Longitudinal Changes in Physical Function and Their Impact on Health Outcomes in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics upon Admission and Disease Severity
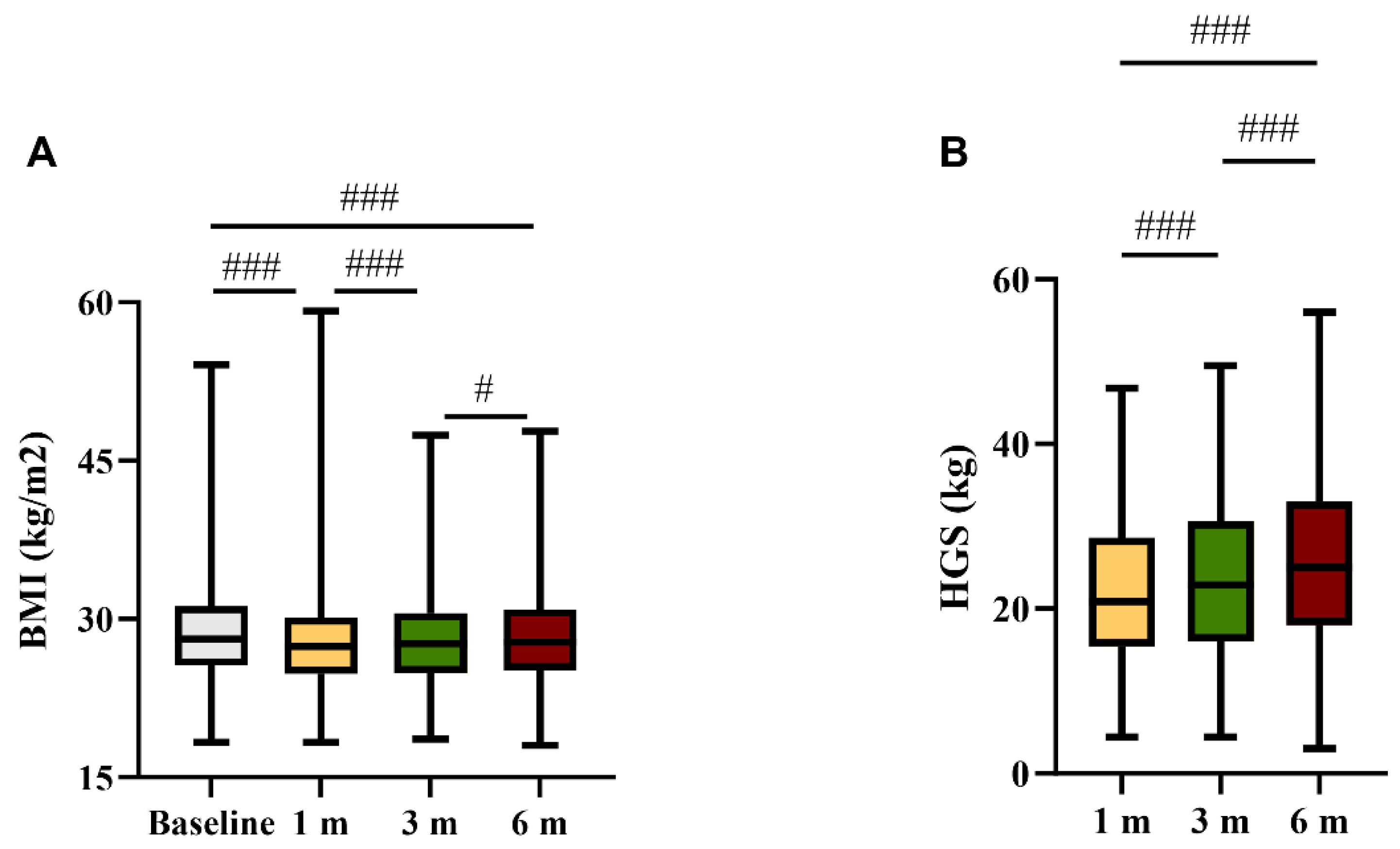
3.2. Anthropometrics and Physical Evaluations during Follow-Up
3.3. Comparison between Participants with Normal and Low HGS
3.4. Comparison between Participants with and without HGS Improvement
3.5. Predictors of HGS Improvement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Zhao, Y.-M.; Yan, W.; Li, C.; Lu, Q.-D.; Liu, L.; Ni, S.-Y.; Mei, H.; Yuan, K.; Shi, L.; et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action. Mol. Psychiatry 2023, 28, 423–433. [Google Scholar] [CrossRef]
- Kelly, J.D.; Curteis, T.; Rawal, A.; Murton, M.; Clark, L.J.; Jafry, Z.; Shah-Gupta, R.; Berry, M.; Espinueva, A.; Chen, L.; et al. SARS-CoV-2 post-acute sequelae in previously hospitalised patients: Systematic literature review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220254. [Google Scholar] [CrossRef]
- Yuan, N.; Lv, Z.-H.; Sun, C.-R.; Wen, Y.-Y.; Tao, T.-Y.; Qian, D.; Tao, F.-P.; Yu, J.-H. Post-acute COVID-19 symptom risk in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1112383. [Google Scholar] [CrossRef] [PubMed]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O.; et al. Long COVID: Mechanisms, risk factors and recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef] [PubMed]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2021, 40, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S.; Ravaioli, F.; Baracco, B.; Battaiola, C.; Bocedi, G.; Brodosi, L.; Leoni, L.; Mari, G.A.; Musio, A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin. Nutr. 2021, 40, 1330–1337. [Google Scholar] [CrossRef]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.S.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Cinel, E.; Falbo, E.; Ferrante, M.; Cilla, M.; Martinenghi, S.; Vitali, G.; Bosi, E.; Giustina, A.; et al. Weight trajectories and abdominal adiposity in COVID-19 survivors with overweight/obesity. Int. J. Obes. 2021, 45, 1986–1994. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef]
