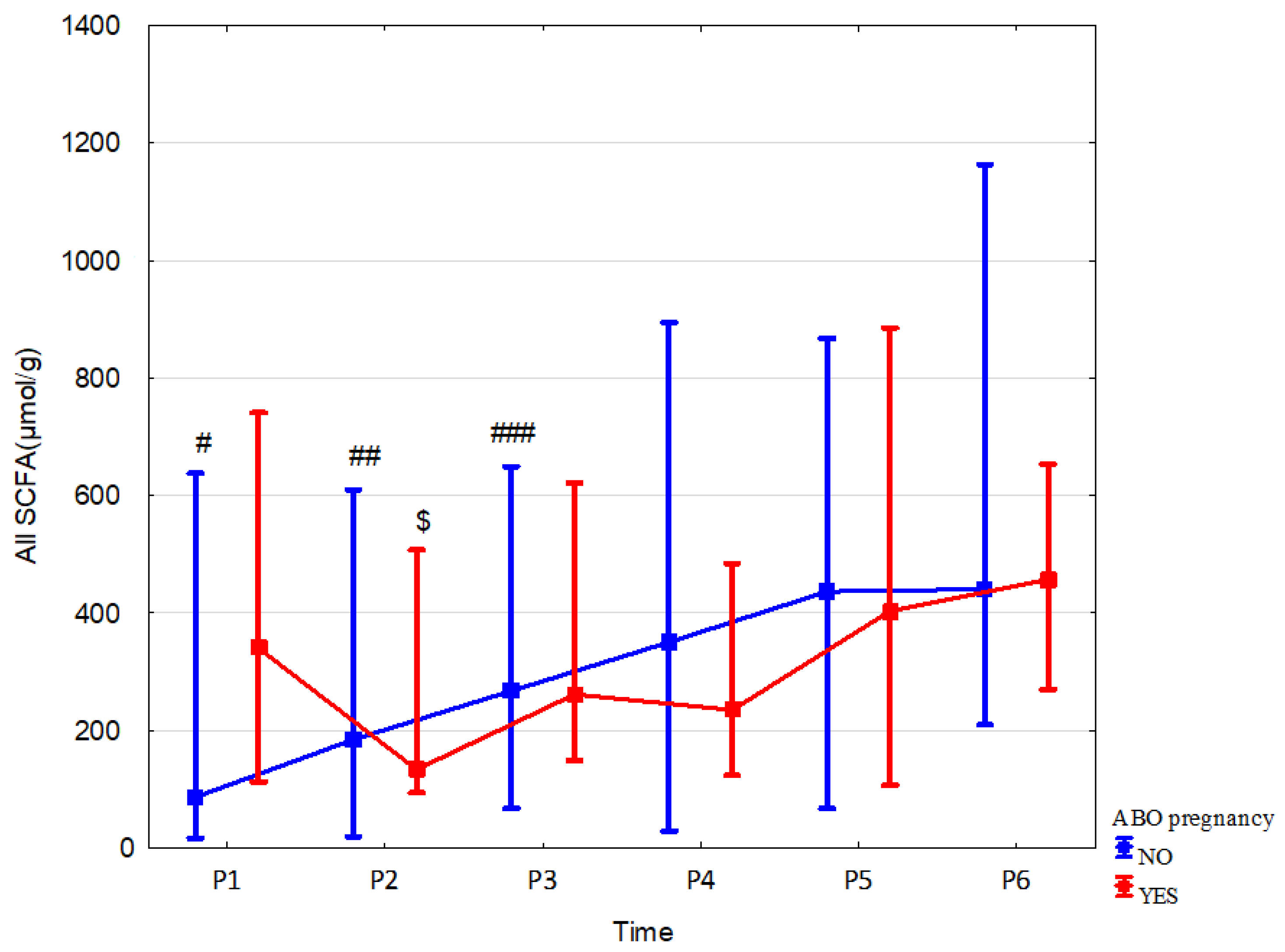
3.2. Effect of Antibiotic Therapy during Pregnancy
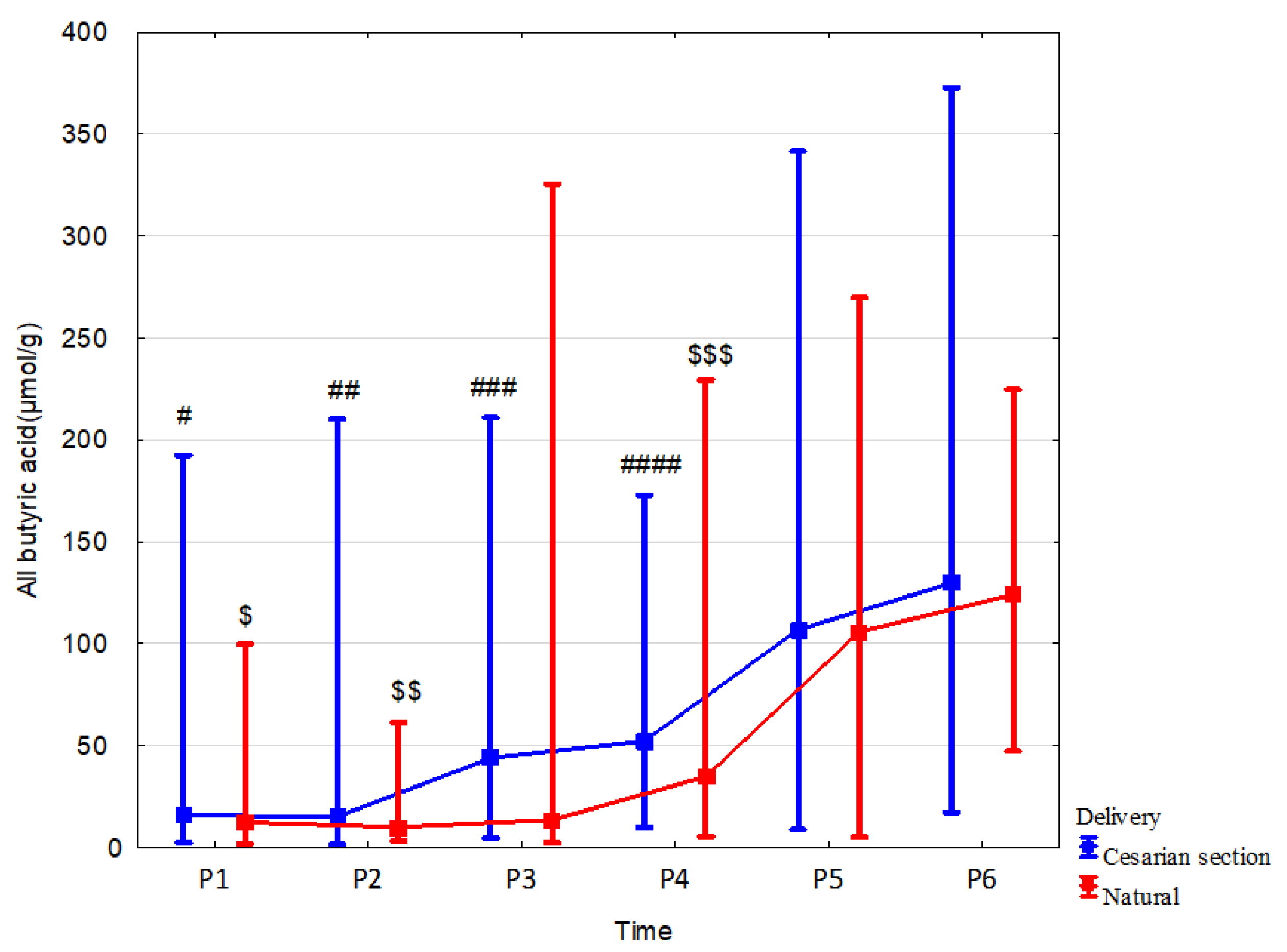
3.3. Effect of Delivery Mode
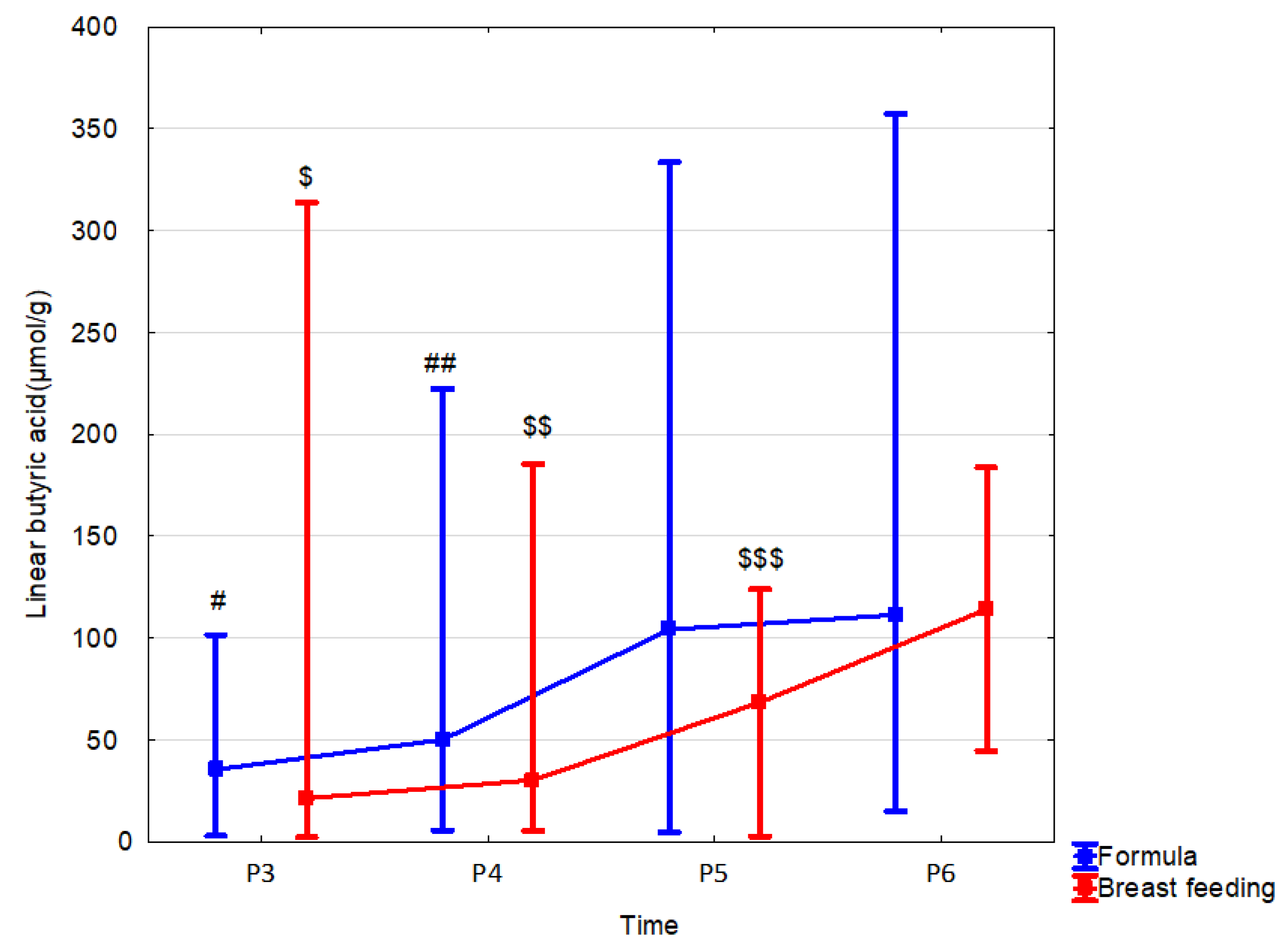
3.4. Effect of Feeding
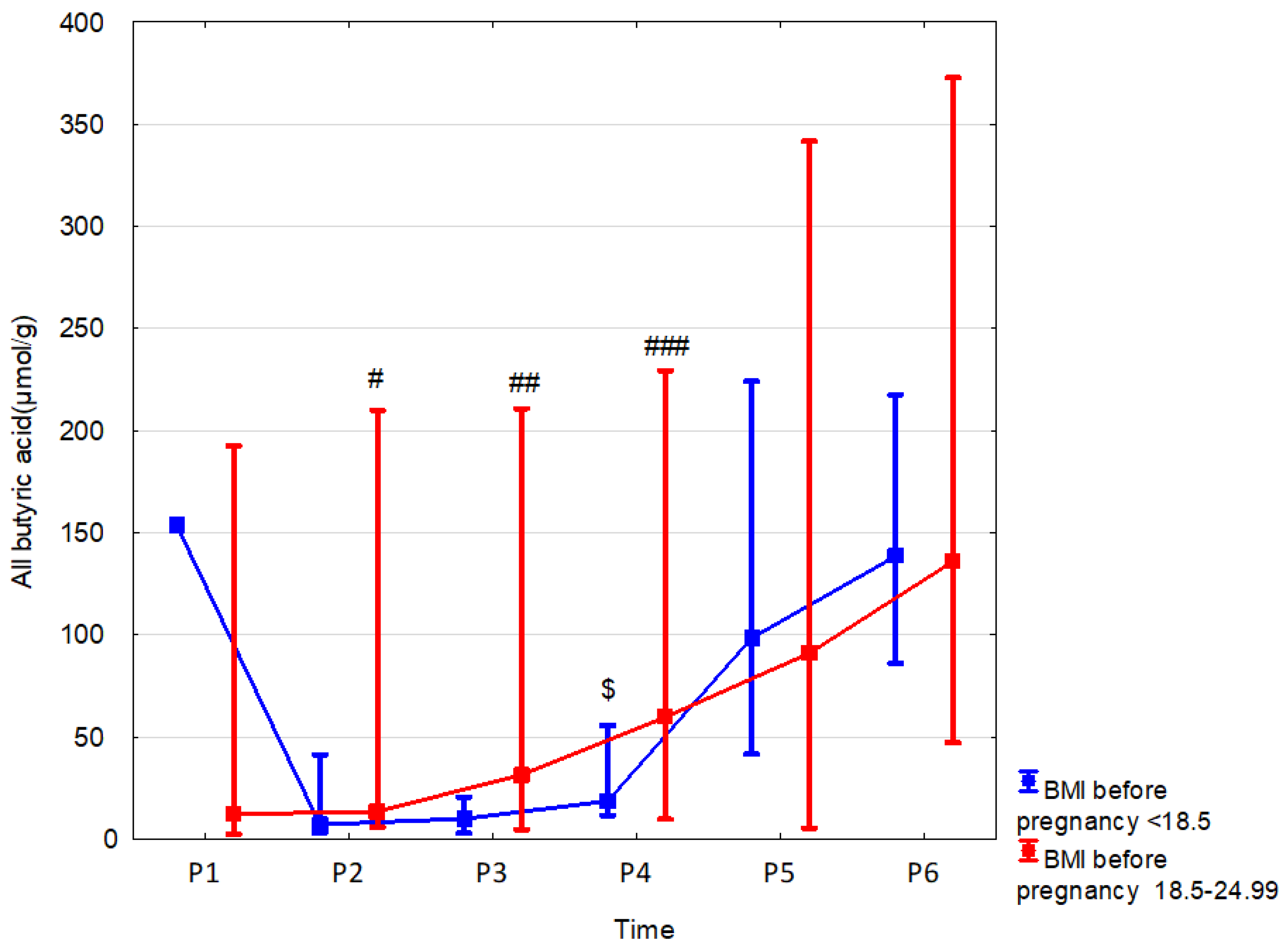
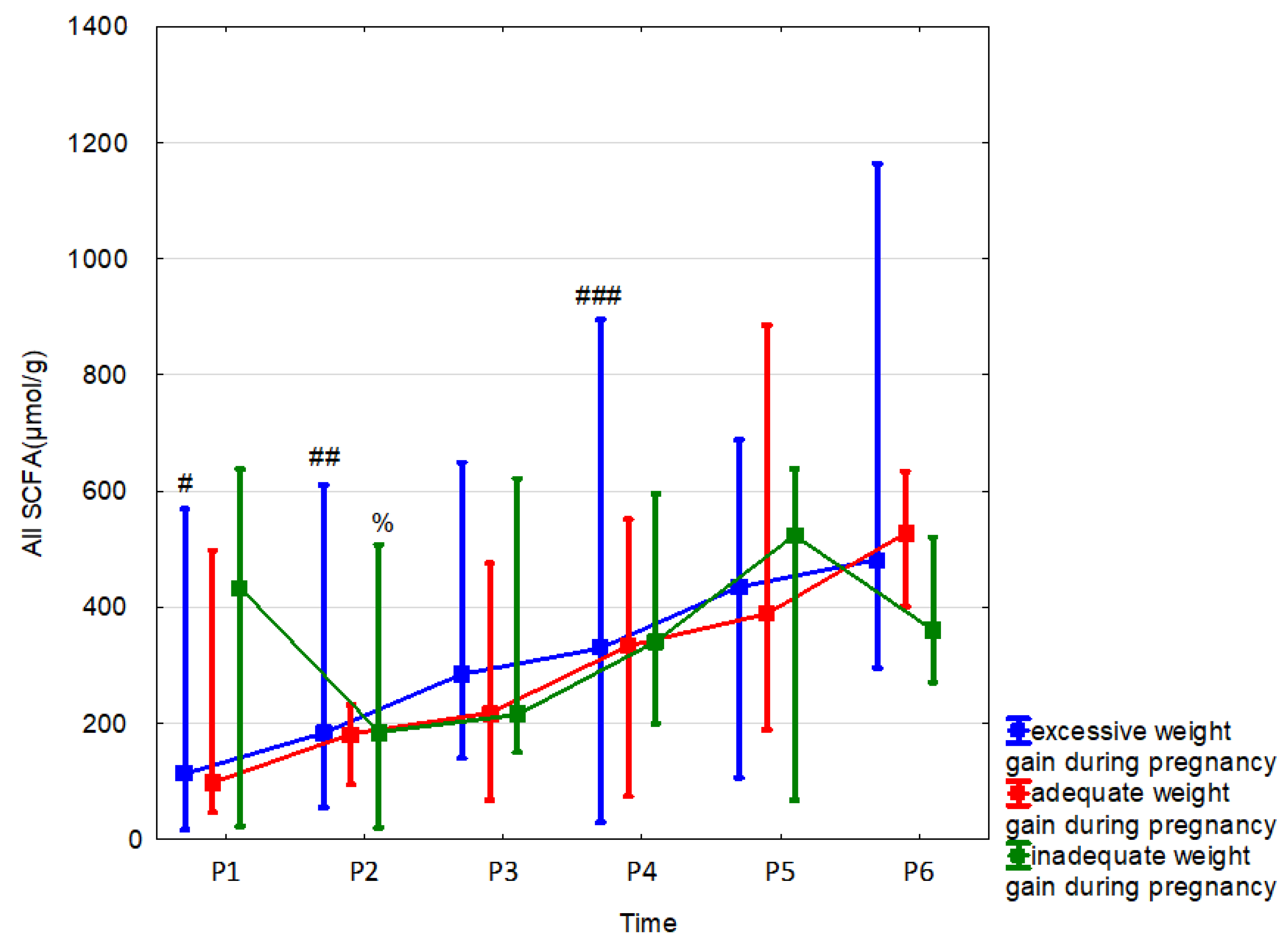
3.5. Effect of Weight
3.6. Effects of Sex
3.7. Multivariable Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- RodrÍguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Stewart, C.J.; Steward, C.; Ajami, N.J.; O’Brien, J.L.; ORein, J.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the TEDDY Study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalile, B.; Oudenhove, L.V.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Ishizawa, R.; Masuda, K.; Sakata, S.; Nakatani, A. Effects of Different Fatty Acid Chain Lengths on Fatty Acid Oxidation-Related Protein Expression Levels in Rat Skeletal Muscles. J. Oleo Sci. 2015, 64, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Erny, D.; Hrabě de Angelis, A.L.; Prinz, M. Communicating Systems in the Body: How Microbiota and Microglia Cooperate. Immunology 2017, 150, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-Host Systems Interactions: Protective Effects of Propionate upon the Blood-Brain Barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? eBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Bourriaud, C.; Robins, R.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate Is Mainly Fermented to Butyrate by Human Intestinal Microfloras but Inter-Individual Variation Is Evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [Green Version]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of Diet and Individual Variation on Intestinal Microbiota Composition and Fermentation Products in Obese Men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a Model Human Gut Microbiota Composed of Members of Its Two Dominant Bacterial Phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [Green Version]
- Czajkowska, A.; Szponar, B. Krótkołańcuchowe Kwasy Tłuszczowe (SCFAs) Jako Produkty Metabolizmu Bakterii Jelitowych Oraz Ich Znaczenie Dla Organizmu Gospodarza. Postępy Hihieny Med. Doświadczalnej 2018, 72, 131–142. [Google Scholar] [CrossRef]
- Łoniewski, I.; Skonieczna-Żydecka, K.; Stachowska, L.; Fraszczyk-Tousty, M.; Tousty, P.; Łoniewska, B. Breastfeeding Affects Concentration of Faecal Short Chain Fatty Acids During the First Year of Life: Results of the Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 939194. [Google Scholar] [CrossRef] [PubMed]
- Łoniewska, B.; Węgrzyn, D.; Adamek, K.; Kaczmarczyk, M.; Skonieczna-Żydecka, K.; Adler, G.; Jankowska, A.; Uzar, I.; Kordek, A.; Celewicz, M.; et al. The Influence of Maternal-Foetal Parameters on Concentrations of Zonulin and Calprotectin in the Blood and Stool of Healthy Newborns during the First Seven Days of Life. An Observational Prospective Cohort Study. J. Clin. Med. 2019, 8, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łoniewska, B.; Adamek, K.; Węgrzyn, D.; Kaczmarczyk, M.; Skonieczna-Żydecka, K.; Clark, J.; Adler, G.; Tousty, J.; Uzar, I.; Tousty, P.; et