Nutritional Value of Yogurt as a Protein Source: Digestibility/Absorbability and Effects on Skeletal Muscle
Abstract
:1. Introduction
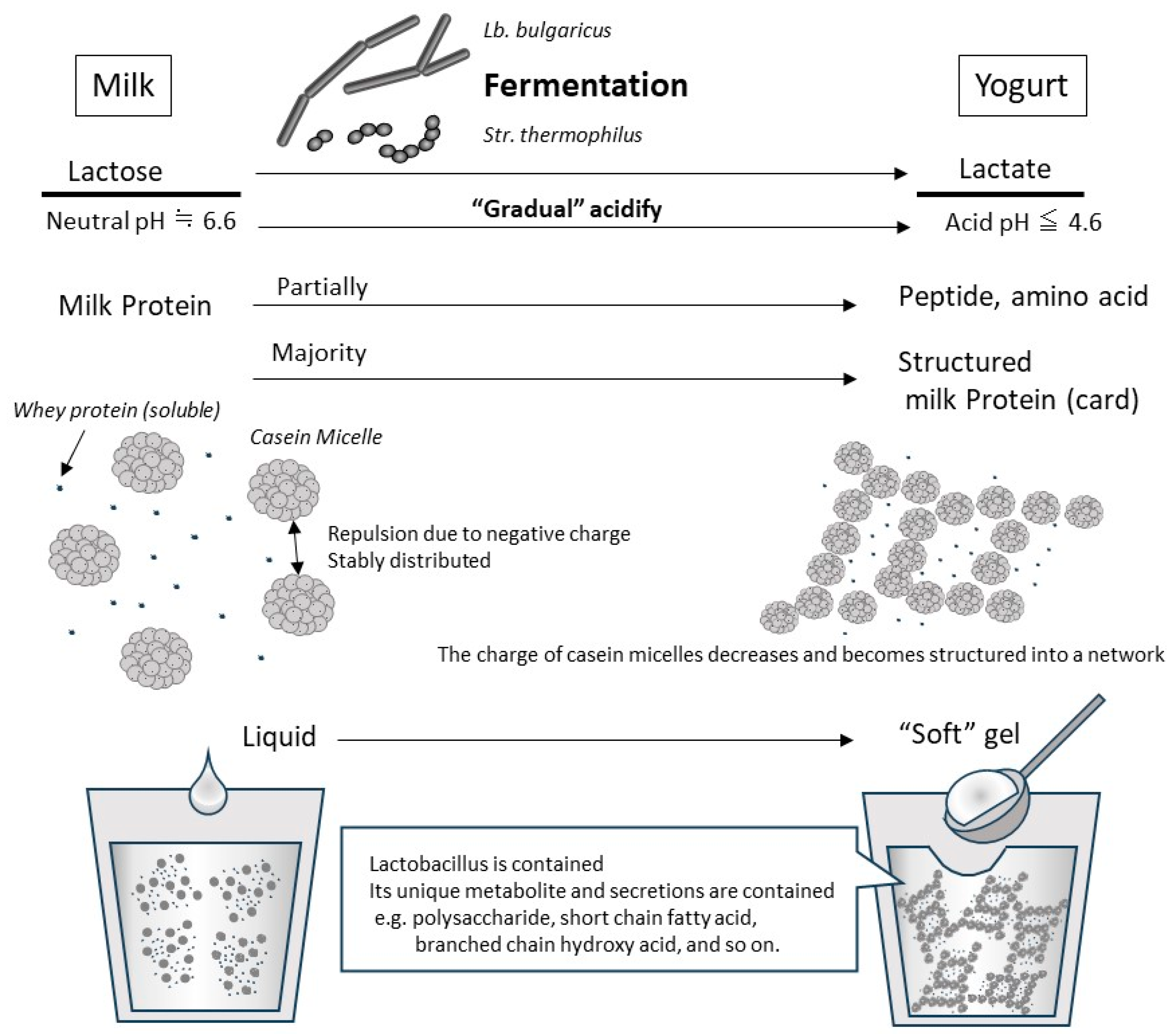
1.1. What Is Yogurt?
1.2. Benefits of Yogurt for Human Health
1.3. Physical/Biological Properties and Digestion/Absorption of Milk Protein
1.4. Milk Protein and Skeletal Muscle Protein Synthesis
2. Digestion/Absorption Properties of Protein in Yogurt
2.1. Digestion/Absorption Effectors of Yogurt Protein
2.2. Slower Rate of Digestion/Absorption of Yogurt Protein Compared with Milk Protein
2.3. Faster Rate of Digestion/Absorption of Yogurt Protein Compared with Milk Protein
3. The Benefits of Yogurt Protein Intake on Skeletal Muscle Health
3.1. Traditional Evidence
3.2. Acute Effects of Yogurt Ingestion on Skeletal Muscle
3.3. Longitudinal Effects of Yogurt Ingestion on Skeletal Muscle
4. Relationship between Milk Protein and the Biological Regulatory Functions of Yogurt Based on Lactobacillus Fermentation
4.1. Gut–Muscle Axis
4.2. Probiotic Effects
4.3. Effects of Prebiotics and Other Ingredients
5. Current Limitations and Future Prospects
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- CXS 243-2003; WHO Standard for Fermented Milks. Food and Agriculture Organization of the United Nations: Rome, Italy, 2018.
- FAO. Food and Agriculture Organization of the United Nations: Food Energy—Methods of Analysis and Conversion Factors; FAO Food and Nutrition Paper 77; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi-Oyelere, B.L.; Kruger, M.C. The Role of Milk Components, Pro-, Pre-, and Synbiotic Foods in Calcium Absorption and Bone Health Maintenance. Front. Nutr. 2020, 7, 578702. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Kaltenegger, A.; Humer, E.; Zebeli, Q. Effect of inclusion of bakery by-products in the dairy cow’s diet on milk fatty acid composition. J. Dairy Res. 2022, 89, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Sigala-Robles, R.; Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Peptides, Exopolysaccharides, and Short-Chain Fatty Acids from Fermented Milk and Perspectives on Inflammatory Bowel Diseases. Dig. Dis. Sci. 2022, 67, 4654–4665. [Google Scholar] [CrossRef] [PubMed]
- Bhavadharini, B.; Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; et al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147,812 individuals from 21 countries. BMJ Open Diabetes Res. Care 2020, 8, e000826. [Google Scholar] [CrossRef] [PubMed]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef] [PubMed]
- Kendler, S.; Thornes, F.W.; Jakobsen, A.N.; Lerfall, J. Nutritional profiling and contaminant levels of five underutilized fish species in Norway. Front. Nutr. 2023, 10, 1118094. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef]
- Amaro-Hernández, J.C.; Olivas, G.I.; Acosta-Muñiz, C.H.; Gutiérrez-Méndez, N.; Sepulveda, D.R. Structure rearrangement during rennet coagulation of milk modifies curd density. J. Dairy Sci. 2020, 103, 3088–3094. [Google Scholar] [CrossRef]
- Rasic, J.L.; Kurmann, J.A. Yoghurt. Scientific Grounds, Technology, Manufacture and Preparations; Technical Dairy Publishing House: Copenhagen, Denmark, 1978; 466p. [Google Scholar]
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef] [PubMed]