- van Gassel, R.J.J.; Bels, J.; Remij, L.M.; van Bussel, B.C.T.; Posthuma, R.; Gietema, H.A.; Verbunt, J.; van der Horst, I.C.C.; Damink, S.W.M.O.; van Santen, S.; et al. Functional Outcomes and Their Association with Physical Performance in Mechanically Ventilated Coronavirus Disease 2019 Survivors at 3 Months Following Hospital Discharge: A Cohort Study. Crit. Care Med. 2021, 49, 1726–1738. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’brien, K.; Sheill, G.; Dyer, A.H.; O’kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- Baricich, A.; Borg, M.B.; Cuneo, D.; Cadario, E.; Azzolina, D.; Balbo, P.E.; Bellan, M.; Zeppegno, P.; Pirisi, M.; Cisari, C.; et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2021, 57, 199–207. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical Sequelae among Patients with COVID-19 Four Months after Hospital Discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Gong, H.S. Measurement and Interpretation of Handgrip Strength for Research on Sarcopenia and Osteoporosis. J. Bone Metab. 2020, 27, 85–96. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef]
- Soysal, P.; Hurst, C.; Demurtas, J.; Firth, J.; Howden, R.; Yang, L.; Tully, M.A.; Koyanagi, A.; Ilie, P.C.; López-Sánchez, G.F.; et al. Handgrip strength and health outcomes: Umbrella review of systematic reviews with meta-analyses of observational studies. J. Sport Health Sci. 2021, 10, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Pérez, P.; Recalde, B.Y.; Costa, A.F.; Sedler, M.J. Hand grip strength before and after SARS-CoV-2 infection in community-dwelling older adults. J. Am. Geriatr. Soc. 2021, 69, 2722–2731. [Google Scholar] [CrossRef]
- de Blasio, F.; Scalfi, L.; Castellucci, B.; Sacco, A.M.; Berlingieri, G.M.; Capitelli, L.; Alicante, P.; Sanduzzi, A.; Bocchino, M. Poor Nutritional Status and Dynapenia Are Highly Prevalent in Post-Acute COVID-19. Front. Nutr. 2022, 9, 888485. [Google Scholar] [CrossRef]
- Battistella, L.R.; Imamura, M.; De Pretto, L.R.; A A Van Cauwenbergh, S.K.H.; Ramos, V.D.; Uchiyama, S.S.T.; Matheus, D.; Kuhn, F.; de Oliveira, A.A.A.; Naves, G.S.; et al. Long-term functioning status of COVID-19 survivors: A prospective observational evaluation of a cohort of patients surviving hospitalisation. BMJ Open 2022, 12, e057246. [Google Scholar] [CrossRef] [PubMed]
- Querini, P.R.; De Lorenzo, R.; Conte, C.; Brioni, E.; Lanzani, C.; Yacoub, M.R.; Chionna, R.; Martinenghi, S.; Vitali, G.; Tresoldi, M.; et al. Post-COVID-19 follow-up clinic: Depicting chronicity of a new disease. Acta Biomed. 2020, 91, 22–28. [Google Scholar] [CrossRef]
