Effects of Interaction between SLC35F3 and Carbohydrate Intake on the Incidence of Metabolic Syndrome in Korean Middle-Aged Adults
Abstract
:1. Introduction
2. Materials and Methods
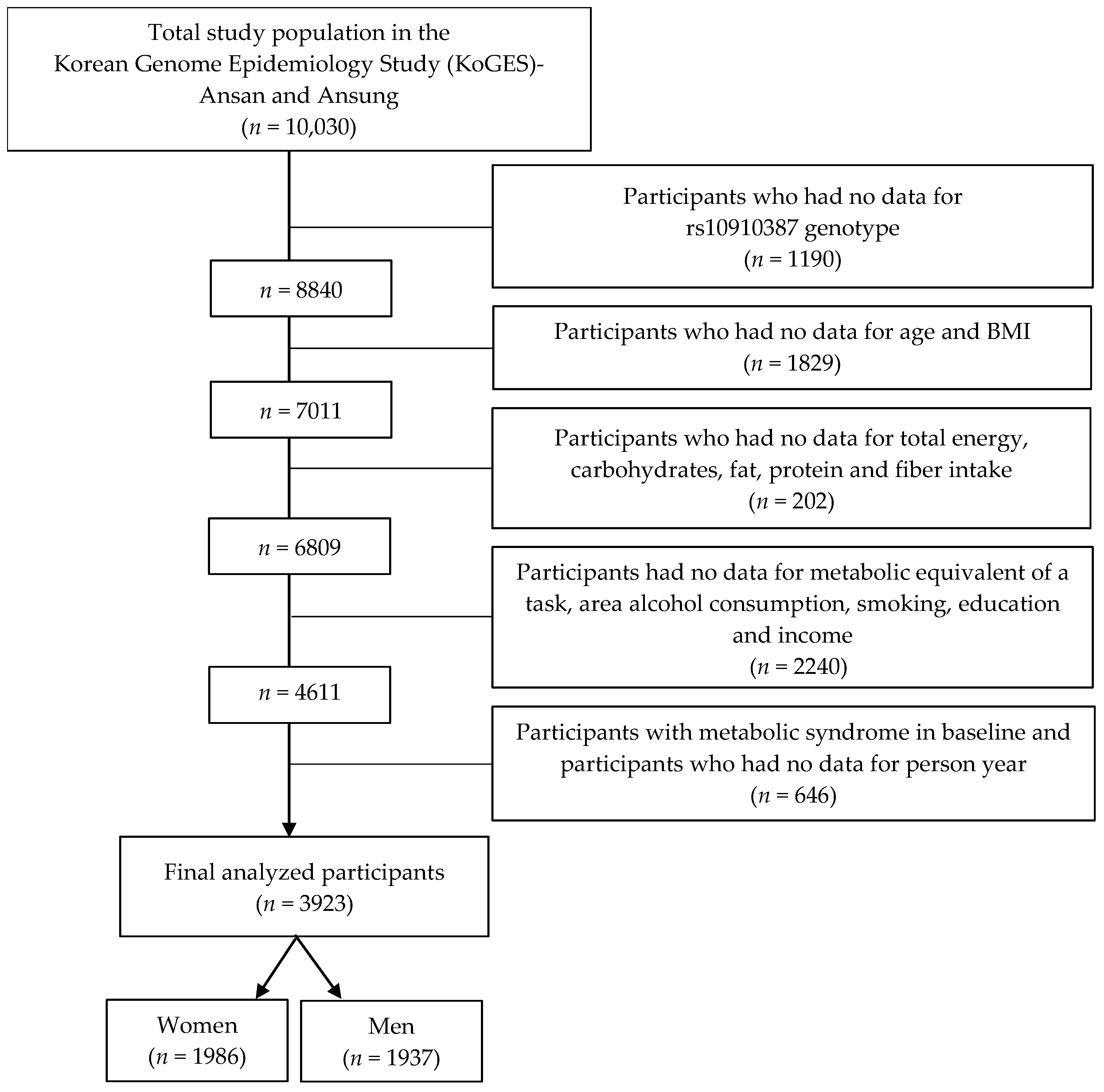
2.1. Data Source and Study Participants
2.2. Dietary Assessment
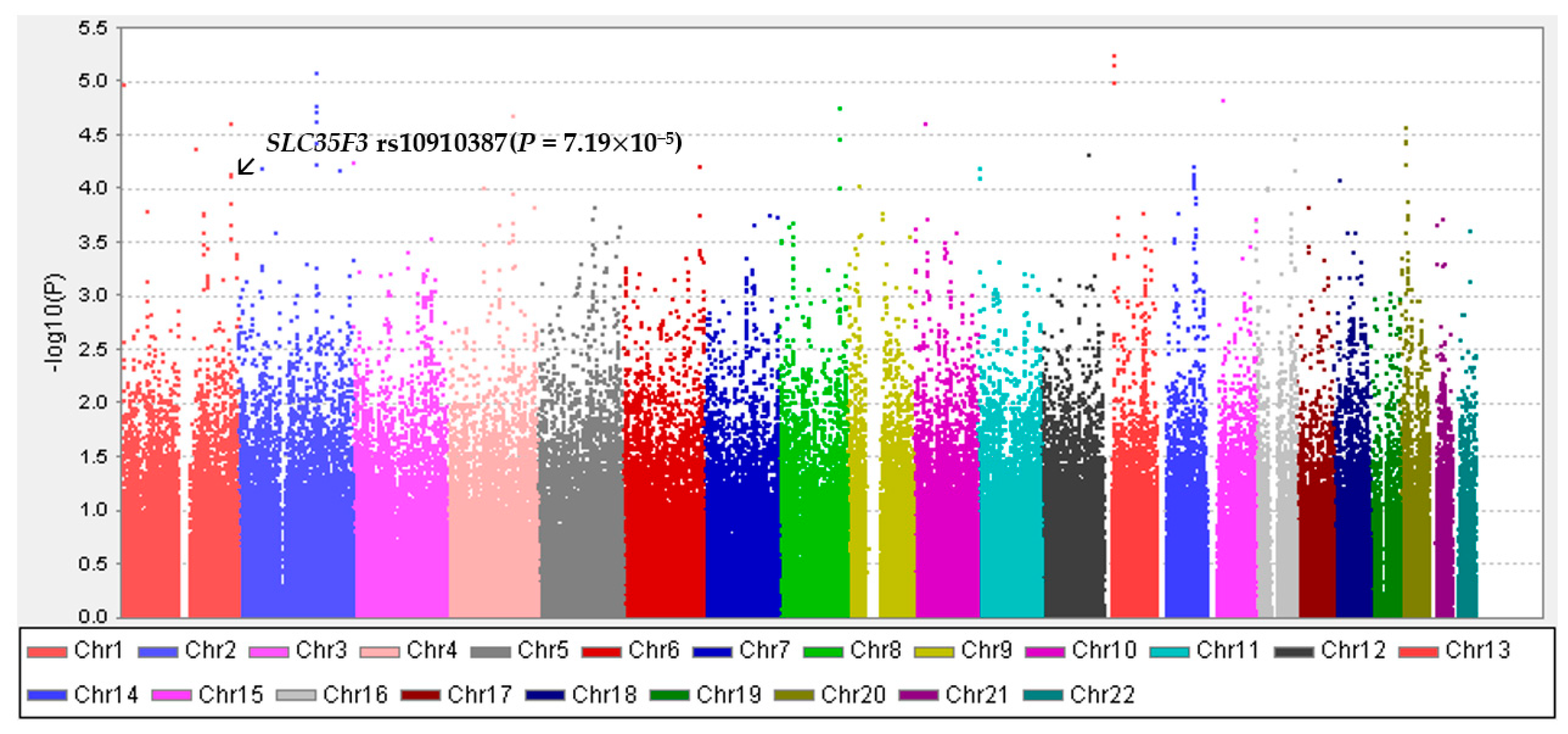
2.3. Genotyping and Imputation
2.4. Assessment of Metabolic Syndrome (MetS)
2.5. Assessment of Other Variables
2.6. Statistical Analyses
3. Results
3.1. General Characteristics of the Study Participants Based on the Presence of Metabolic Syndrome
3.2. General Characteristics of the Study Participants Based on Genetic Variation
3.3. Association of SLC35F3 rs10910387 with Metabolic Syndrome Components
3.4. Metabolic Syndrome Incidence Depending on Genotypes of SLC35F3 rs10910387
3.5. Association of Carbohydrate Intake with Metabolic Syndrome Components
3.6. Metabolic Syndrome Incidence Depending on Carbohydrate Intake
3.7. Metabolic Syndrome Incidence Depending on the Genotype of rs10910387 by Carbohydrate Intake
3.8. Metabolic Syndrome Components Incidence Depending on the Genotype of rs10910387 by Carbohydrate Intake
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azeez, T.A.; Adeleye, J.; Enigbokan, O.A.; Adejimi, B.; Oladapo, J.S. Metabolic syndrome among Nigerians with type 2 diabetes mellitus: A comparative study of the diagnostic criteria. J. Cardio-Diabetes Metab. Disord. 2021, 1, 51. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation; Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Meigs, J.B. Metabolic Syndrome (Insulin Resistance Syndrome or Syndrome X). 2019. Available online: https://www.uptodate.com/contents/metabolic-syndrome-insulin-resistance-syndrome-or-syndrome-x (accessed on 8 June 2022).
- Korean Society of Cardiometabolic Syndrome. Metabolic Syndrome Fact Sheet in Korea 2021. 2021. Available online: http://www.kscms.org/bbs//uploads/2021-04-28/6088c0095b48f.pdf (accessed on 30 March 2022).