al. Analysis of Faecal Zonulin and Calprotectin Concentrations in Healthy Children During the First Two Years of Life. An Observational Prospective Cohort Study. J. Clin. Med. 2020, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.; Löber, U.; Adamek, K.; Węgrzyn, D.; Skonieczna-Żydecka, K.; Malinowski, D.; Łoniewski, I.; Markó, L.; Ulas, T.; Forslund, S.K.; et al. The Gut Microbiota Is Associated with the Small Intestinal Paracellular Permeability and the Development of the Immune System in Healthy Children during the First Two Years of Life. J. Transl. Med. 2021, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Apgar Score: MedlinePlus Medical Encyclopedia. Available online: https://medlineplus.gov/ency/article/003402.htm (accessed on 26 December 2022).
- Zhao, G.; Nyman, M.; Jönsson, J. Rapid determination of short chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Collection: Reports Funded by National Institutes of Health: Washington, DC, USA; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13113-1.
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-Chain Fatty Acids: Ready for Prime Time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef]
- Midtvedt, A.C.; Midtvedt, T. Production of Short Chain Fatty Acids by the Intestinal Microflora during the First 2 Years of Human Life. J. Pediatr. Gastroenterol. Nutr. 1992, 15, 395–403. [Google Scholar] [CrossRef]
- Cummings, J.H. Short Chain Fatty Acids in the Human Colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of Microbial Consortia in the Developing Infant Gut Microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sghir, A.; Gramet, G.; Suau, A.; Rochet, V.; Pochart, P.; Dore, J. Quantification of Bacterial Groups within Human Fecal Flora by Oligonucleotide Probe Hybridization. Appl. Environ. Microbiol. 2000, 66, 2263–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.H.; Macfarlane, G.T. The Control and Consequences of Bacterial Fermentation in the Human Colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; van Sinderen, D.; Ventura, M. Glycan Utilization and Cross-Feeding Activities by Bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut Microbiota of Healthy Canadian Infants: Profiles by Mode of Delivery and Infant Diet at 4 Months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Llorente, C.; Plaza-Diaz, J.; Aguilera, M.; Muñoz-Quezada, S.; Bermudez-Brito, M.; Peso-Echarri, P.; Martinez-Silla, R.; Vasallo-Morillas, M.I.; Campaña-Martin, L.; Vives-Piñera, I.; et al. Three Main Factors Define Changes in Fecal Microbiota Associated with Feeding Modality in Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 461–466. [Google Scholar] [CrossRef]