- Rovere-Querini, P.; Tresoldi, C.; Conte, C.; Ruggeri, A.; Ghezzi, S.; DE Lorenzo, R.; DI Filippo, L.; Farina, N.; Ramirez, G.A.; Ripa, M.; et al. Biobanking for COVID-19 research. Panminerva Med. 2022, 64, 244–252. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Martone, A.M.; Salini, S.; Zazzara, M.B.; Candeloro, M.; Coelho-Junior, H.J.; Tosato, M.; Picca, A.; Marzetti, E. Normative values of muscle strength across ages in a ‘real world’ population: Results from the longevity check-up 7+ project. J. Cachexia Sarcopenia Muscle 2020, 11, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories; American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Casanova, C.; Celli, B.R.; Barria, P.; Casas, A.; Cote, C.; de Torres, J.P.; Jardim, J.; Lopez, M.V.; Marin, J.M.; de Oca, M.M.; et al. The 6-min walk distance in healthy subjects: Reference standards from seven countries. Eur. Respir. J. 2011, 37, 150–156. [Google Scholar] [CrossRef]
- Reis, D.O.; Oliveira, P.; Gomes, J.; Lima, R.; Guimarães, M.; Ladeira, I. Applying reference equations for 6-minute walking test in COPD and ILD patients. Eur. Respir. J. 2020, 56 (Suppl. 64), 540. [Google Scholar] [CrossRef]
- EuroQol Research Foundation. EQ-5D. Available online: https://euroqol.org/ (accessed on 26 December 2021).
- De Lorenzo, R.; Magnaghi, C.; Cinel, E.; Vitali, G.; Martinenghi, S.; Mazza, M.G.; Nocera, L.; Cilla, M.; Damanti, S.; Compagnone, N.; et al. A Nomogram-Based Model to Predict Respiratory Dysfunction at 6 Months in Non-Critical COVID-19 Survivors. Front. Med. 2022, 9, 781410. [Google Scholar] [CrossRef]
- Tanguay, P.; Décary, S.; Lemaire-Paquette, S.; Léonard, G.; Piché, A.; Dubois, M.-F.; Kairy, D.; Bravo, G.; Corriveau, H.; Marquis, N.; et al. Trajectories of health-related quality of life and their predictors in adult COVID-19 survivors: A longitudinal analysis of the Biobanque Québécoise de la COVID-19 (BQC-19). Qual. Life Res. 2023, 32, 2707–2717. [Google Scholar] [CrossRef]
- Steinmetz, A.; Gross, S.; Lehnert, K.; Lücker, P.; Friedrich, N.; Nauck, M.; Bahlmann, S.; Fielitz, J.; Dörr, M. Longitudinal Clinical Features of Post-COVID-19 Patients—Symptoms, Fatigue and Physical Function at 3- and 6-Month Follow-Up. J. Clin. Med. 2023, 12, 3966. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef] [PubMed]
- A Karvonen-Gutierrez, C.; Peng, Q.; Peterson, M.; Duchowny, K.; Nan, B.; Harlow, S. Low grip strength predicts incident diabetes among mid-life women: The Michigan Study of Women’s Health Across the Nation. Age Ageing 2018, 47, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Sun, J.; Han, M.; Park, S.; Nam, C.M. Impact of handgrip strength on cardiovascular, cancer and all-cause mortality in the Korean longitudinal study of ageing. BMJ Open 2019, 9, e027019. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Tan, K.C.B.; Bow, C.H.; Soong, C.S.S.; Loong, C.H.N.; Kung, A.W.-C. Low handgrip strength is a predictor of osteoporotic fractures: Cross-sectional and prospective evidence from the Hong Kong Osteoporosis Study. AGE 2012, 34, 1239–1248. [Google Scholar] [CrossRef]
