Association of Blood Urea Nitrogen with Cardiovascular Diseases and All-Cause Mortality in USA Adults: Results from NHANES 1999–2006
Abstract
:1. Introduction
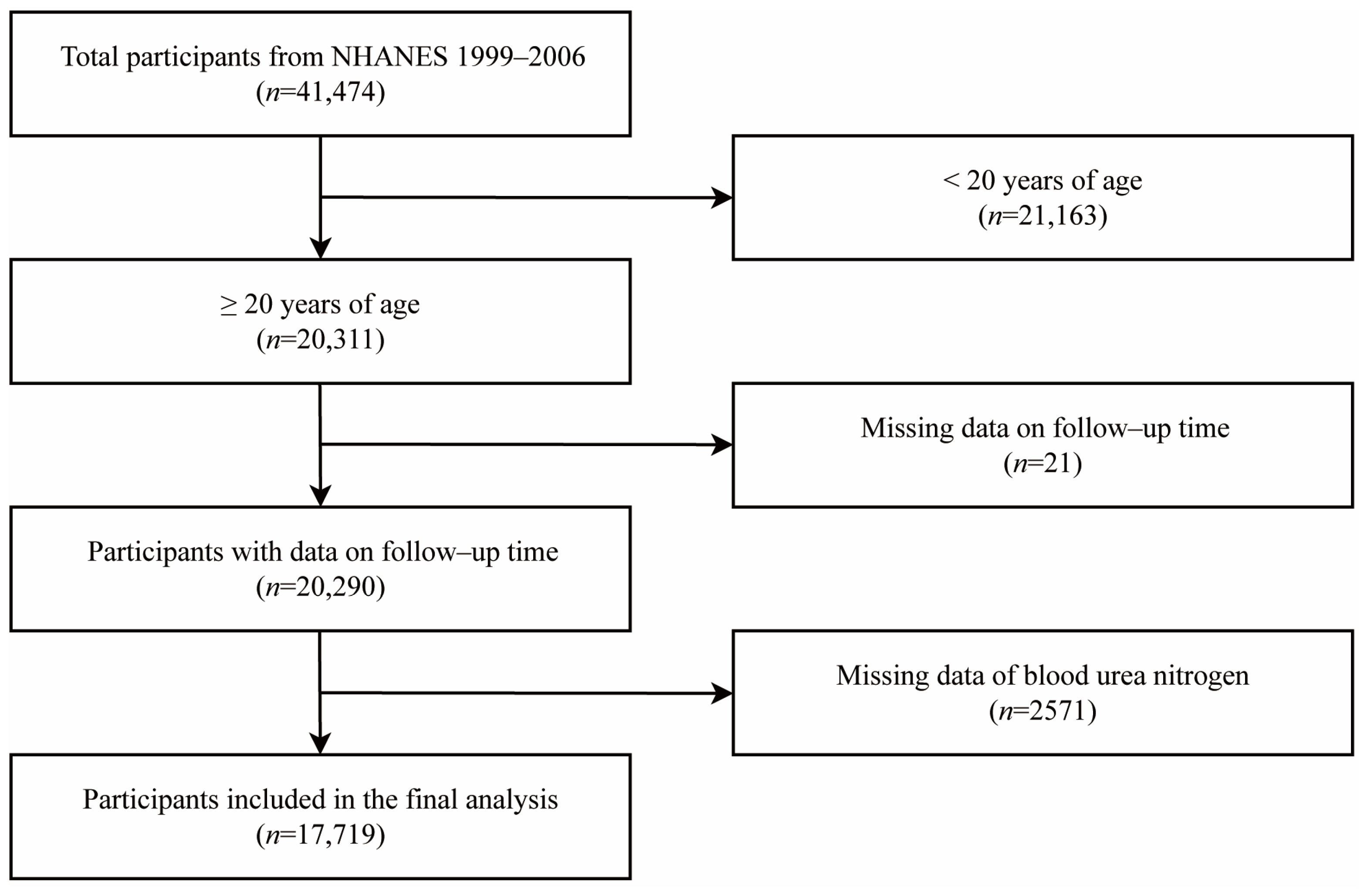
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment and Confounding Factors
2.3. Mortality Ascertainment and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. CVD and All-Cause Mortality
3.3. Stratification Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 315 Diseases and Injuries and Healthy Life Expectancy (HALE), 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [CrossRef] [PubMed] [Green Version]
- Omran, A.R. The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. Milbank Meml. Fund Q. 1971, 49, 509. [Google Scholar] [CrossRef]
- Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [CrossRef] [PubMed] [Green Version]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and Epidemiologic Drivers of Global Cardiovascular Mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- WHO. Cardiovascular diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 27 July 2022).