- Park, S.; Yang, S.J. Factors Affecting Health Promotion Behavior among Workers with High Risk of Metabolic Syndrome: Based on Theory of Planned Behavior. J. Korean Acad. Community Health Nurs. 2015, 26, 128–139. [Google Scholar] [CrossRef]
- Feldeisen, S.E.; Tucker, K.L. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C.; Lopez-Miranda, J.; Perez-Jimenez, F.; McManus, R.; Roche, H.M. Genetic and nutrient determinants of the metabolic syndrome. Curr. Opin. Cardiol. 2006, 21, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Lee, H.-S.; Lee, J.-W. Association of carbohydrate and fat intake with metabolic syndrome. Clin. Nutr. 2018, 37, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, J.E.; Song, W.O.; Paik, H.-Y.; Song, Y. Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. J. Acad. Nutr. Diet. 2014, 114, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-A.; Choi, J.-H. Association between carbohydrate intake and the prevalence of metabolic syndrome in Korean women. Nutrients 2021, 13, 3098. [Google Scholar] [CrossRef]
- Weiss, S.T.; Silverman, E.K. Pro: Genome-wide association studies (GWAS) in asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 631–633. [Google Scholar] [CrossRef]
- Ortigoza-Escobar, J.D.; Molero-Luis, M.; Arias, A.; Martí-Sánchez, L.; Rodriguez-Pombo, P.; Artuch, R.; Pérez-Dueñas, B. Treatment of genetic defects of thiamine transport and metabolism. Expert Rev. Neurother. 2016, 16, 755–763. [Google Scholar] [CrossRef]
- Zang, X.-L.; Han, W.-Q.; Yang, F.-P.; Ji, K.-D.; Wang, J.-G.; Gao, P.-J.; He, G.; Wu, S.-N. Association of a SNP in SLC35F3 gene with the risk of hypertension in a Chinese han population. Front. Genet. 2016, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-Y.; Choi, J.-H. Genetic Variations in Thiamin Transferase SLC35F3 and the Risk of Hypertension in Koreans. Clin. Nutr. Res. 2021, 10, 140. [Google Scholar] [CrossRef]
- Hong, J.T.; Cho, Y.S. Identification of genetic loci associated with abdominal visceral adiposity in Korean populations. Genes Genom. 2017, 39, 541–548. [Google Scholar] [CrossRef]
- Sedel, F.; Challe, G.; Mayer, J.-M.; Boutron, A.; Fontaine, B.; Saudubray, J.M.; Brivet, M. Thiamine responsive pyruvate dehydrogenase deficiency in an adult with peripheral neuropathy and optic neuropathy. J. Neurol. Neurosurg. Psychiatry 2008, 79, 846–847. [Google Scholar] [CrossRef]
- Masuda, H.; Masuda, T.; Hatta, H. Effect of thiamin (vitamin B1) on carbohydrate metabolism at rest and during exercise. J. Sport. Med. Phys. Fit. 2015, 4, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Seheult, J.; Fitzpatrick, G.; Boran, G. Lactic acidosis: An update. Clin. Chem. Lab. Med. 2017, 55, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huentelman, M.J.; Rao, F.; Sun, E.I.; Corneveaux, J.J.; Schork, A.J.; Wei, Z.; Waalen, J.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; et al. Genetic implication of a novel thiamine transporter in human hypertension. J. Am. Coll. Cardiol. 2014, 63, 1542–1555. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.H. Metabolic syndrome. J. Korean Med. Assoc. 2012, 55, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Han, B.-G.; KoGES Group. Cohort profile: The Korean Genome and Epidemiology Study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar]
- Choi, J.W.; Park, J.-S.; Lee, C.H. Genetically determined hypoalbuminemia as a risk factor for hypertension: Instrumental variable analysis. Sci. Rep. 2021, 11, 11290. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.; Jin, H.-S. MACROD2 polymorphisms are associated with hypertension in Korean population. Korean J. Clin. Lab. Sci. 2019, 51, 57–63. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-S.; Jin, H.-S. Genetic polymorphisms of SLC8A1 are associated with hypertension and left ventricular hypertrophy in the korean population. Korean J. Clin. Lab. Sci. 2019, 51, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Ahn, C.W.; Cha, B.S.; Chung, Y.-S.; Lee, K.W.; Lee, H.C.; Huh, K.B.; Kim, D.J. The prevalence of the metabolic syndrome in Korean adults: Comparison of WHO and NCEP criteria. Yonsei Med. J. 2005, 46, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, H.S.; Kim, S.M.; Kwon, H.S.; Kim, D.Y.; Kim, D.J.; Cho, G.J.; Han, J.H.; Kim, S.R.; Park, C.Y.; et al. Cut-off Points of Waist Circumference for Defining Abdominal Obesity in the Korean Population. J. Korean Soc. Study Obes. 2006, 15, 1–9. [Google Scholar]