- Yang, L.; Koyanagi, A.; Smith, L.; Hu, L.; Colditz, G.A.; Toriola, A.T.; Sánchez, G.F.L.; Vancampfort, D.; Hamer, M.; Stubbs, B.; et al. Hand grip strength and cognitive function among elderly cancer survivors. PLoS ONE 2018, 13, e0197909. [Google Scholar] [CrossRef]
- Smith, L.; White, S.; Stubbs, B.; Hu, L.; Veronese, N.; Vancampfort, D.; Hamer, M.; Gardner, B.; Yang, L. Depressive symptoms, handgrip strength, and weight status in US older adults. J. Affect. Disord. 2018, 238, 305–310. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Z.; He, X.; Wang, X.; Yuan, C.; Huang, L.; Song, R.; Wu, Y. Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5267. [Google Scholar] [CrossRef]
- Bradbury, J.; Wilkinson, S.; Schloss, J. Nutritional Support During Long COVID: A Systematic Scoping Review. J. Integr. Complement. Med. 2023. [Google Scholar] [CrossRef]
- Detopoulou, P.; Tsouma, C.M.; Papamikos, V.M. COVID-19 and Nutrition: Summary of Official Recommendations. Top. Clin. Nutr. 2022, 37, 187–202. [Google Scholar] [CrossRef]
- van Zwienen-Pot, J.I.; Reinders, I.; de Groot, L.C.P.G.M.; Beck, A.M.; Feldblum, I.; Jobse, I.; Neelemaat, F.; de van der Schueren, M.A.E.; Shahar, D.R.; Smeets, E.T.H.C.; et al. Effects of Nutritional Interventions in Older Adults with Malnutrition or at Risk of Malnutrition on Muscle Strength and Mortality: Results of Pooled Analyses of Individual Participant Data from Nine RCTs. Nutrients 2023, 15, 2025. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Santamaría, G.; Sánchez-Serrano, N.; Caeiro, E.L.; Seco-Calvo, J. Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome. Viruses 2022, 14, 2797. [Google Scholar] [CrossRef] [PubMed]
- Tamburlani, M.; Cuscito, R.; Servadio, A.; Galeoto, G. Effectiveness of Respiratory Rehabilitation in COVID-19′s Post-Acute Phase: A Systematic Review. Healthcare 2023, 11, 1071. [Google Scholar] [CrossRef]
- Pouliopoulou, D.V.; Macdermid, J.C.; Saunders, E.; Peters, S.; Brunton, L.; Miller, E.; Quinn, K.L.; Pereira, T.V.; Bobos, P. Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2333838. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chen, Y.-C.; Wang, C.-H. Early Rehabilitation in Acute Care Inpatient Wards May Be Crucial to Functional Recovery 3 Months After Ischemic Stroke. Phys. Ther. 2021, 101, pzaa197. [Google Scholar] [CrossRef]