- Peng, R.; Liu, K.; Li, W.; Yuan, Y.; Niu, R.; Zhou, L.; Xiao, Y.; Gao, H.; Yang, H.; Zhang, C.; et al. Blood Urea Nitrogen, Blood Urea Nitrogen to Creatinine Ratio and Incident Stroke: The Dongfeng-Tongji Cohort. Atherosclerosis 2021, 333, 1–8. [Google Scholar] [CrossRef]
- You, S.; Zheng, D.; Zhong, C.; Wang, X.; Tang, W.; Sheng, L.; Zheng, C.; Cao, Y.; Liu, C.-F. Prognostic Significance of Blood Urea Nitrogen in Acute Ischemic Stroke. Circ. J. 2018, 82, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Smilde, T.D.; Damman, K.; van der Harst, P.; Navis, G.; Westenbrink, B.D.; Voors, A.A.; Boomsma, F.; van Veldhuisen, D.J.; Hillege, H.L. Differential Associations between Renal Function and “Modifiable” Risk Factors in Patients with Chronic Heart Failure. Clin. Res. Cardiol. 2009, 98, 121–129. [Google Scholar] [CrossRef]
- Smith, G.L.; Lichtman, J.H.; Bracken, M.B.; Shlipak, M.G.; Phillips, C.O.; DiCapua, P.; Krumholz, H.M. Renal Impairment and Outcomes in Heart Failure: Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2006, 47, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Matsue, Y.; van der Meer, P.; Damman, K.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Blood Urea Nitrogen-to-Creatinine Ratio in the General Population and in Patients with Acute Heart Failure. Heart 2017, 103, 407–413. [Google Scholar] [CrossRef]
- Bhatia, K.; Mohanty, S.; Tripathi, B.K.; Gupta, B.; Mittal, M.K. Predictors of Early Neurological Deterioration in Patients with Acute Ischaemic Stroke with Special Reference to Blood Urea Nitrogen (BUN)/Creatinine Ratio & Urine Specific Gravity. Indian J. Med. Res. 2015, 141, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.J.; Chao, C.L.; Chien, K.L.; Ho, Y.L.; Lee, C.M.; Lin, Y.H.; Wu, Y.W.; Hsu, R.B.; Chou, N.K.; Wang, S.S.; et al. Elevated Blood Urea Nitrogen-to-Creatinine Ratio Increased the Risk of Hospitalization and All-Cause Death in Patients with Chronic Heart Failure. Clin. Res. Cardiol. 2009, 98, 487–492. [Google Scholar] [CrossRef]
- Kirtane, A.J.; Leder, D.M.; Waikar, S.S.; Chertow, G.M.; Ray, K.K.; Pinto, D.S.; Karmpaliotis, D.; Burger, A.J.; Murphy, S.A.; Cannon, C.P.; et al. Serum Blood Urea Nitrogen as an Independent Marker of Subsequent Mortality among Patients with Acute Coronary Syndromes and Normal to Mildly Reduced Glomerular Filtration Rates. J. Am. Coll. Cardiol. 2005, 45, 1781–1786. [Google Scholar] [CrossRef] [Green Version]
- Arihan, O.; Wernly, B.; Lichtenauer, M.; Franz, M.; Kabisch, B.; Muessig, J.; Masyuk, M.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. Blood Urea Nitrogen (BUN) Is Independently Associated with Mortality in Critically Ill Patients Admitted to ICU. PLoS ONE 2018, 13, e0191697. [Google Scholar] [CrossRef] [Green Version]
- Gary, T.; Pichler, M.; Schilcher, G.; Hafner, F.; Hackl, G.; Rief, P.; Eller, P.; Brodmann, M. Elevated Blood Urea Nitrogen Is Associated with Critical Limb Ischemia in Peripheral Arterial Disease Patients. Medicine 2015, 94, e948. [Google Scholar] [CrossRef]