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.; Créminon, C.; Peltre, G.; Wal, J. Allergy to bovine β-lactoglobulin: Specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 1999, 29, 1055–1063. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. Formation of a structured clot during the gastric digestion of milk: Impact on the rate of protein hydrolysis. Food Hydrocoll. 2016, 52, 478–486. [Google Scholar] [CrossRef]
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins. Biol. Chem. 2021, 402, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, E.; Thomassen, G.G.M.; Renes, I.B.; Wierenga, P.A.; Hettinga, K.A. Assessment of milk protein digestion kinetics: Effects of denaturation by heat and protein type used. Food Funct. 2022, 13, 5715–5729. [Google Scholar] [CrossRef] [PubMed]
- Jochems, P.G.M.; Garssen, J.; van Keulen, A.M.; Masereeuw, R.; Jeurink, P.V. Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients 2018, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Gaudichon, C.; Roos, N.; Mahé, S.; Sick, H.; Bouley, C.; Tomé, D. Gastric emptying regulates the kinetics of nitrogen absorption from 15N-labeled milk and 15N-labeled yogurt in miniature pigs. J. Nutr. 1994, 124, 1970–1977. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Pennings, B.; Groen, B.B.L.; Wall, B.T.; Churchward-Venne, T.A.; Horstman, A.M.H.; Koopman, R.; et al. Protein Type, Protein Dose, and Age Modulate Dietary Protein Digestion and Phenylalanine Absorption Kinetics and Plasma Phenylalanine Availability in Humans. J. Nutr. 2020, 150, 2041–2050. [Google Scholar] [CrossRef]
- Kanda, A.; Nakayama, K.; Sanbongi, C.; Nagata, M.; Ikegami, S.; Itoh, H. Effects of whey, caseinate, or milk protein ingestion on muscle protein synthesis after exercise. Nutrients 2016, 8, 339. [Google Scholar] [CrossRef]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kawamoto, K.; Dankel, S.J.; Bell, Z.W.; Spitz, R.W.; Wong, V.; Loenneke, J.P. Longitudinal associations between changes in body composition and changes in sprint performance in elite female sprinters. Eur. J. Sport Sci. 2020, 20, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Travis, S.K.; Stone, M.H. Associations of Body Composition, Maximum Strength, Power Characteristics with Sprinting, Jumping, and Intermittent Endurance Performance in Male Intercollegiate Soccer Players. J. Funct. Morphol. Kinesiol. 2021, 6, 7. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Karlamangla, A.S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 2014, 127, 547–553. [Google Scholar] [CrossRef]
- Phillips, S.M. Protein requirements and supplementation in strength sports. Nutrition 2004, 20, 689–695. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk-and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef]
- Linder, M.C. Nutrition and Metabolism of Carbohydrates. In Nutritional Biochemistry and Metabolism: With Clinical Applications; Elsevier: Amsterdam, The Netherlands, 1991; Chapter 2. [Google Scholar]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef]