- Gregory, K.E.; Samuel, B.S.; Houghteling, P.; Shan, G.; Ausubel, F.M.; Sadreyev, R.I.; Walker, W.A. Influence of Maternal Breast Milk Ingestion on Acquisition of the Intestinal Microbiome in Preterm Infants. Microbiome 2016, 4, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordillo, J.E.; Zhou, Y.; McGeachie, M.J.; Ziniti, J.; Lange, N.; Laranjo, N.; Savage, J.R.; Carey, V.; O’Connor, G.; Sandel, M.; et al. Factors Influencing the Infant Gut Microbiome at Age 3-6 Months: Findings from the Ethnically Diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy Clin. Immunol. 2017, 139, 482–491.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmerman, H.M.; Rutten, N.B.M.M.; Boekhorst, J.; Saulnier, D.M.; Kortman, G.A.M.; Contractor, N.; Kullen, M.; Floris, E.; Harmsen, H.J.M.; Vlieger, A.M.; et al. Intestinal Colonisation Patterns in Breastfed and Formula-Fed Infants during the First 12 Weeks of Life Reveal Sequential Microbiota Signatures. Sci. Rep. 2017, 7, 8327. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota Profile in Feces of Breast- and Formula-Fed Newborns by Using Fluorescence in Situ Hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-Analysis of Effects of Exclusive Breastfeeding on Infant Gut Microbiota across Populations. Nat. Commun. 2018, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.F.; Brown, B.P.; Lennard, K.; Karaoz, U.; Havyarimana, E.; Passmore, J.-A.S.; Hesseling, A.C.; Edlefsen, P.T.; Kuhn, L.; Mulder, N.; et al. Feeding-Related Gut Microbial Composition Associates With Peripheral T-Cell Activation and Mucosal Gene Expression in African Infants. Clin. Infect. Dis. 2018, 67, 1237–1246. [Google Scholar] [CrossRef]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and Solid-Feeding Practices and Daycare Attendance Are Associated with Differences in Bacterial Diversity, Predominant Communities, and Metabolic and Immune Function of the Infant Gut Microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Persaud, R.R.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Sears, M.R.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Impact of Maternal Intrapartum Antibiotics, Method of Birth and Breastfeeding on Gut Microbiota during the First Year of Life: A Prospective Cohort Study. BJOG 2016, 123, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Hesla, H.M.; Stenius, F.; Jäderlund, L.; Nelson, R.; Engstrand, L.; Alm, J.; Dicksved, J. Impact of Lifestyle on the Gut Microbiota of Healthy Infants and Their Mothers—The ALADDIN Birth Cohort. FEMS Microbiol. Ecol. 2014, 90, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of Complementary Feeding Is Associated with Gut Microbiota Diversity and Composition and Short Chain Fatty Acid Concentrations over the First Year of Life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [Green Version]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal Diet Alters Fecal Microbiota and Metabolome Profiles at Different Ages in Infants Fed Breast Milk or Formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Bridgman, S.L.; Azad, M.B.; Field, C.J.; Haqq, A.M.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; Turvey, S.E.; Sears, M.R.; Scott, J.A.; et al. Fecal Short-Chain Fatty Acid Variations by Breastfeeding Status in Infants at 4 Months: Differences in Relative versus Absolute Concentrations. Front. Nutr. 2017, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.A.; Parrett, A.M.; Balmer, S.E.; Wharton, B.A. Faecal Short Chain Fatty Acids in Breast-Fed and Formula-Fed Babies. Acta Paediatr. 1994, 83, 459–462. [Google Scholar] [CrossRef]