- Ha, K.H.; Kim, D.J. Current status of managing diabetes mellitus in Korea. Korean J. Intern. Med. 2016, 31, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Park, S.-H.; Lee, K.-S.; Park, H.-Y. Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: Analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). Int. J. Cardiol. 2010, 139, 234–240. [Google Scholar] [CrossRef]
- Sakurai, M.; Nakagawa, H.; Kadota, A.; Yoshita, K.; Nakamura, Y.; Okuda, N.; Nishi, N.; Miyamoto, Y.; Arima, H.; Ohkubo, T.; et al. Macronutrient intake and socioeconomic status: NIPPON DATA2010. J. Epidemiol. 2018, 28 (Suppl. 3), S17–S22. [Google Scholar] [CrossRef] [Green Version]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.F.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Kokubo, Y.; Saito, I.; Yatsuya, H.; Sawada, N.; Inoue, M.; Tsugane, S. Rice consumption is not associated with risk of cardiovascular disease morbidity or mortality in Japanese men and women: A large population-based, prospective cohort study. Am. J. Clin. Nutr. 2014, 100, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Taylor, A.W.; Hu, G.; Gill, T.; Wittert, G.A. Rice intake, weight change and risk of the metabolic syndrome development among Chinese adults: The Jiangsu Nutrition Study (JIN). Asia Pac. J. Clin. Nutr. 2012, 21, 35–43. [Google Scholar] [PubMed]
- Dam, R.V.; Visscher, A.; Feskens, E.; Verhoef, P.; Kromhout, D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: The Zutphen Elderly Study. Eur. J. Clin. Nutr. 2000, 54, 726–731. [Google Scholar] [PubMed]
- Kim, K.; Yun, S.H.; Choi, B.Y.; Kim, M.K. Cross-sectional relationship between dietary carbohydrate, glycaemic index, glycaemic load and risk of the metabolic syndrome in a Korean population. Br. J. Nutr. 2008, 100, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdi, M.; Imani, H.; Bazshahi, E.; Hosseini, F.; Djafarian, K.; Lesani, A.; Akbarzade, Z.; Shab-Bidar, S. Habitual-and Meal-Specific Carbohydrate Quality Index and Their Relation to Metabolic Syndrome in a Sample of Iranian Adults. Front. Nutr. 2022, 9, 763345. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Steemburgo, T.; de Mello, V.D.; Tonding, S.F.; Gross, J.L.; Azevedo, M.J. High dietary glycemic index and low fiber content are associated with metabolic syndrome in patients with type 2 diabetes. J. Am. Coll. Nutr. 2011, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Sohrab, G.; Hosseini-Esfahani, F.; Azizi, F. Inverse association between fruit, legume, and cereal fiber and the risk of metabolic syndrome: Tehran Lipid and Glucose Study. Diabetes Res. Clin. Pract. 2011, 94, 276–283. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chen, G.-C.; Wang, X.-P.; Qin, L.; Bai, Y. Dietary fiber and metabolic syndrome: A meta-analysis and review of related mechanisms. Nutrients 2017, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Heaton, K.W. Food fibre as an obstacle to energy intake. Lancet 1973, 302, 1418–1421. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Nyman, M.; Fåk, F. Designing future prebiotic fiber to target metabolic syndrome. Nutrition 2014, 30, 497–502. [Google Scholar] [CrossRef]
- Zazpe, I.; Sánchez-Taínta, A.; Santiago, S.; Fuente-Arrillaga, C.D.L.; Bes-Rastrollo, M.; Martínez, J.A.; Martínez-González, M.Á. Association between dietary carbohydrate intake quality and micronutrient intake adequacy in a Mediterranean cohort: The SUN (Seguimiento Universidad de Navarra) Project. Br. J. Nutr. 2014, 111, 2000–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suara, S.B.; Siassi, F.; Saaka, M.; Foroshani, A.R.; Sotoudeh, G. Association between Carbohydrate Quality Index and general and abdominal obesity in women: A cross-sectional study from Ghana. BMJ Open 2019, 9, e033038. [Google Scholar] [CrossRef] [PubMed]