- Ward, A.; Gutenbrunner, C.; Damjan, H.; Giustini, A.; Delarque, A. European union of medical specialists (UEMS) section of Physical & Rehabilitation Medicine: A Position Paper on Physical and Rehabilitation Medicine in Acute Settings. J. Rehabil. Med. 2010, 42, 417–424. [Google Scholar] [CrossRef]



| Variables | |
|---|---|
| Age, median (IQR) years | 67 (56–74.3) |
| Males, n (%) | 144 (61.5%) |
Race/Ethnicity, n (%)
| 218 (93.2) 10 (4.2) 3 (1.3) 3 (1.3) |
| Active smokers, n (%) | 53 (22.6%) |
| Arterial hypertension, n (%) | 136 (58.1%) |
| Diabetes mellitus, n (%) | 50 (21.4%) |
| Ischemic heart disease, n (%) | 39 (16.7%) |
| COPD/asthma, n (%) | 23 (9.8%) |
| Chronic kidney disease, n (%) | 18 (7.7%) |
| Active malignancy, n (%) | 7 (3%) |
| BMI, median (IQR) kg/m2 | 28.2 (25.6–31.2) |
| Length of stay, median (IQR) days | 14 (9–24) |
| Low-flow oxygen, n (%) | 138 (59%) |
| NIV, n (%) | 62 (26.5%) |
| ICU, n (%) | 34 (14.5%) |
| Variable | Low HGS (n = 82) | Normal HGS (n = 152) | p Value |
|---|---|---|---|
| Age, years | 60.0 (54.0; 69.0) | 70.0 (60.0; 77.0) | <0.001 |
| Female sex, n (%) | 24 (29.3) | 66 (43.4) | 0.034 |
Ethnicity, n (%)
| 72 (87.8) 5 (6.1) 2 (2.4) 3 (3.7) | 146 (96.1) * 5 (3.3) 1 (0.7) 0 (0) | 0.031 |
| Smoke, n (%) | 20 (24.2) | 33 (21.9) | 0.659 |
| BMI (baseline), kg/m2 | 28.5 (25.8; 31.4) | 28.0 (25.3; 31.2) | 0.395 |
BMI category †, n (%)
| 0 (0) 14 (17.3) 37 (45.7) 30 (37.0) | 1 (0.6) 33 (22.0) 64 (42.7) 52 (34.7) | 0.800 |
| Arterial hypertension, n (%) | 46 (56.1) | 90 (59.2) | 0.645 |
| Diabetes mellitus, n (%) | 22 (26.8) | 28 (18.4) | 0.134 |
| Coronary artery disease, n (%) | 17 (20.7) | 22 (14.5) | 0.220 |
| Chronic kidney disease, n (%) | 7 (8.5) | 11 (7.2) | 0.722 |
| COPD/asthma, n (%) | 8 (9.8) | 15 (9.9) | 0.978 |
| Malignancy, n (%) | 1 (1.2) | 6 (3.9) | 0.426 |
Treatment modality, n (%)
| 30 (36.6) 27 (32.9) 25 (30.5) | 108 (71.1) ** 35 (23.0) 9 (5.9) ** | <0.001 |
| Length of stay, days | 21.0 (10.0; 40.5) | 12.0 (8.0; 20.0) | <0.001 |
| Follow-up: 1 month | |||
| Time from discharge, days | 34.5 (29.0; 39.8) | 34.0 (29.0; 39.0) | 0.970 |
| SBP, mmHg | 130.0 (120.0; 140.0) | 130.0 (120.0; 140.0) | 0.163 |
| DBP, mmHg | 80.0 (70.0; 80.0) | 80.0 (70.0; 80.0) | 0.555 |
| SatO2, % | 98.0 (97.0; 99.0) | 98.0 (97.0; 99.0) | 0.624 |
| Dyspnoea ‡, n (%) | 20 (27.4) | 28 (19.9) | 0.286 |