- Garrido, D.; Dallas, D.C.; Mills, D.A. Consumption of Human Milk Glycoconjugates by Infant-Associated Bifidobacteria: Mechanisms and Implications. Microbiol. (Read.) 2013, 159, 649–664. [Google Scholar] [CrossRef]
- Newburg, D.S.; Morelli, L. Human Milk and Infant Intestinal Mucosal Glycans Guide Succession of the Neonatal Intestinal Microbiota. Pediatr. Res. 2015, 77, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Kozak, K.; Charbonneau, D.; Sanozky-Dawes, R.; Klaenhammer, T. Characterization of Bacterial Isolates from the Microbiota of Mothers’ Breast Milk and Their Infants. Gut Microbes 2015, 6, 341–351. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal Microbiota Composition of Breast-Fed Infants Is Correlated with Human Milk Oligosaccharides Consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashiardes, S.; Thaiss, C.A.; Elinav, E. It’s in the Milk: Feeding the Microbiome to Promote Infant Growth. Cell Metab. 2016, 23, 393–394. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Sabir, S.; Alhawaj, A.F. Physiology, Breast Milk. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Duale, A.; Singh, P.; Al Khodor, S. Breast Milk: A Meal Worth Having. Front. Nutr. 2022, 8, 800927. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martín-Cabrejas, M.A.; López de Pablo, Á.L.; Sáenz de Pipaón, M.; Ramiro-Cortijo, D. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Sadelhoff, J.H.J.; Wiertsema, S.P.; Garssen, J.; Hogenkamp, A. Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection Against Neonatal Allergies and Infections. Front. Immunol. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Page, M.G.P. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin. Infect. Dis. 2019, 69, S529–S537. [Google Scholar] [CrossRef]
- Teixeira, T.F.S.; Grześkowiak, Ł.; Franceschini, S.C.C.; Bressan, J.; Ferreira, C.L.L.F.; Peluzio, M.C.G. Higher Level of Faecal SCFA in Women Correlates with Metabolic Syndrome Risk Factors. Br. J. Nutr. 2013, 109, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.A. The Importance of Appropriate Initial Bacterial Colonization of the Intestine in Newborn, Child, and Adult Health. Pediatr. Res. 2017, 82, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Sitarik, A.; Woodcroft, K.; Zoratti, E.; Johnson, C. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations with the Gut Microbiome and Sensitization in Children. Curr. Allergy Asthma Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Arnon, D.L.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, Birth Mode, and Diet Shape Microbiome Maturation during Early Life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the Infant Microbiome Community Structure and Function Across Multiple Body Sites and in Relation to Mode of Delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.T.; Differding, M.K.; Østbye, T.; Hoyo, C.; Benjamin-Neelon, S.E. Association of Birth Mode of Delivery with Infant Faecal Microbiota, Potential Pathobionts, and Short Chain Fatty Acids: A Longitudinal Study over the First Year of Life. BJOG 2021, 128, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, N.; Garrido, D. Species Deletions from Microbiome Consortia Reveal Key Metabolic Interactions between Gut Microbes. mSystems 2019, 4, e00185-19. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Pivrncova, E.; Kotaskova, I.; Thon, V. Neonatal Diet and Gut Microbiome Development After C-Section During the First Three Months After Birth: A Systematic Review. Front. Nutr. 2022, 9, 941549. [Google Scholar] [CrossRef]