- McKinnell, I.W.; Rudnicki, M.A. Molecular Mechanisms of Muscle Atrophy. Cell 2004, 119, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Saris, W.H.M.; van Baak, M.A. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolysed proteins. Br. J. Nutr. 2008, 100, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Amigo-Benavent, M.; Power-Grant, O.; FitzGerald, R.J.; Jakeman, P. The insulinotropic and incretin response to feeding a milk based protein matrix in healthy young women. J. Funct. Foods 2020, 72, 104056. [Google Scholar] [CrossRef]
- Floyd, J.C.; Fajans, S.S.; Conn, J.W.; Knopf, R.F.; Rull, J. Stimulation of insulin secretion by amino acids. J. Clin. Investig. 1966, 45, 1487–1502. [Google Scholar] [CrossRef]
- Kuhara, T.; Ikeda, S.; Ohneda, A.; Sasaki, Y. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Am. J. Physiol. Endocrinol. Metab. 1991, 260, E21–E26. [Google Scholar] [CrossRef]
- Suryawan, A.; Rudar, M.; Fiorotto, M.L.; Davis, T.A. Differential regulation of mTORC1 activation by leucine and β-hydroxy-β-methylbutyrate in skeletal muscle of neonatal pigs. J. Appl. Physiol. 2020, 128, 286–295. [Google Scholar] [CrossRef]
- Anthony, J.C.; Lang, C.H.; Crozier, S.J.; Anthony, T.G.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1092–E1101. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Hall, M.N. Multiple amino acid sensing inputs to mTORC1. Cell Res. 2016, 26, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Perez-Schindler, J.; Philp, A.; Smith, K.; Atherton, P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: Impact of nutrition and exercise. Acta Physiol. 2016, 216, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Ato, S.; Fujita, S. Regulation of muscle protein metabolism by nutrition and exercise. J. Phys. Fit. Sports Med. 2017, 6, 119–124. [Google Scholar] [CrossRef]
- Joanisse, S.; McKendry, J.; Lim, C.; Nunes, E.A.; Stokes, T.; McLeod, J.C.; Phillips, S.M. Understanding the effects of nutrition and post-exercise nutrition on skeletal muscle protein turnover: Insights from stable isotope studies. Clin. Nutr. Open Sci. 2021, 36, 56–77. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient timing revisited: Is there a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 5. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Paulussen, K.J.M.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021, 8, 615849. [Google Scholar] [CrossRef]
- Phillips, S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. 2016, 13, 64. [Google Scholar] [CrossRef]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef]
- Dardevet, D.; Mosoni, L.; Savary-Auzeloux, I.; Peyron, M.A.; Polakof, S.; Rémond, D. Important determinants to take into account to optimize protein nutrition in the elderly: Solutions to a complex equation. Proc. Nutr. Soc. 2021, 80, 207–220. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Gathercole, J.L.; Day, L.; Dalziel, J.E. Differences in peptide generation following in vitro gastrointestinal digestion of yogurt and milk from cow, sheep and goat. Food Chem. 2020, 317, 126419. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Osada, T.; Yonekura, K.; Horiuchi, H. A new method for producing superior set yogurt, focusing on heat treatment and homogenization. J. Dairy Sci. 2022, 105, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, F.; Kessler, H.G. Effect of denaturation of beta-lactoglobulin on texture properties of set-style nonfat yoghurt. 2. Firmness and flow properties. Milchwiss. Milk Sci. Int. 1988, 43, 700–704. [Google Scholar]