- Faisst, M.; Wellner, U.F.; Utzolino, S.; Hopt, U.T.; Keck, T. Elevated Blood Urea Nitrogen Is an Independent Risk Factor of Prolonged Intensive Care Unit Stay Due to Acute Necrotizing Pancreatitis. J. Crit. Care 2010, 25, 105–111. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. Vital Health Stat. 2 2013, 161, 1–24. [Google Scholar]
- Rong, S.; Snetselaar, L.G.; Xu, G.; Sun, Y.; Liu, B.; Wallace, R.B.; Bao, W. Association of Skipping Breakfast with Cardiovascular and All-Cause Mortality. J. Am. Coll. Cardiol. 2019, 73, 2025–2032. [Google Scholar] [CrossRef]
- Khoury, J.; Bahouth, F.; Stabholz, Y.; Elias, A.; Mashiach, T.; Aronson, D.; Azzam, Z.S. Blood Urea Nitrogen Variation upon Admission and at Discharge in Patients with Heart Failure. ESC Heart Fail. 2019, 6, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Kajimoto, K.; Minami, Y.; Sato, N.; Takano, T. Serum Sodium Concentration, Blood Urea Nitrogen, and Outcomes in Patients Hospitalized for Acute Decompensated Heart Failure. Int. J. Cardiol. 2016, 222, 195–201. [Google Scholar] [CrossRef]
- Saygitov, R.T.; Glezer, M.G.; Semakina, S.V. Blood Urea Nitrogen and Creatinine Levels at Admission for Mortality Risk Assessment in Patients with Acute Coronary Syndromes. Emerg. Med. J. 2010, 27, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.; Nasir, S.A.R.; Merchant, A.Z.; Rizvi, A.H.; Rehan, A.; Shaikh, A.T.; Abbas, A.H.; Godil, A.; Khetpal, A.; Mallick, M.S.A.; et al. Efficacy of Serum Blood Urea Nitrogen, Creatinine and Electrolytes in the Diagnosis and Mortality Risk Assessment of Patients with Acute Coronary Syndrome. Indian Heart J. 2018, 70, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Giamouzis, G.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Agha, S.A.; Rashad, M.A.; Laskar, S.R.; Smith, A.L.; Butler, J. Incremental Value of Renal Function in Risk Prediction with the Seattle Heart Failure Model. Am. Heart J. 2009, 157, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Mittleman, M.A.; Burger, A.J. Elevated Blood Urea Nitrogen Level as a Predictor of Mortality in Patients Admitted for Decompensated Heart Failure. Am. J. Med. 2004, 116, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Massie, B.M.; Leimberger, J.D.; O’Connor, C.M.; Pina, I.L.; Adams, K.F.; Califf, R.M.; Gheorghiade, M.; Optime-Chf Investigators. Admission or Changes in Renal Function During Hospitalization for Worsening Heart Failure Predict Postdischarge Survival Results From the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF). Circ. Heart Fail. 2008, 1, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazory, A. Emergence of Blood Urea Nitrogen as a Biomarker of Neurohormonal Activation in Heart Failure. Am. J. Cardiol. 2010, 106, 694–700. [Google Scholar] [CrossRef]
- Shenkman, H.J.; Zareba, W.; Bisognano, J.D. Comparison of Prognostic Significance of Amino-Terminal pro-Brain Natriuretic Peptide versus Blood Urea Nitrogen for Predicting Events in Patients Hospitalized for Heart Failure. Am. J. Cardiol. 2007, 99, 1143–1145. [Google Scholar] [CrossRef]
- Solomon, S.D.; Pfeffer, M.A. Renin-Angiotensin System and Cardiac Rupture after Myocardial Infarction. Circulation 2002, 106, 2167–2169. [Google Scholar] [CrossRef] [Green Version]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-Induced ROS Generation Causes Insulin Resistance in Mice with Chronic Renal Failure. J. Clin. Investig. 2010, 120, 932. [Google Scholar] [CrossRef] [Green Version]