- Moore, C.; Negrusz, A.; Lewis, D. Determination of Drugs of Abuse in Meconium. J. Chromatogr. B Biomed. Sci. Appl. 1998, 713, 137–146. [Google Scholar] [CrossRef]
- Ostrea, E.M.; Romero, A.; Yee, H. Adaptation of the Meconium Drug Test for Mass Screening. J. Pediatr. 1993, 122, 152–154. [Google Scholar] [CrossRef]
- Ostrea, E.M. Testing for Exposure to Illicit Drugs and Other Agents in the Neonate: A Review of Laboratory Methods and the Role of Meconium Analysis. Curr. Probl. Pediatr. 1999, 29, 37–56. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B.; Skarżyńska, E.; Bakun, M. Meconium Proteins as a Source of Biomarkers for the Assessment of the Intrauterine Environment of the Fetus. J. Dev. Orig. Heal. Dis. 2018, 9, 329–337. [Google Scholar] [CrossRef]
- Harries, J.T. Meconium in Health and Disease. Br. Med. Bull. 1978, 34, 75–78. [Google Scholar] [CrossRef]
- Friel, J.K.; Matthew, D.; Andrews, W.L.; Skinner, C.T. Trace Elements in Meconium from Preterm and Full-Term Infants. NEO 1989, 55, 214–217. [Google Scholar] [CrossRef]
- Rasmussen, H.S.; Holtug, K.; Ynggård, C.; Mortensen, P.B. Faecal Concentrations and Production Rates of Short Chain Fatty Acids in Normal Neonates. Acta Paediatr. Scand. 1988, 77, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Høverstad, T. Studies of Short-Chain Fatty Acid Absorption in Man. Scand. J. Gastroenterol. 1986, 21, 257–260. [Google Scholar] [CrossRef]
- Fitzsimmons, E.D.; Bajaj, T. Embryology, Amniotic Fluid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The Maternal Microbiota Drives Early Postnatal Innate Immune Development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Sener-Aydemir, A.; Sharma, S.; Lerch, F. Postnatal Development of Gut Microbial Activity and Their Importance for Jejunal Motility in Piglets. J. Anim. Sci. 2021, 99. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, S.K.; Ten Have, G.A.M.; Engelen, M.P.K.J.; Deutz, N.E.P. Transorgan Short-Chain Fatty Acid Fluxes in the Fasted and Postprandial State in the Pig. Am. J. Physiol. Metab. 2021, 321, E665–E673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Theil, P.K.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Zhuo, Y.; Wu, F.; Jiang, X.; et al. The Differences in Energy Metabolism and Redox Status between Sows with Short and Long Farrowing Duration. Animal 2021, 15, 100355. [Google Scholar] [CrossRef]
- Willers, M.; Ulas, T.; Völlger, L.; Vogl, T.; Heinemann, A.S.; Pirr, S.; Pagel, J.; Fehlhaber, B.; Halle, O.; Schöning, J.; et al. S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants. Gastroenterology 2020, 159, 2130–2145.e5. [Google Scholar] [CrossRef] [PubMed]
- Ampicillin Use During Pregnancy. Available online: https://www.drugs.com/pregnancy/ampicillin.html (accessed on 29 December 2022).