- Breslaw, E.S.; Kleyn, D.H. In Vitro Digestibility of Protein in Yogurt at Various Stages of Processing. J. Food Sci. 1973, 38, 1016–1021. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Tzvetkova, I.; Dalgalarrondo, M.; Danova, S.; Iliev, I.; Ivanova, I.; Chobert, J.-M.; Haertlé, T. Hydrolysis of Major Dairy Proteins by Lactic Acid Bacteria from Bulgarian Yogurts. J. Food Biochem. 2007, 31, 680–702. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L. The Ratio of Casein to Whey Protein Impacts Yogurt Digestion In Vitro. Food Dig. 2012, 3, 25–35. [Google Scholar] [CrossRef]
- Dupont, D.; Mandalari, G.; Mollé, D.; Jardin, J.; Rolet-Répécaud, O.; Duboz, G.; Léonil, J.; Mills, C.E.N.; Mackie, A.R. Food processing increases casein resistance to simulated infant digestion. Mol. Nutr. Food Res. 2010, 54, 1677–1689. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT—Food Sci. Technol. 2014, 57, 99–105. [Google Scholar] [CrossRef]
- Gaudichon, C.; Mahé, S.; Roos, N.; Benamouzig, R.; Luengo, C.; Huneau, J.F.; Sick, H.; Bouley, C.; Rautureau, J.; Tome, D. Exogenous and endogenous nitrogen flow rates and level of protein hydrolysis in the human jejunum after [15N]milk and [15N]yoghurt ingestion. Br. J. Nutr. 1995, 74, 251–260. [Google Scholar] [CrossRef]
- Sanggaard, K.M.; Holst, J.J.; Rehfeld, J.F.; Sandström, B.; Raben, A.; Tholstrup, T. Different effects of whole milk and a fermented milk with the same fat and lactose content on gastric emptying and postprandial lipaemia, but not on glycaemic response and appetite. Br. J. Nutr. 2004, 92, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.H.; Ganzevles, R.A.; Kudla, U.; Kardinaal, A.F.M.; van den Borne, J.J.G.C.; Huppertz, T. Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix. Int. Dairy J. 2021, 113, 104890. [Google Scholar] [CrossRef]
- Sumi, K.; Osada, K.; Ashida, K.; Nakazato, K. Lactobacillus-fermented milk enhances postprandial muscle protein synthesis in Sprague-Dawley rats. J. Funct. Foods 2020, 66, 103789. [Google Scholar] [CrossRef]
- Sumi, K.; Osada, K.; Sakuda, M.; Ashida, K.; Nakazato, K. Fermented milk retains beneficial effects on skeletal muscle protein anabolism after processing by centrifugation and supernatant removal. J. Dairy Sci. 2021, 104, 1336–1350. [Google Scholar] [CrossRef]
- Nakayama, K.; Kanda, A.; Tagawa, R.; Sanbongi, C.; Ikegami, S.; Itoh, H. Post-exercise muscle protein synthesis in rats after ingestion of acidified bovine milk compared with skim milk. Nutrients 2017, 9, 1071. [Google Scholar] [CrossRef]
- Morell, P.; Fiszman, S.; Llorca, E.; Hernando, I. Designing added-protein yogurts: Relationship between in vitro digestion behavior and structure. Food Hydrocoll. 2017, 72, 27–34. [Google Scholar] [CrossRef]
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Nyakayiru, J.; van Lieshout, G.A.A.; Trommelen, J.; van Kranenburg, J.; Verdijk, L.B.; Bragt, M.C.E.; van Loon, L.J.C. The glycation level of milk protein strongly modulates post-prandial lysine availability in humans. Br. J. Nutr. 2020, 123, 545–552. [Google Scholar] [CrossRef]