- Biolo, A.; Shibata, R.; Ouchi, N.; Kihara, S.; Sonoda, M.; Walsh, K.; Sam, F. Determinants of Adiponectin Levels in Patients with Chronic Systolic Heart Failure. Am. J. Cardiol. 2010, 105, 1147–1152. [Google Scholar] [CrossRef] [Green Version]
- Jalali, Z.; Khademalhosseini, M.; Soltani, N.; Esmaeili Nadimi, A. Smoking, Alcohol and Opioids Effect on Coronary Microcirculation: An Update Overview. BMC Cardiovasc. Disord. 2021, 21, 185. [Google Scholar] [CrossRef]
- Mayyas, F.; Alzoubi, K.H. Impact of Cigarette Smoking on Kidney Inflammation and Fibrosis in Diabetic Rats. Inhal. Toxicol. 2019, 31, 45–51. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Yang, X.; Peng, Q.; Wang, J.; Mo, C.; Wu, J.; Sui, J.; Liu, Y.; Lu, Y.; et al. Establishing Reference Values for Blood Urea Nitrogen and Serum Creatinine in Chinese Han Ethnic Adult Men. Clin. Lab. 2014, 60, 1123–1128. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Villareal, D.T.; Frimel, T.N.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Differences in Muscle Protein Synthesis and Anabolic Signaling in the Postabsorptive State and in Response to Food in 65–80 Year Old Men and Women. PLoS ONE 2008, 3, e1875. [Google Scholar] [CrossRef] [Green Version]
- Henderson, G.C.; Dhatariya, K.; Ford, G.C.; Klaus, K.A.; Basu, R.; Rizza, R.A.; Jensen, M.D.; Khosla, S.; O’Brien, P.; Nair, K.S. Higher Muscle Protein Synthesis in Women than Men across the Lifespan, and Failure of Androgen Administration to Amend Age-related Decrements. FASEB J. 2008, 23, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Varga, Z.V.; Matyas, C.; Paloczi, J.; Pacher, P. Alcohol Misuse and Kidney Injury: Epidemiological Evidence and Potential Mechanisms. Alcohol Res. Curr. Rev. 2017, 38, 283–288. [Google Scholar]
- Klatsky, A.L. Alcohol and Cardiovascular Health. Physiol. Behav. 2010, 100, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Higher Blood Urea Nitrogen Is Associated with Increased Risk of Incident Diabetes Mellitus. Kidney Int. 2018, 93, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.; Tang, C.Y.; Li, S.Y.; Ma, W.Z.; Zhai, X.B.; Liu, K.Y.; Sheerah, H.A.; Cao, J.H. Association of Lung Function with the Risk of Cardiovascular Diseases and All-Cause Mortality in Patients with Diabetes: Results from NHANES III 1988–1994. Front. Cardiovasc. Med. 2022, 9, 976817. [Google Scholar] [CrossRef]
- Anavekar, N.S.; McMurray, J.J.V.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.-L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation between Renal Dysfunction and Cardiovascular Outcomes after Myocardial Infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef]
