Reduced Relapse-Free Survival in Colorectal Cancer Patients with Elevated Soluble CD40 Ligand Levels Improved by Vitamin D Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Randomization and Blinding
2.4. Intervention
2.5. Follow-Up and Outcomes
2.6. Measurement of Serum sCD40L Levels
2.7. Vitamin D Measurement
2.8. Sample Size
2.9. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of Patients Randomized into Vitamin D and Placebo Groups
3.3. Serum sCD40L Levels
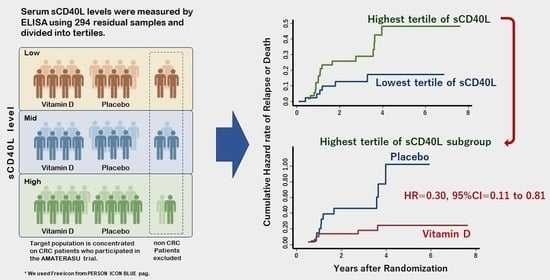
3.4. Characteristics of Patients Stratified into Tertiles According to Serum sCD40L Levels
3.5. Survival Analyses of Patients Stratified According to Tertiles of Serum sCD40L
3.6. Effect of Vitamin D Supplementation in the Subgroup of CRC Patients in the Highest sCD40L Tertile
4. Discussion
Study Limitations
- Sample size: Our analysis included a relatively small number of CRC patients in the highest sCD40L tertile subgroup, as well as in the esophageal cancer group. This limitation might increase the risk of type II errors.
- Timing of measurements: Serum sCD40L levels were exclusively measured postoperatively, omitting preoperative and post-supplement-initiation measurements. A comprehensive evaluation of whether vitamin D supplementation genuinely reduces sCD40L levels requires a comparison of levels before and after the initiation of vitamin D supplementation and placebo.
- Exploratory nature: It is crucial to recognize that our study involved exploratory analyses, which were not predefined in the original AMATERASU trial protocol. Consequently, a degree of caution is warranted when interpreting these findings.
- Subgroup analyses: Subgroup analyses based on tertiles might heighten the probability of type I errors due to multiple comparisons.
- Population specificity: The AMATERASU trial predominantly featured a Japanese population with specific cancer subtypes. Given the variation in cancer types and genetics across populations, generalizing our study’s outcomes to different populations should be undertaken with caution.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Estimated Number of New Cases from 2020 to 2040, Both Sexes, Age [0–85+]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype (accessed on 27 September 2023).
- Akutsu, T.; Kitamura, H.; Himeiwa, S.; Kitada, S.; Akasu, T.; Urashima, M. Vitamin D and Cancer Survival: Does Vitamin D Supplementation Improve the Survival of Patients with Cancer? Curr. Oncol. Rep. 2020, 22, 62. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. VITAL Research Group. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 38, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef] [PubMed]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M.; et al. Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Shaw, P.G.; Buijs, L.F.; Blackmur, J.P.; Theodoratou, E.; Zgaga, L.; Din, F.V.N.; Farrington, S.M.; Dunlop, M.G. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2020, 123, 1705–1712. [Google Scholar] [CrossRef]
- Urashima, M.; Ohdaira, H.; Akutsum, T.; Okada, S.; Yoshida, M.; Kitajima, M.; Suzuki, Y. Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients with Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA 2019, 321, 1361–1369. [Google Scholar] [CrossRef]