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffredo, M.; Mass, K.; Parks, E.J.; Wagner, D.A.; McClure, E.A.; Graf, J.; Savoye, M.; Pierpont, B.; Cline, G.; Santoro, N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J. Clin. Endocrinol. Metab. 2016, 101, 4367–4376. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.H.; Smith, D.M.; Arch, J.R.S. Roles of GPR41 and GPR43 in Leptin Secretory Responses of Murine Adipocytes to Short Chain Fatty Acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [Green Version]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
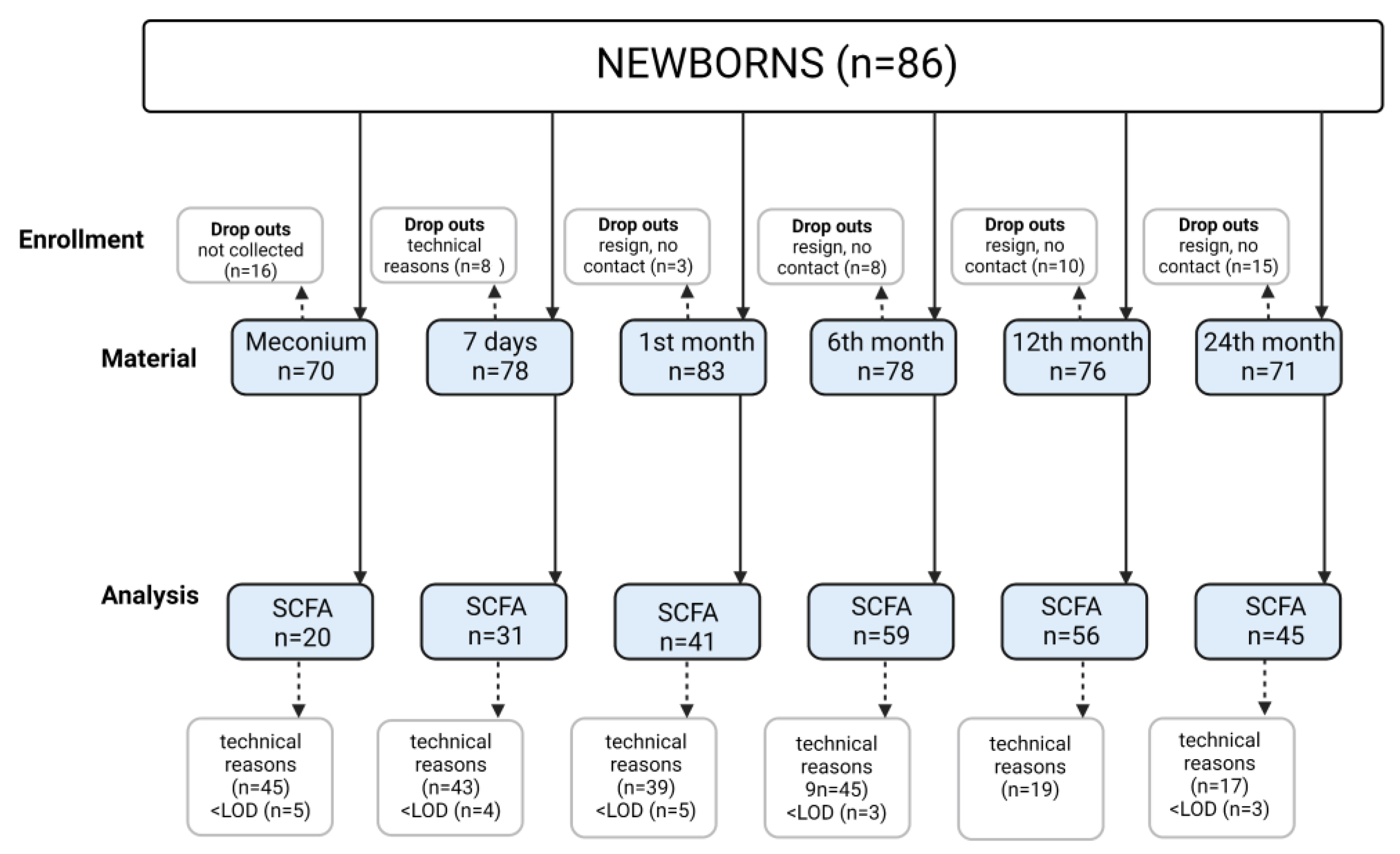


| Characteristic | Newborns (N = 86) | |||
|---|---|---|---|---|
| Gender: (% male) | 54% | |||
| Birth weight (g) | ||||
| (mean ± SD) | 3441 ± 463 | |||
| (range) | 2140–4960 | |||
| ≤15th percentile | 15% (n = 13) | |||
| ≥85th percentile | 15% (n = 13) | |||
| Characteristic | Mothers (N = 86) | |||
| Vaginal childbirth | 37% (n = 32) | |||
| Antibiotic therapy during pregnancy | 30% (n = 26) | |||
| Antibiotic therapy at delivery | 81% (n = 69) | |||
| BMI before pregnancy (%) | <18.5 | 18.5 < 25 | 25 < 30 | >30 |
| 13.2% | 53.0% | 20.5% | 13.3% | |
| BMI before delivery (%) | <18.5 | 18.5 < 25 | 25 < 30 | >30 |
| 0% | 16.9% | 33.7% | 49.4% | |
| Gestational weight gain | inadequate | adequate | excessive | |
| 22.8% | 17.7% | 59.5% | ||
| Characteristic | Age of Children (Months) | |||
|---|---|---|---|---|
| 1 | 6 | 12 | 24 | |
| n = 83 | n = 78 | n = 76 | n = 71 | |
| Gender (% male) | 49.3% (n = 41) | 48.7% (n = 38) | 47.3% (n = 36) | 43.7% (n = 31) |
| Method of delivery (% vaginal) | 33.7% (n = 28) | 34.6% (n = 27) | 34.2% (n = 26) | 32.4% (n = 23) |
| Antibiotics (%) | 2.53% (n = 2) | 21.25% (n = 17) | 40.8% (n = 31) | 74.6% (n = 53) |
| Birth weight: | ||||
| ≤15th percentile | 14.5% (n = 12) | 12.8% (n = 10) | 13.2% (n = 10) | 12.7% (n = 9) |
| ≥85th percentile | 14.5% (n = 12) | 14.1% (n = 11) | 13.2% (n = 10) | 12.7% (n = 9) |
| Mass (kg) at each age: | ||||
| (mean ± SD) | 4.49 ± 0.612 | 7.907 ± 1.099 | 10.07 ± 1.08 | 12.89 ± 1.684 |
| (range) | (2.780, 5.970) | (6, 10) | (7.89, 12.5) | (10, 17) |
| ≤15th percentile * | 12.0% (n = 10) | 21.8% (n = 17) | 1.3% (n = 1) | 2.8% (n = 2) |
| >15–<85th percentile * | 63.9% (n = 53) | 53.8% (n = 42) | 65.8% (n = 50) | 62% (n = 44) |
| ≥85th percentile * | 24.1% (n = 20) | 24.4% (n = 19) | 32.9% (n = 25) | 35.2% (n = 25) |
| Feeding method (% non-breast-fed) | 17.1% (n = 14) | 51.2% (n = 40) | 85.4% (n = 65) | 95.7% (n = 68) |
| Stage | Meconium (P1) n = 20 | 7 Days (P2) n = 31 | 1 Month (P3) n = 41 | 6 Months (P4) n = 59 | 12 Months (P5) n = 56 | 24 Months (P6) n = 45 |
|---|---|---|---|---|---|---|
| Acetic acid (µmol/g) median (range) | 88.49 (12.42–393.51) | 129.26 (4.11–430.51) | 182.21 (55.12–466.02) | 213.1 (12.55–516.45) | 233.49 (55.12–511.2) | 207.5 (114.7–686.93) |
| Propionic acid (µmol/g) median (range) | 13.93 (1.57–234.50) | 14.71 (4.76–61.74) | 32.87 (5.07–106.6) | 55.69 (3.16–310.69) | 87.48 (2.60–187.6) | 86.36 (14.58–285.45) |
| Branched butyric acid (µmol/g) median (range) | 5.81 (0.07–94.75) | 1.95 (0.087–35.71) | 3.24 (0.18–72.18) | 3.25 (0.07–31.6) | 5.52 (0.59–4.23) | 11.3 (1.26–140.2) |
| Linear butyric acid (µmol/g) median (range) | 8.96 (1.19–191.3) | 11 (1.47–195.5) | 22.95 (2.18–313.87) | 45.9 (5.34–222.33) | 95.19 (2.54–333.58) | 111.34 (14.9–357.45) |