| Weight change (0–1 month) | −5.7 (−9.1; −0.6) | −3.2(−5.7; 0.0) | 0.004 |
| BMI, kg/m2 | 27.2 (25.2; 29.6) | 27.6 (24.6; 30.7) | 0.574 |
| Abdominal obesity, n (%) | 38 (46.3) | 96 (63.2) | 0.013 |
| Handgrip strength, kg | 18.6 (13.8; 25.4) | 23.4 (16.6; 31.5) | <0.001 |
| 6-MWT, m | 460.0 (400.0; 500.0) | 460.0 (440.0; 500.0) | 0.486 |
| 6-MWT, % predicted | 91.0 (81.0; 96.0) | 93.0 (86.6; 101.0) | 0.012 |
| Follow-up: 3 months | |||
| Time from discharge, days | 90.0 (90.0; 96.0) | 90.0 (90.0; 93.0) | 0.978 |
| SBP, mmHg | 130.0 (120.0; 135.0) | 130.0 (120.0; 140.0) | 0.036 |
| DBP, mmHg | 80.0 (73.8; 80.0) | 80.0 (70.0; 80.0) | 0.600 |
| SatO2, % | 98.0 (97.0; 98.0) | 98.0 (98.0; 98.0) | 0.360 |
| Dyspnoea ‡, n (%) | 13 (15.9) | 16 (10.5) | 0.053 |
| BMI, kg/m2 | 27.5 (25.4; 29.9) | 27.7 (24.6; 30.8) | 0.923 |
| Abdominal obesity, n (%) | 45 (54.9) | 98 (64.5) | 0.151 |
| Handgrip strength, kg | 21.5 (14.7; 27.6) | 24.2 (16.6; 33.1) | 0.013 |
| 6-MWT, m | 480.0 (410.0; 500.0) | 480.0 (460.0; 500.0) | 0.979 |
| 6-MWT, % predicted | 95.7 (84.0; 102.0) | 100.0 (92.9; 105.0) | 0.007 |
| Follow-up: 6 months | |||
| Time from discharge, days | 180.0 (179.0; 181.3) | 180.0 (180.0; 188.0) | 0.136 |
| SBP, mmHg | 125.0 (120.0; 135.0) | 130.0 (120.0; 140.0) | 0.041 |
| DBP, mmHg | 80.0 (75.0; 80.0) | 80.0 (80.0; 80.0) | 0.729 |
| SatO2, % | 98.0 (97.0; 98.0) | 98.0 (98.0; 98.0) | 0.189 |
| Dyspnoea ‡, n (%) | 10 (12.2) | 14 (9.2) | 0.449 |
| BMI (1 month), kg/m2 | 27.9 (25.5; 30.8) | 27.8 (24.8; 31.2) | 0.824 |
| Abdominal obesity, n (%) | 50 (61.0) | 103 (67.8) | 0.298 |
| Handgrip strength, kg | 24.5 (15.8; 33.3) | 25.0 (18.4; 32.9) | 0.433 |
| 6-MWT, m | 500.0 (460.0; 520.0) | 480.0 (460.0; 500.0) | 0.881 |
| 6-MWT, % predicted | 100.0 (89.2; 105.0) | 102 (96.3; 109.0) | 0.003 |
| Variable | Stable/Worse HGS (n = 31) | Improved HGS (n = 51) | p Value |
|---|---|---|---|
| Age, years | 56.0 (51.0; 67.0) | 62.0 (56.0; 72.0) | 0.094 |
| Female sex, n (%) | 11 (35.5) | 13 (25.5) | 0.335 |
| Smoke, n (%) | 8 (25.8) | 12 (23.5) | 0.816 |
| BMI (baseline), kg/m2 | 28.3 (25.6 (31.9) | 28.5 (25.8; 31.3) | 0.977 |
BMI category †, n (%)
| - 5 (16.1) 13 (41.9) 12 (38.7) | - 9 (17.6) 24 (47.1) 18 (35.3) | 0.543 |
| Arterial hypertension, n (%) | 19 (61.3) | 27 (52.9) | 0.460 |
| Diabetes mellitus, n (%) | 9 (29.0) | 13 (25.5) | 0.726 |
| Coronary artery disease, n (%) | 9 (29.0) | 8 (15.7) | 0.148 |
| Chronic kidney disease, n (%) | 3 (9.7) | 4 (7.8) | 1.000 |
| COPD/asthma, n (%) | 5 (16.1) | 3 (5.9) | 0.129 |