- Morita, M.; Okuyama, M.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Vitamin D Supplementation Regulates Postoperative Serum Levels of PD-L1 in Patients with Digestive Tract Cancer and Improves Survivals in the Highest Quintile of PD-L1: A Post Hoc Analysis of the AMATERASU Randomized Controlled Trial. Nutrients 2021, 13, 1987. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman., E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Sadeghlar, F.; Vogt, A.; Mohr, R.U.; Mahn, R.; van Beekum, K.; Kornek, M.; Weismüller, T.J.; Branchi, V.; Matthaei, H.; Toma, M.; et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol. Immunother. 2021, 70, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Sadeghlar, F.; Ayub, T.H.; Schneider, C.; Möhring, C.; Zhou, T.; Mahn, R.; Bartels, A.; Praktiknjo, M.; Kornek, M.T.; et al. Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma. Cancers 2021, 13, 3375. [Google Scholar] [CrossRef] [PubMed]
- Ohe, G.; Kudo, Y.; Kamada, K.; Mouri, Y.; Takamaru, N.; Kudoh, K.; Kurio, N.; Miyamoto, Y. The Soluble Factor from Oral Cancer Cell Lines Inhibits Interferon-γ Production by OK-432 via the CD40/CD40 Ligand Pathway. Cancer 2021, 13, 3301. [Google Scholar] [CrossRef]
- Labeur, M.S.; Roters, B.; Pers, B.; Mehling, A.; Luger, T.A.; Schwarz, T.; Grabbe, S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J. Immunol. 1999, 162, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.J., Jr.; Fisher, D.A.; Wallace, P.K.; Channon, J.Y.; Noelle, R.J.; Gui, J.; Ernstoff, M.S. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: Tumor-specific immune responses are associated with improved survival. Clin. Cancer Res. 2010, 16, 5548–5556. [Google Scholar] [CrossRef]
- Clark, E.A. CD40: A cytokine receptor in search of a ligand. Tissue Antigens 1990, 36, 33–36. [Google Scholar] [CrossRef]
- Armitage, R.J.; Fanslow, W.C.; Strockbine, L.; Sato, T.A.; Clifford, K.N.; Macduff, B.M.; Anderson, D.M.; Gimpel, S.D.; Davis-Smith, T.; Maliszewski, C.R.; et al. Molecular and biological characterization of a murine ligand for CD40. Nature 1992, 357, 80–82. [Google Scholar] [CrossRef]
- Aruffo, A.; Farrington, M.; Hollenbaugh, D.; Li, X.; Milatovich, A.; Nonoyama, S.; Bajorath, J.; Grosmaire, L.S.; Stenkamp, R.; Neubauer, M.; et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 1993, 72, 291–300. [Google Scholar] [CrossRef]
- DiSanto, J.P.; Bonnefoy, J.Y.; Gauchat, J.F.; Fischer, A.; de Saint Basile, G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature 1993, 361, 541–543. [Google Scholar] [CrossRef]
- Korthäuer, U.; Graf, D.; Mages, H.W.; Brière, F.; Padayachee, M.; Malcolm, S.; Ugazio, A.G.; Notarangelo, L.D.; Levinsky, R.J.; Kroczek, R.A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature 1993, 361, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C.; Armitage, R.J.; Conley, M.E.; Rosenblatt, H.; Jenkins, N.A.; Copeland, N.G.; Bedell, M.A.; Edelhoff, S.; Disteche, C.M.; Simoneaux, D.K.; et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science 1993, 259, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Geha, R.S.; Rosen, F.S. The genetic basis of immunoglobulin-class switching. N. Engl. J. Med. 1994, 330, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Durandy, A.; Schiff, C.; Bonnefoy, J.Y.; Forveille, M.; Rousset, F.; Mazzei, G.; Milili, M.; Fischer, A. Induction by anti-CD40 antibody or soluble CD40 ligand and cytokines of IgG, IgA and IgE production by B cells from patients with X-linked hyper IgM syndrome. Eur. J. Immunol. 1993, 23, 2294–2299. [Google Scholar] [CrossRef]