- Liu, E.; Zeng, C. Blood Urea Nitrogen and In-Hospital Mortality in Critically Ill Patients with Cardiogenic Shock: Analysis of the MIMIC-III Database. BioMed Res. Int. 2021, 2021, 5948636. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Schrier, R.W. Blood Urea Nitrogen. J. Am. Coll. Cardiol. 2011, 58, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The Elephant in Uremia: Oxidant Stress as a Unifying Concept of Cardiovascular Disease in Uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, G.; Dal Canton, A.; Terribile, M.; Cianciaruso, B.; Di Minno, G.; Pannain, M.; Russo, D.; Andreucci, V.E. Renal Handling of Urea in Subjects with Persistent Azotemia and Normal Renal Function. Kidney Int. 1987, 32, 721–727. [Google Scholar] [CrossRef]
- Martin, P.Y.; Schrier, R.W. Sodium and Water Retention in Heart Failure: Pathogenesis and Treatment. Kidney Int. Suppl. 1997, 59, S57–S61. [Google Scholar]

| Characteristic | Blood Urea Nitrogen (mmol/L) | p Value | |||
|---|---|---|---|---|---|
| Q1 <3.57 | Q2 3.57–4.59 | Q3 4.60–5.70 | Q4 ≥5.71 | ||
| Number of participants | 4047 | 4661 | 4349 | 4662 | |
| Age, y | <0.001 | ||||
| 20–40 | 2400 (54.4) | 2039 (46.5) | 1315 (35.6) | 655 (19.7) | |
| 40–60 | 1107 (36.9) | 1561 (39.4) | 1462 (41.1) | 1160 (36.6) | |
| ≥60 | 540 (8.7) | 1061 (14.1) | 1572 (23.3) | 2847 (43.7) | |
| Gender | <0.001 | ||||
| Men | 1185 (31.8) | 2115 (44.5) | 2397 (55.3) | 2769 (59.0) | |
| Women | 2862 (68.2) | 2546 (55.5) | 1952 (44.7) | 1893 (41.0) | |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic white | 900 (8.8) | 1152 (8.8) | 997 (7.0) | 878 (5.3) | |
| Non-Hispanic black | 339 (9.5) | 401 (11.6) | 349 (10.1) | 294 (8.0) | |
| Mexican American | 1765 (65.4) | 2102 (68.1) | 2232 (74.2) | 2863 (80.3) | |
| Other | 1043 (16.3) | 1006 (11.5) | 771 (8.7) | 627 (6.4) | |
| Education | <0.001 | ||||
| <High school | 446 (6.0) | 631 (6.4) | 657 (6.2) | 880 (9.0) | |
| High school | 1766 (42.5) | 1807 (35.6) | 1676 (37.7) | 1845 (39.0) | |
| >High school | 1830 (51.5) | 2215 (58.0) | 2010 (56.1) | 1924 (52.0) | |
| BMI, kg/m2 | <0.001 | ||||
| <25.0 (Normal) | 1432 (40.2) | 1593 (36.2) | 1341 (33.2) | 1501 (32.3) | |
| 25.0–29.9 (Overweight) | 1273 (29.5) | 1590 (32.8) | 1581 (35.4) | 1720 (36.3) | |
| ≥30.0 (Obese) | 1342 (30.3) | 1478 (31.0) | 1427 (31.4) | 1441 (31.4) | |
| Alcohol drinking status | <0.001 | ||||
| Never drinker | 2982 (72.8) | 3293 (70.5) | 2997 (68.9) | 3377 (71.6) | |
| Moderate drinking | 336 (8.6) | 496 (11.9) | 559 (13.8) | 541 (13.2) | |
| Heavy drinking | 532 (18.6) | 694 (17.6) | 609 (17.3) | 531 (15.2) | |
| Smoking status | <0.001 | ||||
| Never smoker | 2187 (48.7) | 2443 (51.3) | 2193 (49.6) | 2313 (51.3) | |
| Former smoker | 713 (16.7) | 1021 (21.7) | 1266 (28.4) | 1686 (33.5) | |
| Current smoker | 1143 (34.6) | 1189 (27.0) | 885 (22.0) | 657 (15.2) | |
| Total cholesterol (mg/dL) | 201.06 ± 46.07 | 201.39 ± 42.09 | 203.81 ± 41.45 | 203.38 ± 43.81 | 0.004 |
| ALT (U/L) | 22.98 ± 17.82 | 26.65 ± 23.01 | 27.08 ± 38.91 | 24.92 ± 31.52 | <0.001 |
| Total protein (g/dL) | 7.16 ± 0.60 | 7.34 ± 0.50 | 7.35 ± 0.49 | 7.29 ± 0.51 | <0.001 |
| Albumin (g/L) | 40.58 ± 4.86 | 42.87 ± 3.51 | 43.18 ± 3.20 | 42.47 ± 3.49 | <0.001 |
| Globulin (g/L) | 31.03 ± 4.73 | 30.51 ± 4.58 | 30.33 ± 4.48 | 30.44 ± 4.74 | <0.001 |
| HDL (mmol/L) | 1.47 ± 0.45 | 1.37 ± 0.41 | 1.35 ± 0.40 | 1.34 ± 0.41 | <0.001 |
| History of hypertension | <0.001 | ||||
| Yes | 833 (20.0) | 1184 (22.9) | 1377 (27.7) | 2173 (40.9) | |