- Bulló, M.; Papandreou, C.; Ruiz-Canela, M.; Guasch-Ferré, M.; Li, J.; Hernández-Alonso, P.; Toledo, E.; Liang, L.; Razquin, C.; Corella, D.; et al. Plasma metabolomic profiles of glycemic index, glycemic load, and carbohydrate quality index in the PREDIMED study. J. Nutr. 2021, 151, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Suara, S.B.; Siassi, F.; Saaka, M.; Rahimiforoushani, A.; Sotoudeh, G. Relationship between dietary carbohydrate quality index and metabolic syndrome among type 2 diabetes mellitus subjects: A case-control study from Ghana. BMC Public Health 2021, 21, 526. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R.; Singleton, C.K.; Hiller-Sturmhöfel, S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res. Health 2003, 27, 134. [Google Scholar]
- Page, G.L.J.; Laight, D.; Cummings, M.H. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 2011, 65, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Lai, C.-Q.; Mattei, J.; Ordovas, J.M.; Tucker, K.L. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: The Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 2010, 91, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J.; Babaei-Jadidi, R.; Ali, H.A.; Rabbani, N.; Antonysuni, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 2007, 50, 2164–2170. [Google Scholar] [CrossRef] [Green Version]
- Luong, K.V.Q.; Nguyena, L.T.H. The impact of thiamine treatment in the diabetes mellitus. J. Clin. Med. Res. 2012, 4, 153. [Google Scholar] [CrossRef] [Green Version]
- Fattal-Valevski, A.; Azouri-Fattal, I.; Greenstein, Y.J.; Guindy, M.; Blau, A.; Zelnik, N. Delayed language development due to infantile thiamine deficiency. Dev. Med. Child Neurol. 2009, 51, 629–634. [Google Scholar] [CrossRef]
- Elmadfa, I.; Majchrzak, D.; Rust, P.; Genser, D. The thiamine status of adult humans depends on carbohydrate intake. Int. J. Vitam. Nutr. Res. 2001, 71, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Carrodeguas, L.; Kaidar-Person, O.; Szomstein, S.; Antozzi, P.; Rosenthal, R. Preoperative thiamine deficiency in obese population undergoing laparoscopic bariatric surgery. SOARD 2005, 1, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.C.; Arundel, C.; Chawla, L.S. Thiamin deficiency in people with obesity. Adv. Nutr. 2015, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Mahdavifard, S.; Nakhjavani, M. Thiamine pyrophosphate improved vascular complications of diabetes in rats with type 2 diabetes by reducing glycation, oxidative stress, and inflammation markers. Med. J. Islam. Repub. Iran 2020, 34, 331–336. [Google Scholar] [CrossRef]
- Alaei-Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: A randomised, double-blind cross-over trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 213–217. [Google Scholar] [CrossRef]
- Araújo, J.; Cai, J.; Stevens, J. Prevalence of optimal metabolic health in American adults: National Health and Nutrition Examination Survey 2009–2016. Metab. Syndr. Relat. Disord. 2019, 17, 46–52. [Google Scholar] [CrossRef]
| Variables | Men | p Value | Women | p Value | ||
|---|---|---|---|---|---|---|
| Metabolic Syndrome (n = 747) | No Metabolic Syndrome (n = 1239) | Metabolic Syndrome (n = 706) | No Metabolic Syndrome (n = 1231) | |||
| rs10910387 | 0.0002 | 0.05 | ||||
| CC | 470 (62.9%) | 864 (69.7%) | 479 (67.9%) | 870 (70.7%) | ||
| TC | 236 (31.6%) | 344 (27.8%) | 201 (28.5%) | 337 (27.4%) | ||
| TT | 41 (5.5%) | 31 (2.5%) | 26 (3.7%) | 24 (2.0%) | ||
| Age (years) | 50.4 ± 8.2 | 50.1 ± 8.6 | 0.47 | 52.7 ± 8.6 | 48.2 ± 7.7 | <0.0001 |
| BMI (kg/m2) | 24.7 ± 2.4 | 23.1 ± 2.5 | <0.0001 | 25.1 ± 2.9 | 23.4 ± 2.7 | <0.0001 |
| Waist circumference (cm) | 84.5 ± 5.8 | 79.7 ± 6.4 | <0.0001 | 81.2 ± 7.5 | 75.2 ± 7.5 | <0.0001 |