- Van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Weijzen, M.E.G.; van Kranenburg, J.; Ganzevles, R.A.; Beelen, M.; Verdijk, L.B.; van Loon, L.J.C. Casein Protein Processing Strongly Modulates Post-Prandial Plasma Amino Acid Responses In Vivo in Humans. Nutrients 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Simhaee, E.; Keshavarz, K. Comparison of Gross Protein Value and Metabolizable Energy of Dried Skim Milk and Dried Yoghurt. Poult. Sci. 1974, 53, 184–191. [Google Scholar] [CrossRef]
- Hargrove, R.E.; Alford, J.A. Growth rate and feed efficiency of rats fed yogurt and other fermented milks. J. Dairy Sci. 1978, 61, 11–19. [Google Scholar] [CrossRef]
- Hermans, W.J.H.; Fuchs, C.J.; Nyakayiru, J.; Hendriks, F.K.; Houben, L.H.P.; Senden, J.M.; van Loon, L.J.C.; Verdijk, L.B. Acute Quark Ingestion Increases Muscle Protein Synthesis Rates at Rest with a Further Increase after Exercise in Young and Older Adult Males in a Parallel-Group Intervention Trial. J. Nutr. 2023, 153, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; Van Loon, L.J.C. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef]
- Zemel, M.B.; Richards, J.; Mathis, S.; Milstead, A.; Gebhardt, L.; Silva, E. Dairy augmentation of total and central fat loss in obese subjects. Int. J. Obes. 2005, 29, 391–397. [Google Scholar] [CrossRef]
- Thomas, D.T.; Wideman, L.; Lovelady, C.A. Effects of a dairy supplement and resistance training on lean mass and insulin-like growth factor in women. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 181–188. [Google Scholar] [CrossRef]
- Bridge, A.; Brown, J.; Snider, H.; Nasato, M.; Ward, W.E.; Roy, B.D.; Josse, A.R. Greek Yogurt and 12 Weeks of Exercise Training on Strength, Muscle Thickness and Body Composition in Lean, Untrained, University-Aged Males. Front. Nutr. 2019, 6, 55. [Google Scholar] [CrossRef]
- Bagheri, R.; Hooshmand Moghadam, B.; Candow, D.G.; Elliott, B.T.; Wong, A.; Ashtary-Larky, D.; Forbes, S.C.; Rashidlamir, A. Effects of Icelandic yogurt consumption and resistance training in healthy untrained older males. Br. J. Nutr. 2021, 127, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, J. Commentary: Greek Yogurt and 12 Weeks of Exercise Training on Strength, Muscle Thickness and Body Composition in Lean, Untrained, University-Aged Males. Front. Nutr. 2019, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the gut microbiota and sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Murphy, C.H.; Roche, H.M. Targeting the Gut Microbiota to Improve Dietary Protein Efficacy to Mitigate Sarcopenia. Front. Nutr. 2021, 8, 656730. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Phillips, S.M. Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. Biofactors 2014, 40, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Chang, S.S.; Chang, H.Y.; Wu, C.H.; Pan, C.H.; Chang, C.C.; Chan, C.H.; Huang, H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 2022, 13, 515–531. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Zhang, Y.; Wang, S.; Li, F. Effect of Lactobacillus plantarum KSFY01 on the exercise capacity of D-galactose-induced oxidative stress-aged mice. Front. Microbiol. 2022, 13, 1030833. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Park, S.D.; Shim, J.J.; Lee, J.L. Lactobacillus plantarum HY7715 Ameliorates Sarcopenia by Improving Skeletal Muscle Mass and Function in Aged Balb/c Mice. Int. J. Mol. Sci. 2021, 22, 10023. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, Z.Y.; Li, Y.H.; Yang, L.Y.; Yin, J.; He, J.H.; Hou, D.X.; Liu, Y.L.; Huang, X.G. Effects of Dietary Supplementation of Lactobacillus delbrueckii on Gut Microbiome and Intestinal Morphology in Weaned Piglets. Front. Vet. Sci. 2021, 8, 692389. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; de Goede, J. Dairy Consumption and Cardiometabolic Diseases: Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Curr. Nutr. Rep. 2018, 7, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Nachbar, R.T.; Tran, T.T.T.; Ouellette, A.; Varin, T.V.; Cotillard, A.; Quinquis, L.; Gagné, A.; St-Pierre, P.; Trottier, J.; et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat. Commun. 2022, 13, 1343. [Google Scholar] [CrossRef] [PubMed]
- Sumi, K.; Sakuda, M.; Munakata, K.; Nakamura, K.; Ashida, K. α-Hydroxyisocaproic Acid Decreases Protein Synthesis but Attenuates TNFα/IFNγ Co-Exposure-Induced Protein Degradation and Myotube Atrophy via Suppression of iNOS and IL-6 in Murine C2C12 Myotube. Nutrients 2021, 13, 2391. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Aiso, C.; Tabata, Y.; Gotoh, Y.; Kosaka, H.; Suzuki, T. Lactococcus cremoris subsp. cremoris FC-fermented milk activates protein synthesis and increases skeletal muscle mass in middle-aged mice. Biochem. Biophys. Res. Commun. 2022, 612, 176–180. [Google Scholar] [CrossRef]
- Horstman, A.M.H.; Huppertz, T. Milk proteins: Processing, gastric coagulation, amino acid availability and muscle protein synthesis. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Loveday, S.M. Protein digestion and absorption: The influence of food processing. Nutr. Res. Rev. 2022, 1–50. [Google Scholar] [CrossRef]
- Lim, M.T.; Pan, B.J.; Toh, D.W.; Sutanto, C.N.; Kim, J.E. Animal Protein versus Plant Protein in Supporting Lean Mass and Muscle Strength: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 661. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumi, K.; Tagawa, R.; Yamazaki, K.; Nakayama, K.; Ichimura, T.; Sanbongi, C.; Nakazato, K. Nutritional Value of Yogurt as a Protein Source: Digestibility/Absorbability and Effects on Skeletal Muscle. Nutrients 2023, 15, 4366. https://doi.org/10.3390/nu15204366
Sumi K, Tagawa R, Yamazaki K, Nakayama K, Ichimura T, Sanbongi C, Nakazato K. Nutritional Value of Yogurt as a Protein Source: Digestibility/Absorbability and Effects on Skeletal Muscle. Nutrients. 2023; 15(20):4366. https://doi.org/10.3390/nu15204366
Chicago/Turabian StyleSumi, Koichiro, Ryoichi Tagawa, Kae Yamazaki, Kyosuke Nakayama, Takefumi Ichimura, Chiaki Sanbongi, and Koichi Nakazato. 2023. "Nutritional Value of Yogurt as a Protein Source: Digestibility/Absorbability and Effects on Skeletal Muscle" Nutrients 15, no. 20: 4366. https://doi.org/10.3390/nu15204366
APA StyleSumi, K., Tagawa, R., Yamazaki, K., Nakayama, K., Ichimura, T., Sanbongi, C., & Nakazato, K. (2023). Nutritional Value of Yogurt as a Protein Source: Digestibility/Absorbability and Effects on Skeletal Muscle. Nutrients, 15(20), 4366. https://doi.org/10.3390/nu15204366