- Graf, D.; Müller, S.; Korthäuer, U.; van Kooten, C.; Weise, C.; Kroczek, R.A. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur. J. Immunol. 1995, 25, 1749–1754. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Heeschen, C.; Dimmeler, S.; Hamm, C.W.; van den Brand, M.J.; Boersma, E.; Zeiher, A.M.; Simoons, M.L. CAPTURE Study Investigators. Soluble CD40 ligand in acute coronary syndromes. N. Engl. J. Med. 2003, 348, 1104–1111. [Google Scholar] [CrossRef]
- Pereira-da-Silva, T.; Ferreira, V.; Castelo, A.; Caldeira, D.; Napoleão, P.; Pinheiro, T.; Ferreira, R.C.; Carmo, M.M. Soluble CD40 ligand expression in stable atherosclerosis: A systematic review and meta-analysis. Atherosclerosis 2021, 319, 86–100. [Google Scholar] [CrossRef]
- Percario, R.; Panaccio, P.; di Mola, F.F.; Grottola, T.; Di Sebastiano, P. The complex network between inflammation and colorectal cancer: A systematic review of the literature. Cancers 2021, 13, 6237. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Manninen, P.; Karvonen, A.L.; Huhtala, H.; Aitola, P.; Hyöty, M.; Nieminen, I.; Hemminki, H.; Collin, P. The risk of colorectal cancer in patients with inflammatory bowel diseases in Finland: A follow-up of 20 years. J. Crohn’s Colitis 2013, 11, e551–e557. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Wei, S.; Xu, J.; Jiang, X.; Yang, L. The ratio of platelets to lymphocytes predicts the prognosis of metastatic colorectal cancer: A review and meta-analysis. Gastroenterol. Res. Pract. 2021, 2021, 9699499. [Google Scholar] [CrossRef] [PubMed]
- Dymicka-Piekarska, V.; Korniluk, A.; Gryko., M.; Siergiejko, E.; Kemona, H. Potential role of soluble CD40 ligand as inflammatory biomarker in colorectal cancer patients. Int. J. Biol. Markers 2014, 29, e261–e267. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Mineo, T.C.; Basili, S.; Martini, F.; Mariotti, S.; Aloe, S.; Del Monte, G.; Ambrogi, V.; Spila, A.; Palmirotta, R.; et al. Soluble CD40 ligand plasma levels in lung cancer. Clin. Cancer Res. 2004, 10, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Lee, J.W.; Park, P.J.; Shin, Y.S.; Lee, W.Y.; Lee, K.A.; Ye, S.; Hyun, H.; Kang, K.N.; Yeo, D.; et al. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009, 11, R22. [Google Scholar] [CrossRef] [PubMed]
- Angelou, A.; Antoniou, E.; Garmpis, N.; Damaskos, C.; Theocharis, S.; Margonis, G.A. The Role of Soluble CD40L Ligand in Human Carcinogenesis. Anticancer Res. 2018, 38, 3199–3201. [Google Scholar] [CrossRef]
- Da Silva, J.P.A.; Martins, M.R.; Dos Santos, R.L.; da Silva, L.M.; Lima, C.A.C.; Torres, L.C.; Forones, N.M. Evaluation of platelet activation marker expression and its correlation with tumorigenesis and tumor progression in patients with gastric cancer. J. Surg. Oncol. 2022, 126, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Azzariti, A.; Brunetti, O.; Porcelli, L.; Graziano, G.; Iacobazzi, R.M.; Signorile, M.; Scarpa, A.; Lorusso, V.; Silvestris, N. Potential predictive role of chemotherapy-induced changes of soluble CD40 ligand in untreated advanced pancreatic ductal adenocarcinoma. OncoTargets Ther. 2016, 9, 4681–4686. [Google Scholar] [CrossRef]
- Zhao, P.; Fang, W.J.; Chai, L.; Ruan, J.; Zheng, Y.; Jiang, W.Q.; Lin, S.; Zhou, S.H.; Zhang, Z.L. The prognostic value of plasma soluble CD40 ligand levels in patients with nasopharyngeal carcinoma. Clin. Chim. Acta 2015, 447, 66–70. [Google Scholar] [CrossRef]