| No | 3180 (80.0) | 3419 (77.1) | 2943 (72.3) | 2460 (59.1) | |
| History of diabetes | <0.001 | ||||
| Yes | 216 (4.3) | 314 (4.9) | 387 (6.0) | 827 (13.3) | |
| No | 3794 (94.9) | 4277 (93.9) | 3892 (92.6) | 3742 (85.2) | |
| Borderline | 35 (0.8) | 69 (1.2) | 64 (1.4) | 91 (1.5) | |
| Characteristic | Blood Urea Nitrogen (mmol/L) | P for Trend | |||
|---|---|---|---|---|---|
| Q1 <3.57 | Q2 3.57–4.59 | Q3 4.60–5.70 | Q4 ≥5.71 | ||
| CVD mortality | |||||
| Deaths, No. (%) | 82 (1.5) | 118 (1.4) | 168 (2.3) | 491 (6.9) | <0.001 |
| Deaths/person-years | 511/47,792 | 799/56,451 | 1222/53,031 | 2930/49,173 | |
| Mortality/per 1000 person | 15 | 14 | 23 | 69 | |
| Unadjusted | 1 [Reference] | 0.93 (0.63,1.39) | 1.52 (1.08,2.13) | 5.01 (3.80,6.59) | <0.001 |
| Model 1 | 1 [Reference] | 0.66 (0.44,0.99) | 0.74 (0.52,1.06) | 1.54 (1.13,2.09) | <0.001 |
| Model 2 | 1 [Reference] | 0.69 (0.46,1.04) | 0.78 (0.54,1.12) | 1.57 (1.13,2.16) | <0.001 |
| Model 3 | 1 [Reference] | 0.77 (0.51,1.15) | 0.87 (0.61,1.24) | 1.65 (1.20,2.28) | <0.001 |
| Model 4 | 1 [Reference] | 0.76 (0.52,1.11) | 0.85 (0.61,1.20) | 1.48 (1.08,2.02) | <0.001 |
| All-cause mortality | |||||
| Deaths, No. (%) | 406 (7.5) | 609 (9.2) | 809 (12.6) | 1804 (27.0) | <0.001 |
| Deaths/person-years | 2743/47,792 | 4458/56,451 | 6158/53,031 | 11,928/49,173 | |
| Mortality/per 1000 person | 75 | 92 | 126 | 270 | |
| Unadjusted | 1 [Reference] | 1.18 (1.01,1.37) | 1.58 (1.36,1.83) | 3.79 (3.23,4.44) | <0.001 |
| Model 1 | 1 [Reference] | 0.89 (0.76,1.03) | 0.85 (0.72,1.00) | 1.34 (1.15,1.58) | <0.001 |
| Model 2 | 1 [Reference] | 0.96 (0.84,1.10) | 0.95 (0.82,1.10) | 1.47 (1.27,1.71) | <0.001 |
| Model 3 | 1 [Reference] | 1.07 (0.94,1.23) | 1.07 (0.92,1.23) | 1.59 (1.37,1.84) | <0.001 |
| Model 4 | 1 [Reference] | 1.06 (0.92,1.21) | 1.05 (0.91,1.22) | 1.48 (1.28,1.72) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.; Zhu, H.; Zhou, X.; Zhai, X.; Li, S.; Ma, W.; Liu, K.; Shirai, K.; Sheerah, H.A.; Cao, J. Association of Blood Urea Nitrogen with Cardiovascular Diseases and All-Cause Mortality in USA Adults: Results from NHANES 1999–2006. Nutrients 2023, 15, 461. https://doi.org/10.3390/nu15020461
Hong C, Zhu H, Zhou X, Zhai X, Li S, Ma W, Liu K, Shirai K, Sheerah HA, Cao J. Association of Blood Urea Nitrogen with Cardiovascular Diseases and All-Cause Mortality in USA Adults: Results from NHANES 1999–2006. Nutrients. 2023; 15(2):461. https://doi.org/10.3390/nu15020461
Chicago/Turabian StyleHong, Canlin, Huiping Zhu, Xiaoding Zhou, Xiaobing Zhai, Shiyang Li, Wenzhi Ma, Keyang Liu, Kokoro Shirai, Haytham A. Sheerah, and Jinhong Cao. 2023. "Association of Blood Urea Nitrogen with Cardiovascular Diseases and All-Cause Mortality in USA Adults: Results from NHANES 1999–2006" Nutrients 15, no. 2: 461. https://doi.org/10.3390/nu15020461
APA StyleHong, C., Zhu, H., Zhou, X., Zhai, X., Li, S., Ma, W., Liu, K., Shirai, K., Sheerah, H. A., & Cao, J. (2023). Association of Blood Urea Nitrogen with Cardiovascular Diseases and All-Cause Mortality in USA Adults: Results from NHANES 1999–2006. Nutrients, 15(2), 461. https://doi.org/10.3390/nu15020461