| Malignancy, n (%) | 0 (0) | 1 (2.0) | 1.000 |
Treatment modality, n (%)
| 11 (35.5) 9 (29.0) 11 (35.5) | 19 (37.3) 18 (35.3) 14 (27.5) | 0.721 |
| Length of stay, days | 19.0 (9.0; 43.0) | 21.0 (10.8; 39.8) | 0.596 |
| Follow-up: 1 month | |||
| Time from discharge, days | 35.0 (29.0; 38.3) | 33.5 (29.0; 40.0) | 0.964 |
| SBP, mmHg | 130.0 (110.0; 140.0) | 130.0 (120.0; 135.0) | 0.399 |
| DBP, mmHg | 80.0 (70.0; 85.0) | 80.0 (70.0, 80.0) | 0.941 |
| SatO2, % | 98.0 (97.0; 99.0) | 98.0 (97.0; 98.0) | 0.477 |
| Dyspnoea ‡, n (%) | 11 (35.5) | 9 (17.6) | 0.140 |
| Weight change 0–1 months, % | −4.7 (−9.8; 0.0) | −5.9 (−8.7; −1.6) | 0.784 |
| MNA-SF | 0.362 | ||
| No malnutrition | 3 (10.0) | 4 (7.8) | |
| Risk of malnutrition | 16 (53.3) | 20 (39.2) | |
| Malnutrition | 11 (36.7) | 27 (52.9) | |
| BMI (1 month), kg/m2 | 27.3 (25.2; 29.0) | 27.1 (25.0; 30.5) | 0.800 |
| Waist circumference (cm) | 95.0 (88.0; 103.0) | 97.0 (90.0; 108.0) | 0.464 |
| Abdominal obesity, n (%) | 17 (54.8) | 21 (41.2) | 0.229 |
| Capillary blood glucose, mg/dL | 108.0 (100.0; 120.0) | 114.0 (101.0; 149.0) | 0.176 |
| Handgrip strength, kg | 15.8 (12.6; 19.6) | 22.2 (13.9; 26.0) | 0.006 |
| 6-MWT, m | 460.0 (320.0; 500.0) | 460.0 (420.0; 500.0) | 0.265 |
| 6-MWT, % predicted | 87.0 (73.0; 93.3) | 91.5 (84.3; 97.8) | 0.006 |
| Follow-up: 3 months | |||
| Time from discharge, days | 90.0 (90.0; 96.0) | 90.0 (90.0; 97.0) | 0.900 |
| SBP, mmHg | 130.0 (120.0; 135.0) | 130.0 (115.0; 130.0) | 0.506 |
| DBP, mmHg | 80.0 (70.0; 80.0) | 80.0 (75.0; 80.0) | 0.649 |
| SatO2, % | 98.0 (97.0; 98.0) | 98.0 (97.0; 98.0) | 0.882 |
| Dyspnoea ‡, n (%) | 9 (29.0) | 4 (7.8) | 0.022 |
| BMI (1 month), kg/m2 | 27.5 (25.0; 29.6) | 27.4 (25.5; 30.2) | 0.992 |
| Waist circumference (cm) | 95.0 (90.0; 110.0) | 97.0 (90.0; 108.0) | 0.989 |
| Abdominal obesity, n (%) | 21 (67.7) | 24 (47.1) | 0.068 |
| Capillary blood glucose, mg/dL | 122.0 (106.0; 145.0) | 116.0 (103.0; 147.0) | 0.681 |
| HGS, kg | 17.0 (12.4; 22.7) | 25.0 (17.3; 29.1) | 0.001 |
| 6-MWT, m | 470.0 (355.0; 505.0) | 500.0 (440.0; 500.0) | 0.205 |
| 6-MWT, % predicted | 87.0 (73.8; 95.7) | 98.8 (92.0; 103.0) | <0.001 |
| Follow-up: 6 months | |||
| Time from discharge, days | 180.0 (179.0; 181.0) | 180.0 (179.0; 188.0) | 0.193 |
| SBP, mmHg | 125.0 (120.0; 130.0) | 125.0 (120.0; 135.0) | 0.301 |
| DBP, mmHg | 80.0 (70.0; 80.0) | 80.0 (80.0; 80.0) | 0.146 |
| SatO2, % | 98.0 (97.0; 98.0) | 98.0 (98.0; 98.0) | 0.408 |
| Dyspnoea ‡, n (%) | 9 (29.0) | 1 (2.0) | <0.001 |