- Huang, J.; Jochems, C.; Talaie, T.; Anderson, A.; Jales, A.; Tsang, K.Y.; Madan, R.A.; Gulley, J.L.; Schlom, J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood 2012, 120, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Vahedi, H.; Honarvar, M.R.; Amiriani, T.; Nikniaz, Z.; Rad, E.Y.; Hosseinzadeh-Attar, M.J. Vitamin D decreases CD40L gene expression in ulcerative colitis patients: A randomized, double-blinded, placebo-controlled trial. Turk. J. Gastroenterol. 2020, 31, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Kamerud, J.Q.; Selvaag, S.R.; Lorenz, J.D.; Napoli, J.L. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin. Chem. 1993, 39, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Mezawa, H.; Sugiura, T.; Watanabe, M.; Norizoe, C.; Takahashi, D.; Shimojimam, A.; Tamez, S.; Tsutsumi, Y.; Yanaga, K.; Urashima, M. Serum vitamin D levels and survival of patients with colorectal cancer: Post-hoc analysis of a prospective cohort study. BMC Cancer 2010, 10, 347. [Google Scholar] [CrossRef]
- Fang, Y.; Doyle, M.F.; Chen, J.; Alosco, M.L.; Mez, J.; Satizabal, C.L.; Qiu, W.Q.; Murabito, J.M.; Lunetta, K.L. Association between inflammatory biomarkers and cognitive aging. PLoS ONE 2022, 17, e0274350. [Google Scholar] [CrossRef] [PubMed]
- Vardon-Bounes, F.; Garcia, C.; Piton, A.; Series, J.; Gratacap, M.P.; Poëtte, M.; Seguin, T.; Crognier, L.; Ruiz, S.; Silva, S.; et al. Evolution of platelet activation parameters during septic shock in intensive care unit. Platelets 2022, 33, 918–925. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Cong, X.; Jiang, S.; Li, S.; Shen, Q.; Chen, L. sCD40L is increased and associated with the risk of gestational diabetes mellitus in pregnant women with isolated TPOAb positivity. Int. J. Endocrinol. 2022, 2022, 2946891. [Google Scholar] [CrossRef] [PubMed]
- Herold, Z.; Herold, M.; Herczeg, G.; Fodor, A.; Szasz, A.M.; Dank, M.; Somogyi, A. High plasma CD40 ligand level is associated with more advanced stages and worse prognosis in colo-rectal cancer. World J. Clin. Cases 2022, 10, 4084–4096. [Google Scholar] [CrossRef]




| Vitamin D n = 181 | Placebo n = 113 | |
|---|---|---|
| 25(OH)D (ng/mL), median IQR (25–75%) | 21 (16–27) | 21 (14–27) |
| Sex, n (%) | ||
| Male | 127 (70.2) | 71 (62.8) |
| Female | 54 (29.8) | 42 (37.2) |
| Age (years), median IQR (25–75%) | 67 (61–75) | 64 (58–71) |
| Body mass index (kg/m2), median IQR (25–75%) | 21.7 (19.6–24.0) | 22.1 (20.1–23.6) |
| History of other cancers, n (%) | 6 (5.3) | 6 (3.3) |
| Comorbidities, n (%) | ||
| Hypertension | 72 (39.8) | 42 (37.2) |
| Diabetes mellitus | 33 (18.2) | 15 (13.3) |
| Endocrine diseases | 22 (12.2) | 10 (8.9) |
| Cardiovascular diseases | 15 (8.3) | 7 (6.2) |
| Chronic kidney diseases | 3 (1.7) | 0 (0.0) |
| Asthma | 3 (1.7) | 0 (0.0) |
| Orthopedic diseases | 0 (0.0) | 1 (0.9) |
| Site of cancer, n (%) | ||
| Esophagus | 16 (8.8) | 12 (10.6) |
| Stomach | 76 (42.0) | 52 (46.0) |
| Colorectal | 89 (49.2) | 49 (43.4) |
| Stage, n (%) | ||
| I | 82 (45.3) | 48 (42.5) |
| II | 46 (25.4) | 34 (30.1) |
| III | 53 (29.3) | 31 (27.4) |
| Pathology | ||
| Adenocarcinoma, n (%) | 165 (91.2) | 102 (90.3) |
| Squamous cell carcinoma, n (%) | 16 (8.8) | 11 (9.7) |