| All butyric acid (µmol/g) median (range) | 14 (1.7–192.5) | 13.6 (1.61–210.1) | 28.39 (2.37–325.46) | 47.7 (5.42–229.39) | 106.33 (5.14–341.71) | 129.72 (17.17–372.79) |
| All SCFA (µmol/g) median (range) | 119.95 (16.5–740.77) | 183.21 (19.44–610.01) | 267.57 (67.32–649.06) | 340.54 (29.27–894.84) | 435.65 (67.32–885.64) | 447.63 (209.65–1163.73) |
| Stage | Meconium (P1) | 7 Days (P2) | 1 Month (P3) | 6 Months (P4) | 12 Months (P5) | 24 Months (P6) |
|---|---|---|---|---|---|---|
| ABO YES | n = 6 | n = 8 | n = 10 | n = 13 | n = 17 | n = 9 |
| All SCFA (µmol/g) media n (range) | 340.09 (113.09–740.77) | 133.73 (94.15–507.52) | 261.69 (149–621.41) | 235.14 (123.94–484.31) | 403.65 (106.55–885.64) | 456.73 (270.29–653.56) |
| ABO NO | n = 14 | n = 23 | n = 31 | n = 46 | n = 39 | n = 36 |
| All SCFA (µmol/g) median (range) | 86.85 (16.5–637.63) | 184.78 (19.64–610.01) | 267.57 (67.32–649.06) | 350.51 (29.27–894.84) | 436.6 (67.32–867.37) | 440.52 (209.65–1163.73) |
| p, r | 0.026 (0.49) | 0.55 (−0.11) | 0.6 (0.08) | 0.019 (−0.03) | 0.49 (−0.1) | 0.92 (−0.01) |
| Stage | Meconium (P1) | 7 Days (P2) | 1 Month (P3) | 6 Months (P4) | 12 Months (P5) | 24 Months (P6) |
|---|---|---|---|---|---|---|
| Cesarean section | n = 13 | n = 20 | n = 25 | n = 38 | n = 37 | n = 29 |
| All butyric acid (µmol/g) median (range) | 15.89 (2.36–192.5) | 15.1 (1.61–210.13) | 43.88 (4.63–210.86) | 52.17 (9.68–172.87) | 106.84 (8.66–341.71) | 129.72 (17.17–372.79) |
| Natural Delivery | n = 7 | n = 11 | n = 16 | n = 21 | n = 19 | n = 16 |
| All butyric acid (µmol/g) median (range) | 12.3 (1.7–99.71) | 9.95 (3.08–61.34) | 13.49 (2.37–325.46) | 34.78 (5.42–229.39) | 105.82 (5.14–269.74) | 123.01 (47.25–224.64) |
| p, r | 0.31 (0.23) | 0.16 (0.26) | 0.037 (0.32) | 0.24 (0.15) | 0.78 (−0.04) | 0.34 (0.14) |
| Stage | 1 Month (P3) | 6 Months (P4) | 12 Months (P5) | 24 Months (P6) |
|---|---|---|---|---|
| Breastfeeding | n = 30 | n = 26 | n = 9 | n = 3 |
| Linear butyric acid (µmol/g) median (range) | 21.26 (2.18–313.87) | 30.21 (5.34–185.35) | 68.34 (2.54–123.9) | 114.11 (44.49–183.73) |
| Formula | n = 10 | n = 32 | n = 46 | n = 41 |
| Linear butyric acid (µmol/g) median (range) | 35.43 (2.74–101.58) | 50.07 (5.6–222.33) | 104.07 (4.39–333.58) | 111.34 (14.9–357.45) |
| p, r | 0.77 (0.04) | 0.29 (−0.14) | 0.049 (−0.27) | 0.80 (−0.04) |
| Stage | Meconium (P1) | 7 Days (P2) | 1 Month (P3) | 6 Months (P4) | 12 Months (P5) | 24 Months (P6) |
|---|---|---|---|---|---|---|
| BMI before pregnancy 18.5–24.99 | n = 10 | n = 12 | n = 18 | n = 32 | n = 28 | n = 26 |
| All butyric acid (µmol/g) median (range) | 12.21 (2.36–192.5) | 13.55 (5.76–210.13) | 31.49 (4.63–210.86) | 59.74 (9.68–229.39) | 90.99 (5.28–341.71) | 135.92 (47.25–372.79) |
| BMI before pregnancy < 18.5 | n = 1 | n = 5 | n = 4 | n = 7 | n = 6 | n = 3 |
| All butyric acid (µmol/g) median (range) | 154.25 (154.25–154.25) | 7.12 (3.08–41.4) | 9.91 (2.97–20.44) | 18.56 (11.52–55.62) | 98.44 (41.68–224.28) | 138.49 (86.02–217.54) |
| p, r | 1.00 (0.00) | 0.08 (0.42) | 0.07 (0.39) | 0.01 (0.41) | 0.46 (−0.13) | 0.97 (0.01) |
| Stage | Meconium (P1) | 7 Days (P2) | 1 Month (P3) | 6 Months (P4) | 12 Months (P5) | 24 Months (P6) |
|---|---|---|---|---|---|---|
| Inadequate | n = 3 | n = 7 | n = 9 | n = 10 | n = 9 | n = 12 |