| Blood pressure | ||||||
| Systolic blood pressure (mmHg) | 121.5 ± 16.3 | 115.9 ± 15.0 | <0.0001 | 120.0 ± 16.6 | 109.5 ± 14.9 | <0.0001 |
| Diastolic blood pressure (mmHg) | 82.0 ± 10.5 | 78.1 ± 10.2 | <0.0001 | 78.4 ± 9.9 | 72.3 ± 9.7 | <0.0001 |
| Triglycerides (mg/dL) | 181.3 ± 128.4 | 132.7 ± 65.6 | <0.0001 | 133.2 ± 67.3 | 109.8 ± 49.0 | <0.0001 |
| Glucose (mg/dL) | 89.9 ± 19.8 | 85.6 ± 15.3 | <0.0001 | 83.9 ± 15.4 | 80.1 ± 9.7 | <0.0001 |
| HDL-cholesterol (mg/dL) | 42.6 ± 8.4 | 47.0 ± 10.0 | <0.0001 | 45.9 ± 9.1 | 49.9 ± 10.4 | <0.0001 |
| Dietary intake | ||||||
| Calorie intake (kcal) | 2038.7 ± 697.2 | 2013.6 ± 594.6 | 0.41 | 1901.9 ± 674.6 | 1881.8 ± 682.4 | 0.53 |
| Carbohydrate intake (g) | 71.5 ± 30.2 | 69.7 ± 26.2 | 0.18 | 64.1 ± 27.0 | 65.4 ± 32.7 | 0.34 |
| Fat intake (g) | 37.3 ± 23.2 | 36.6 ± 18.8 | 0.48 | 29.4 ± 17.3 | 32.3 ± 22.2 | 0.002 |
| Protein intake (g) | 348.7 ± 109.8 | 345.7 ± 97.7 | 0.54 | 340.8 ± 123.3 | 328.4 ± 108.1 | 0.03 |
| Fiber intake (g) | 7.0 ± 3.6 | 6.8 ± 3.0 | 0.11 | 7.2 ± 3.8 | 6.9 ± 3.3 | 0.04 |
| MET (hours/week) | 166.1 ± 102.4 | 159.2 ± 97.3 | 0.13 | 163.4 ± 102.3 | 143.0 ± 82.9 | <0.0001 |
| Area | 0.004 | <0.0001 | ||||
| Ansung | 241 (32.3%) | 325 (26.2%) | 329 (46.6%) | 295 (24.0%) | ||
| Ansan | 506 (67.7%) | 914 (73.8%) | 377 (53.4%) | 936 (76.0%) | ||
| Education | 0.07 | 0.007 | ||||
| Elementary/technical college | 611 (81.8%) | 1017 (82.1%) | 680 (96.3%) | 1143 (92.9%) | ||
| University | 119 (15.9%) | 173 (14.0%) | 23 (3.3%) | 81 (6.6%) | ||
| Graduate school | 17 (2.3%) | 49 (4.0%) | 3 (0.4%) | 7 (0.6%) | ||
| Income (million won/month) | 0.63 | <0.0001 | ||||
| <1 | 160 (21.4%) | 268 (21.6%) | 281 (39.8%) | 282 (22.9%) | ||
| 1–3 | 381 (51.0%) | 653 (52.7%) | 326 (46.2%) | 669 (54.4%) | ||
| >3 | 206 (27.6%) | 318 (25.7%) | 99 (14.0%) | 280 (22.8%) | ||
| Smoking | 0.004 | 0.49 | ||||
| None | 132 (17.7%) | 285 (23.0%) | 675 (95.6%) | 1189 (96.6%) | ||
| Past | 227 (30.4%) | 394 (31.8%) | 8 (1.1%) | 13 (1.1%) | ||
| Current | 388 (51.9%) | 560 (45.2%) | 23 (3.3%) | 29 (2.4%) | ||
| Drinking | 0.16 | 0.61 | ||||
| None | 129 (17.3%) | 237 (19.1%) | 470 (66.6%) | 837 (68.0%) | ||
| Past | 57 (7.6%) | 118 (9.5%) | 24 (3.4%) | 33 (2.7%) | ||
| Current | 561 (75.1%) | 884 (71.4%) | 212 (30.0%) | 361 (29.3%) | ||
| Variables | Men | p Value | Women | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 1334) | TC (n = 580) | TT (n = 72) | CC (n = 1349) | TC (n = 538) | TT (n = 50) | |||
| Age (years) | 50.0 ± 8.3 | 50.6 ± 8.7 | 50.5 ± 9.3 | 0.42 | 49.9 ± 8.4 | 49.7 ± 8.1 | 48.1 ± 7.8 | 0.27 |
| BMI (kg/m2) | 23.6 ± 2.6 | 23.8 ± 2.6 | 24.3 ± 2.4 | 0.06 | 24.0 ± 2.8 | 24.0 ± 3.0 | 24.0 ± 2.8 | 0.98 |
| Waist circumference (cm) | 81.4 ± 6.7 | 81.8 ± 6.4 | 81.7 ± 6.4 | 0.39 | 77.3 ± 7.9 | 77.6 ± 8.3 | 77.1 ± 8.6 | 0.73 |
| Blood pressure | ||||||||
| Systolic blood pressure (mmHg) | 117.5 ± 15.5 | 119.0 ± 15.8 | 119.6 ± 18.9 | 0.09 | 113.2 ± 16.0 | 113.7 ± 17.0 | 112.2 ± 16.8 | 0.75 |
| Diastolic blood pressure (mmHg) | 79.2 ± 10.5 | 80.5 ± 10.5 | 79.2 ± 10.3 | 0.04 | 74.5 ± 10.1 | 74.5 ± 10.6 | 75.1 ± 9.7 | 0.92 |
| Triglycerides (mg/dL) | 148.0 ± 91.7 | 156.8 ± 109.4 | 158.5 ± 86.6 | 0.15 | 118.1 ± 56.9 | 119.5 ± 60.5 | 112.7 ± 37.0 | 0.7 |
| Glucose (mg/dL) | 87.5 ± 18.1 | 86.6 ± 15.1 | 87.3 ± 17.1 | 0.61 | 81.5 ± 12.3 | 81.3 ± 12.2 | 81.1 ± 7.3 | 0.92 |
| HDL-cholesterol (mg/dL) | 45.6 ± 9.6 | 44.8 ± 9.7 | 45.5 ± 10.6 | 0.23 | 48.4 ± 10.2 | 48.6 ± 10.0 | 47.7 ± 9.7 | 0.76 |
| Dietary intake | ||||||||
| Calorie intake (kcal) | 2018.2 ± 649.8 | 2035.0 ± 608.9 | 2018.7 ± 578.7 | 0.87 | 1889.0 ± 669.8 | 1903.8 ± 716.4 | 1735.6 ± 496.1 | 0.25 |
| Protein intake (g) | 70.1 ± 28.0 | 71.1 ± 27.7 | 69.3 ± 24.0 | 0.75 | 64.7 ± 29.4 | 65.9 ± 34.5 | 61.4 ± 22.9 | 0.54 |
| Fat intake (g) | 36.8 ± 20.9 | 37.3 ± 20.2 | 36.0 ± 16.5 | 0.81 | 31.1 ± 19.6 | 31.7 ± 23.1 | 29.4 ± 18.4 | 0.7 |
| Carbohydrate intake (g) | 346.1 ± 104.4 | 348.4 ± 98.8 | 348.8 ± 94.8 | 0.89 | 333.3 ± 113.1 | 334.8 ± 118.2 | 302.5 ± 85.7 | 0.16 |