| Adjuvant chemotherapy, n (%) | 63 (34.8) | 40 (35.4) |
| Total, n = 294 | Lowest Tertile, n = 98 | Middle Tertile, n = 98 | Highest Tertile, n = 98 | p-Value | |
|---|---|---|---|---|---|
| sCD40L (pg/mL), median (IQR) | 117 (67–186) | 49 (32–67) | 117 (98–138) | 229 (186–276) | |
| Vitamin D supplementation, n (%) | 181 (61.6) | 59 (60.2) | 65 (66.3) | 57 (58.2) | 0.47 c |
| 25(OH)D (ng/mL), median (IQR) | 21 (16–27) | 20 (16–27) | 22 (16–29) | 20.5 (15–27) | 0.32 b |
| Female, n (%) | 96 (32.7) | 32 (32.7) | 30 (30.6) | 34 (34.7) | 0.83 c |
| Age (years), median (IQR) | 66 (60–74) | 67 (61–74) | 67 (62–74) | 64 (57–72) | 0.03 b |
| BMI (kg/m2), median (IQR) | 21.8 (19.8–23.8) | 21.3 (19.5–23.3) | 21.8 (20.0–23.6) | 22.2 (20.2–24.5) | 0.04 b |
| History of other cancers, n (%) | 12 (4.1) | 4 (4.1) | 3 (3.1) | 5 (5.1) | 0.77 c |
| Comorbidities, n (%) | |||||
| Hypertension | 114 (38.8) | 38 (38.8) | 36 (36.7) | 40 (40.8) | 0.84 c |
| Diabetes mellitus | 48 (16.3) | 14 (14.3) | 18 (18.4) | 16 (16.3) | 0.74 c |
| Endocrine diseases | 32 (10.9) | 10 (10.2) | 8 (8.2) | 14 (14.3) | 0.38 c |
| Cardiovascular diseases | 22 (7.5) | 7 (7.1) | 5 (5.1) | 10 (10.2) | 0.39 c |
| Chronic kidney diseases | 3 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 1.00 c |
| Asthma | 3 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 1.00 c |
| Orthopedic diseases | 1 (0.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0.37 c |
| Site of cancer, n (%) a | 0.22 c | ||||
| Esophagus | 28 (9.5) | 14 (14.3) | 7 (7.1) | 7 (7.1) | |
| Stomach | 128 (43.5) | 41 (41.8) | 48 (49.0) | 39 (39.8) | |
| Colorectal | 138 (46.9) | 43 (43.9) | 43 (43.9) | 52 (53.1) | |
| Stage, n (%) a | 0.24 c | ||||
| I | 130 (44.2) | 36 (36.7) | 49 (50.0) | 45 (45.9) | |
| II | 80 (27.2) | 31 (31.6) | 27 (27.6) | 22 (22.5) | |
| III | 84 (28.6) | 31 (31.6) | 22 (22.5) | 31 (31.6) | |
| Pathology, n (%) | 0.10 c | ||||
| Adenocarcinoma | 267 (90.8) | 84 (85.7) | 92 (93.9) | 91 (92.9) | |
| Squamous cell carcinoma | 27 (9.2) | 14 (14.3) | 6 (6.1) | 7 (7.1) | |
| Adjuvant chemotherapy, n (%) | 103 (35.0) | 43 (43.9) | 29 (29.6) | 31 (31.6) | 0.08 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, H.; Fukuzato, S.; Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Reduced Relapse-Free Survival in Colorectal Cancer Patients with Elevated Soluble CD40 Ligand Levels Improved by Vitamin D Supplementation. Nutrients 2023, 15, 4361. https://doi.org/10.3390/nu15204361
Fujimoto H, Fukuzato S, Kanno K, Akutsu T, Ohdaira H, Suzuki Y, Urashima M. Reduced Relapse-Free Survival in Colorectal Cancer Patients with Elevated Soluble CD40 Ligand Levels Improved by Vitamin D Supplementation. Nutrients. 2023; 15(20):4361. https://doi.org/10.3390/nu15204361
Chicago/Turabian StyleFujimoto, Hiroshi, Soichiro Fukuzato, Kazuki Kanno, Taisuke Akutsu, Hironori Ohdaira, Yutaka Suzuki, and Mitsuyoshi Urashima. 2023. "Reduced Relapse-Free Survival in Colorectal Cancer Patients with Elevated Soluble CD40 Ligand Levels Improved by Vitamin D Supplementation" Nutrients 15, no. 20: 4361. https://doi.org/10.3390/nu15204361
APA StyleFujimoto, H., Fukuzato, S., Kanno, K., Akutsu, T., Ohdaira, H., Suzuki, Y., & Urashima, M. (2023). Reduced Relapse-Free Survival in Colorectal Cancer Patients with Elevated Soluble CD40 Ligand Levels Improved by Vitamin D Supplementation. Nutrients, 15(20), 4361. https://doi.org/10.3390/nu15204361