| All SCFA (µmol/g) median (range) | 433.05 (22.36–637.63) | 184.78 (19.64–507.52) | 214.55 (149.82–621.4) | 340.92 (199.34–595.13) | 523.65 (67.32–637.63) | 360.54 (270.29–520.16) |
| Adequate | n = 4 | n = 4 | n = 8 | n = 12 | n = 10 | n = 6 |
| All SCFA (µmol/g) median (range) | 97.56 (45.99–497.37) | 181.49 (94.15–231.66) | 217.44 (67.32–475.85) | 333.24 (73.7–551.35) | 388.26 (188.92–885.64) | 527.02 (401.27–633.96) |
| Excessive | n = 11 | n = 17 | n = 21 | n = 33 | n = 33 | n = 25 |
| All SCFA (µmol/g) median (range) | 113.09 (16.5–568.9) | 183.21 (54.97–610.01) | 284.36 (139.93–649.06) | 329.09 (29.27–894.84) | 434.71 (106.55–687.87) | 482.05 (294.96–1163.73) |
| p, r | 0.74 (0.04) | 0.89 (0.01) | 0.42 (0.05) | 0.96 (0.001) | 0.66 (0.02) | 0.02 (0.18) |
| Stage | Meconium (P1) | 7 Days (P2) | 1 Month (P3) | 6 Months (P4) | 12 Months (P5) | 24 Months (P6) |
|---|---|---|---|---|---|---|
| Female | n = 11 | n = 14 | n = 19 | n = 24 | n = 26 | n = 23 |
| Acetic acid (µmol/g) median (range) | 67.33 (12.42–328.8) | 80.66 (32.24–430.51) | 175.9 (55.12–466.02) | 203.24 (74.82–435.63) | 227.25 (84.6–445.09) | 225 (114.7–686.93) |
| Male | n = 9 | n = 17 | n = 22 | n = 35 | n = 30 | n = 22 |
| Acetic acid (µmol/g) median (range) | 96.69 (32.46–393.51) | 144.5 (4.11–264.67) | 186.62 (72.87–337.3) | 226.1 (12.55–516.45) | 233.6 (55.12–511.2) | 187.01 (121.42–304.82) |
| p, r | 0.26 (−0.25) | 0.19 (−0.24) | 0.95 (−0.01) | 0.77 (−0.04) | 0.85 (−0.02) | 0.04 (0.31) |
| Response/ Factors | ABO | Delivery Mode | Feeding Type | BMI before Pregnancy | Weight Gain | Sex |
|---|---|---|---|---|---|---|
| All SCFA | P1U, P4U | P5M | P6UM | P6M | ||
| Acetic | P1U, P4U | P6U | ||||
| Propionic | P1U, P4U | P6M | P6M | |||
| Linear butyric | P3U | P5UM | P2U, P4UM | P6UM | P6M | |
| Branched butyric | P2UM, P5M | P5M | P2M, P6UM | |||
| All butyric | P3U | P5M | P4UM | P6UM | P6M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łoniewska, B.; Fraszczyk-Tousty, M.; Tousty, P.; Skonieczna-Żydecka, K.; Maciejewska-Markiewicz, D.; Łoniewski, I. Analysis of Fecal Short-Chain Fatty Acids (SCFAs) in Healthy Children during the First Two Years of Life: An Observational Prospective Cohort Study. Nutrients 2023, 15, 367. https://doi.org/10.3390/nu15020367
Łoniewska B, Fraszczyk-Tousty M, Tousty P, Skonieczna-Żydecka K, Maciejewska-Markiewicz D, Łoniewski I. Analysis of Fecal Short-Chain Fatty Acids (SCFAs) in Healthy Children during the First Two Years of Life: An Observational Prospective Cohort Study. Nutrients. 2023; 15(2):367. https://doi.org/10.3390/nu15020367
Chicago/Turabian StyleŁoniewska, Beata, Magda Fraszczyk-Tousty, Piotr Tousty, Karolina Skonieczna-Żydecka, Dominika Maciejewska-Markiewicz, and Igor Łoniewski. 2023. "Analysis of Fecal Short-Chain Fatty Acids (SCFAs) in Healthy Children during the First Two Years of Life: An Observational Prospective Cohort Study" Nutrients 15, no. 2: 367. https://doi.org/10.3390/nu15020367
APA StyleŁoniewska, B., Fraszczyk-Tousty, M., Tousty, P., Skonieczna-Żydecka, K., Maciejewska-Markiewicz, D., & Łoniewski, I. (2023). Analysis of Fecal Short-Chain Fatty Acids (SCFAs) in Healthy Children during the First Two Years of Life: An Observational Prospective Cohort Study. Nutrients, 15(2), 367. https://doi.org/10.3390/nu15020367