| Fiber intake (g) | 6.8 ± 3.2 | 7.0 ± 3.3 | 6.8 ± 2.8 | 0.35 | 6.9 ± 3.4 | 7.2 ± 3.8 | 6.4 ± 2.9 | 0.3 |
| MET (hours/week) | 163.2 ± 99.0 | 161.1 ± 101.3 | 142.4 ± 86.6 | 0.22 | 149.7 ± 90.6 | 149.8 ± 91.9 | 178.8 ± 86.8 | 0.08 |
| Area | 0.29 | 0.67 | ||||||
| Ansung | 379 (28.4%) | 172 (29.7%) | 15 (20.8%) | 434 (32.2%) | 171 (31.8%) | 19 (38.0%) | ||
| Ansan | 955 (71.6%) | 408 (70.3%) | 57 (79.2%) | 915 (67.8%) | 367 (68.2%) | 31 (62.0%) | ||
| Education | 0.24 | 0.47 | ||||||
| Elementary/technical college | 1082 (81.1%) | 483 (83.3%) | 63 (87.5%) | 1267 (93.9%) | 506 (94.1%) | 50 (100.0%) | ||
| University | 208 (15.6%) | 75 (12.9%) | 9 (12.5%) | 74 (5.5%) | 30 (5.6%) | 0 (0%) | ||
| Graduate school | 44 (3.3%) | 22 (3.8%) | 0 (0%) | 8 (0.6%) | 2 (0.4%) | 0 (0%) | ||
| Income (million won/month) | 0.78 | 0.43 | ||||||
| <1 | 282 (21.1%) | 130 (22.4%) | 16 (22.2%) | 388 (28.8%) | 161 (29.9%) | 14 (28.0%) | ||
| 1–3 | 688 (51.6%) | 308 (53.1%) | 38 (52.8%) | 691 (51.2%) | 282 (52.4%) | 22 (44.0%) | ||
| >3 | 364 (27.3%) | 142 (24.5%) | 18 (25.0%) | 270 (20.0%) | 95 (17.7%) | 14 (28.0%) | ||
| Smoking | 0.7 | 0.32 | ||||||
| None | 281 (21.1%) | 118 (20.3%) | 18 (25.0%) | 1299 (96.3%) | 519 (96.5%) | 46 (92.0%) | ||
| Past | 410 (30.7%) | 192 (33.1%) | 19 (26.4%) | 12 (0.9%) | 8 (1.5%) | 1 (2.0%) | ||
| Current | 643 (48.2%) | 270 (46.6%) | 35 (48.6%) | 38 (2.8%) | 11 (2.0%) | 3 (6.0%) | ||
| Drinking | 0.81 | 0.61 | ||||||
| None | 243 (18.2%) | 106 (18.3%) | 17 (23.6%) | 907 (67.2%) | 367 (68.2%) | 33 (66.0%) | ||
| Past | 120 (9.0%) | 50 (8.6%) | 5 (6.9%) | 36 (2.7%) | 18 (3.4%) | 3 (6.0%) | ||
| Current | 971 (72.8%) | 424 (73.1%) | 50 (69.4%) | 406 (30.1%) | 153 (28.4%) | 14 (28.0%) | ||
| SNP | rs10910387 (SLC35F3) | |
|---|---|---|
| Minor Allele: T | Beta ± SE | Add p |
| Waist circumference (cm) | 0.23 ± 0.17 | 0.161 |
| Systolic blood pressure (mmHg) | 0.38 ± 1.11 | 0.266 |
| Diastolic blood pressure (mmHg) | 0.46 ± 2.10 | 0.036 |
| Triglycerides (mg/dL) | 5.54 ± 2.68 | 0.007 |
| Glucose (mg/dL) | 0.12 ± 0.27 | 0.784 |
| HDL-cholesterol (mg/dL) | −0.30 ± −1.53 | 0.126 |
| Men | Metabolic Syndrome | ||
| rs10910387 (SLC35F3) | Cases/Total | HR (95% CI) | p Value |
| CC | 470/1334 | 1.00 (Ref) | |
| TC | 236/580 | 1.19 (1.02–1.39) | 0.03 |
| TT | 41/72 | 1.48 (1.07–2.04) | 0.02 |
| Women | Metabolic Syndrome | ||
| rs10910387 (SLC35F3) | Cases/Total | HR (95% CI) | p Value |
| CC | 479/1349 | 1.00 (Ref) | |
| TC | 201/538 | 1.11 (0.94–1.31) | 0.22 |
| TT | 26/50 | 2.15 (1.44–3.21) | 0.0002 |
| Men (n = 1986) | Women (n = 1937) | |||
|---|---|---|---|---|
| Beta ± SE | p Value | Beta ± SE | p Value | |
| Waist circumference (cm) | −0.01 ± 0.01 | 0.082 | 0.01 ± 0.01 | 0.310 |
| Systolic blood pressure (mmHg) | 0.01 ± 0.02 | 0.648 | −0.003 ± 0.02 | 0.896 |
| Diastolic blood pressure (mmHg) | −0.01 ± 0.02 | 0.739 | 0.01 ± 0.01 | 0.375 |
| Triglycerides (mg/dL) | 0.42 ± 0.15 | 0.005 | 0.09 ± 0.09 | 0.284 |
| Fasting blood glucose (mg/dL) | −0.04 ± 0.03 | 0.113 | −0.003 ± 0.02 | 0.863 |
| HDL-cholesterol (mg/dL) | −0.02 ± 0.01 | 0.043 | −0.04 ± 0.02 | 0.004 |
| Men | Metabolic Syndrome | ||
| Carbohydrate (% Energy) | Cases/Total | HR (95% CI) | p Value |
| Tertile 1 | 258/662 | 1.00 (Ref) | |
| Tertile 2 | 231/662 | 0.87 (0.73–1.05) | 0.14 |
| Tertile 3 | 258/662 | 0.98 (0.80–1.19) | 0.83 |
| Women | Metabolic Syndrome | ||
| Carbohydrate (% Energy) | Cases/Total | HR (95% CI) | p Value |
| Tertile 1 | 191/645 | 1.00 (Ref) | |
| Tertile 2 | 233/646 | 1.07 (0.86–1.30) | 0.53 |
| Tertile 3 | 282/646 | 0.95 (0.77–1.17) | 0.64 |
| Men | Carbohydrate (% Energy) | Women | Carbohydrate (% Energy) | ||||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | ||||
| Median | 63.4 | 69.5 | 75.3 | Median | 64.9 | 71.6 | 77.4 | ||
| Person-years | 5599 | 5688 | 5434 | 16,721 | Person-years | 5809 | 5742 | 5235 | 16,786 |
| Incident cases (n) | 258/662 | 231/662 | 258/662 | 747/1986 | Incident cases (n) | 191/645 | 233/646 | 282/646 | 706/1937 |