| BMI (1 month), kg/m2 | 27.7 (25.5; 30.8) | 28.1 (25.6; 30.7) | 0.916 |
| Waist circumference (cm) | 100.0 (92.0; 110) | 101.0 (92.0; 108.0) | 0.912 |
| Abdominal obesity, n (%) | 21 (67.7) | 29 (56.9) | 0.327 |
| Capillary blood glucose, mg/dL | 123.0 (109.0; 163.0) | 116.0 (101.0; 150.0) | 0.341 |
| Handgrip strength, kg | 19.4 (14.4; 24.3) | 31.2 (21.3; 35.9) | <0.001 |
| 6-MWT, m | 480.0 (430.0; 505.0) | 500.0 (460.0; 520.0) | 0.355 |
| 6-MWT, % predicted | 93.3 (78.3; 101.0) | 101.0 (95.0; 107.0) | <0.001 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Odds Ratio (95% C.I.) | p Value | Odds Ratio (95% C.I.) | p Value | |
| Age | 1.03 (0.99; 1.07) | 0.145 | ||
| Sex (female) | 1.61 (0.61; 4.23) | 0.337 | ||
| Race (white) | 4.67 (1.01; 19.67) | 0.036 | 4.37 (0.97; 19.70) | 0.055 |
| BMI (1 M) | 0.97 (0.89; 1.06) | 0.476 | ||
| Abdominal obesity (1 M) | 1.74 (0.71; 4.27) | 0.231 | ||
| Arterial hypertension | 0.711 (0.287; 1.76) | 0.461 | ||
| Diabetes mellitus | 0.84 (0.31; 2.27) | 0.726 | ||
| Coronary artery disease | 0.46 (0.15; 1.34) | 0.154 | ||
| Chronic kidney disease | 0.79 (0.67; 3.81) | 0.774 | ||
| COPD/asthma | 0.33 (0.07; 1.47) | 0.144 | ||
| ICU | 0.69 (0.26; 1.80) | 0.445 | ||
| LoS | 1.00 (0.98; 1.02) | 0.930 | ||
| Handgrip strength 1 M | 1.12 (1.03; 1.21) | 0.006 | 1.11 (1.03; 1.20) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lorenzo, R.; Di Filippo, L.; Scelfo, S.; Merolla, A.; Giustina, A.; Conte, C.; Rovere-Querini, P. Longitudinal Changes in Physical Function and Their Impact on Health Outcomes in COVID-19 Patients. Nutrients 2023, 15, 4474. https://doi.org/10.3390/nu15204474
De Lorenzo R, Di Filippo L, Scelfo S, Merolla A, Giustina A, Conte C, Rovere-Querini P. Longitudinal Changes in Physical Function and Their Impact on Health Outcomes in COVID-19 Patients. Nutrients. 2023; 15(20):4474. https://doi.org/10.3390/nu15204474
Chicago/Turabian StyleDe Lorenzo, Rebecca, Luigi Di Filippo, Sabrina Scelfo, Aurora Merolla, Andrea Giustina, Caterina Conte, and Patrizia Rovere-Querini. 2023. "Longitudinal Changes in Physical Function and Their Impact on Health Outcomes in COVID-19 Patients" Nutrients 15, no. 20: 4474. https://doi.org/10.3390/nu15204474
APA StyleDe Lorenzo, R., Di Filippo, L., Scelfo, S., Merolla, A., Giustina, A., Conte, C., & Rovere-Querini, P. (2023). Longitudinal Changes in Physical Function and Their Impact on Health Outcomes in COVID-19 Patients. Nutrients, 15(20), 4474. https://doi.org/10.3390/nu15204474