| Adjusted HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | p interaction | Adjusted HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | p interaction |
| CC | 1.00 (Ref) | 0.83 (0.66–1.04) | 0.90 (0.71–1.14) | 0.16 | CC | 1.00 (Ref) | 1.01 (0.80–1.28) | 0.95 (0.75–1.21) | 0.79 |
| TC | 1.02 (0.77–1.34) | 1.03 (0.78–1.36) | 1.19 (0.90–1.57) | TC | 1.11 (0.80–1.54) | 1.26 (0.94–1.69) | 0.97 (0.72–1.30) | ||
| TT | 1.48 (0.86–2.56) | 1.02 (0.60–1.74) | 1.88 (1.03–3.41) | TT | 1.45 (0.59–3.57) | 2.22 (1.12–4.42) | 2.53 (1.38–4.61) | ||
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Carbohydrate (% Energy) | Carbohydrate (% Energy) | ||||||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | p Interaction | HR (95% CI) | HR (95% CI) | HR (95% CI) | p Interaction | ||
| Abdominal obesity | 0.89 | Abdominal obesity | 0.21 | ||||||
| CC | 1.00 (Ref) | 1.00 (0.78–1.29) | 0.85 (0.65–1.11) | CC | 1.00 (Ref) | 0.97 (0.76–1.25) | 1.04 (0.81–1.34) | ||
| TC | 1.10 (0.80–1.52) | 1.21 (0.90–1.62) | 0.96 (0.69–1.33) | TC | 1.33 (0.95–1.86) | 1.06 (0.77–1.46) | 0.92 (0.66–1.27) | ||
| TT | 1.32 (0.64–2.71) | 1.38 (0.79–2.40) | 1.17 (0.63–2.17) | TT | 0.70 (0.22–2.19) | 1.72 (0.70–4.22) | 1.00 (0.50–1.99) | ||
| Elevated blood pressure | 0.53 | Elevated blood pressure | 0.74 | ||||||
| CC | 1.00 (Ref) | 0.82 (0.65–1.04) | 0.90 (0.71–1.14) | CC | 1.00 (Ref) | 1.10 (0.87–1.41) | 0.98 (0.77–1.27) | ||
| TC | 0.92 (0.68–1.24) | 0.86 (0.64–1.16) | 0.98 (0.73–1.32) | TC | 1.02 (0.73–1.44) | 1.21 (0.89–1.64) | 1.01 (0.74–1.37) | ||
| TT | 0.89 (0.42–1.91) | 0.93 (0.52–1.68) | 0.79 (0.35–1.81) | TT | 1.10 (0.48–2.50) | 1.67 (0.80–3.47) | 0.78 (0.32–1.91) | ||
| Elevated fasting glucose | 0.68 | Elevated fasting glucose | 0.17 | ||||||
| CC | 1.00 (Ref) | 0.90 (0.74–1.08) | 0.83 (0.68–1.02) | CC | 1.00 (Ref) | 0.96 (0.77–1.19) | 0.87 (0.69–1.09) | ||
| TC | 1.05 (0.83–1.33) | 0.92 (0.72–1.18) | 1.03 (0.81–1.31) | TC | 1.34 (1.02–1.75) | 1.09 (0.83–1.43) | 0.95 (0.72–1.25) | ||
| TT | 1.28 (0.71–2.29) | 0.87 (0.50–1.49) | 0.74 (0.36–1.52) | TT | 1.93 (1.04–3.57) | 0.92 (0.38–2.26) | 1.03 (0.55–1.92) | ||
| Elevated triglycerides | 0.84 | Elevated triglycerides | 0.1 | ||||||
| CC | 1.00 (Ref) | 0.95 (0.74–1.23) | 1.06 (0.81–1.38) | CC | 1.00 (Ref) | 1.12 (0.89–1.41) | 1.02 (0.80–1.30) | ||
| TC | 1.03 (0.74–1.42) | 1.11 (0.80–1.55) | 0.96 (0.68–1.37) | TC | 0.80 (0.57–1.12) | 1.19 (0.88–1.61) | 1.26 (0.93–1.71) | ||
| TT | 1.35 (0.55–3.32) | 0.87 (0.44–1.73) | 1.59 (0.76–3.31) | TT | 1.30 (0.57–2.96) | 1.05 (0.42–2.60) | 1.50 (0.80–2.79) | ||
| Low HDL cholesterol | 0.56 | Low HDL cholesterol | 0.76 | ||||||
| CC | 1.00 (Ref) | 0.95 (0.77–1.19) | 0.98 (0.79–1.23) | CC | 1.00 (Ref) | 0.99 (0.75–1.29) | 1.01 (0.76–1.35) | ||
| TC | 0.81 (0.60–1.09) | 1.11 (0.84–1.46) | 1.10 (0.83–1.47) | TC | 1.03 (0.72–1.48) | 1.17 (0.83–1.67) | 0.96 (0.67–1.37) | ||
| TT | 1.79 (1.02–3.16) | 0.79 (0.42–1.51) | 1.16 (0.60–2.29) | TT | 1.01 (0.37–2.75) | 1.32 (0.53–3.33) | 1.96 (0.91–4.24) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Shin, D. Effects of Interaction between SLC35F3 and Carbohydrate Intake on the Incidence of Metabolic Syndrome in Korean Middle-Aged Adults. Nutrients 2023, 15, 469. https://doi.org/10.3390/nu15020469
Park H, Shin D. Effects of Interaction between SLC35F3 and Carbohydrate Intake on the Incidence of Metabolic Syndrome in Korean Middle-Aged Adults. Nutrients. 2023; 15(2):469. https://doi.org/10.3390/nu15020469
Chicago/Turabian StylePark, Haeun, and Dayeon Shin. 2023. "Effects of Interaction between SLC35F3 and Carbohydrate Intake on the Incidence of Metabolic Syndrome in Korean Middle-Aged Adults" Nutrients 15, no. 2: 469. https://doi.org/10.3390/nu15020469
APA StylePark, H., & Shin, D. (2023). Effects of Interaction between SLC35F3 and Carbohydrate Intake on the Incidence of Metabolic Syndrome in Korean Middle-Aged Adults. Nutrients, 15(2), 469. https://doi.org/10.3390/nu15020469






