Effects of Dietary Supplementation with a Ferulic Acid-Rich Bioactive Component of Wheat Bran in a Murine Model of Graft-Versus-Host Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredient Preparation
2.2. Generation of a GvHD Murine Model
2.3. GvHD Scoring
2.4. Histopathological Analysis of GvHD
2.5. Cytokines Profiles Analysis
2.6. Statistical Analysis
3. Results
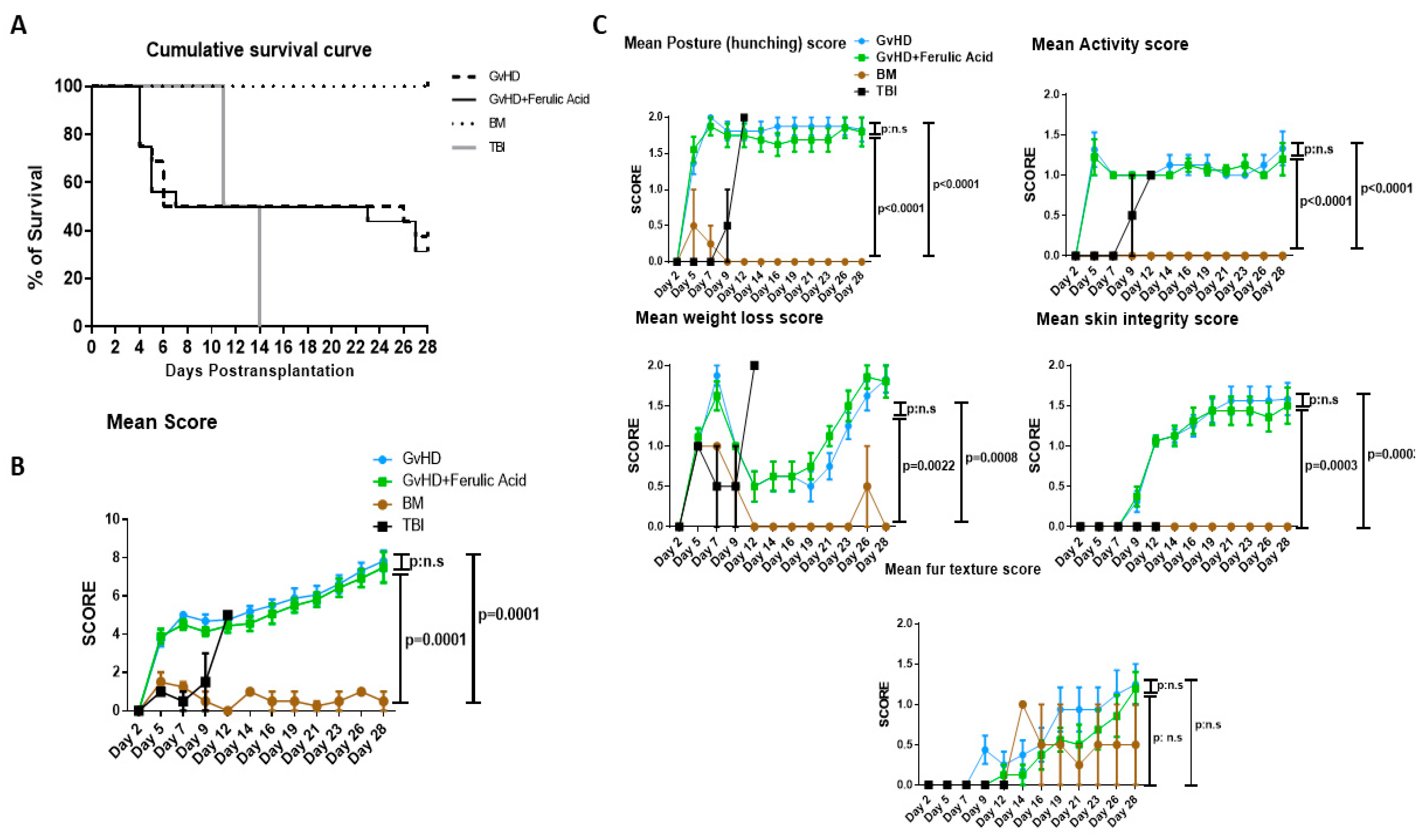
3.1. Survival and Clinical GvHD Score
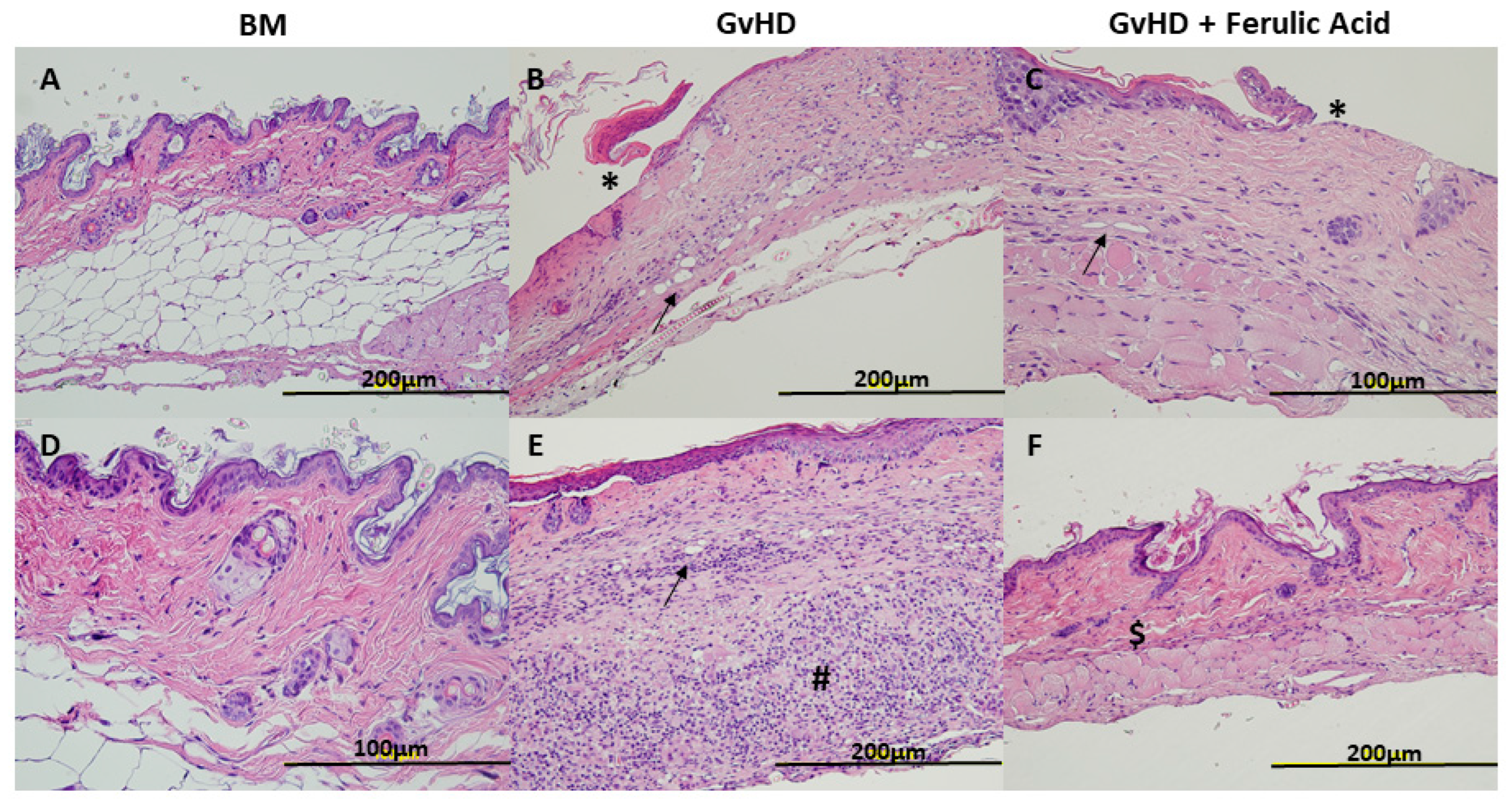
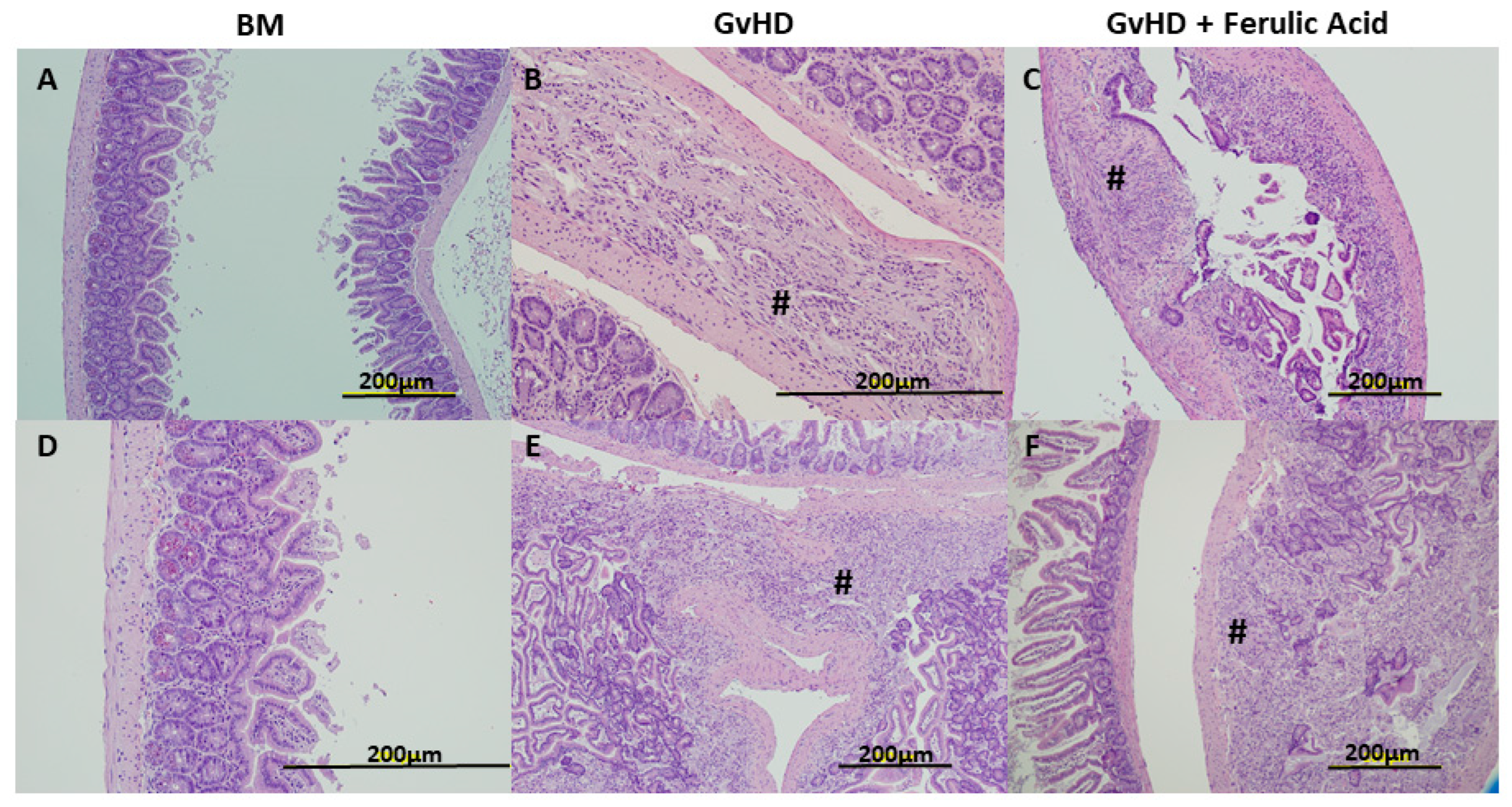
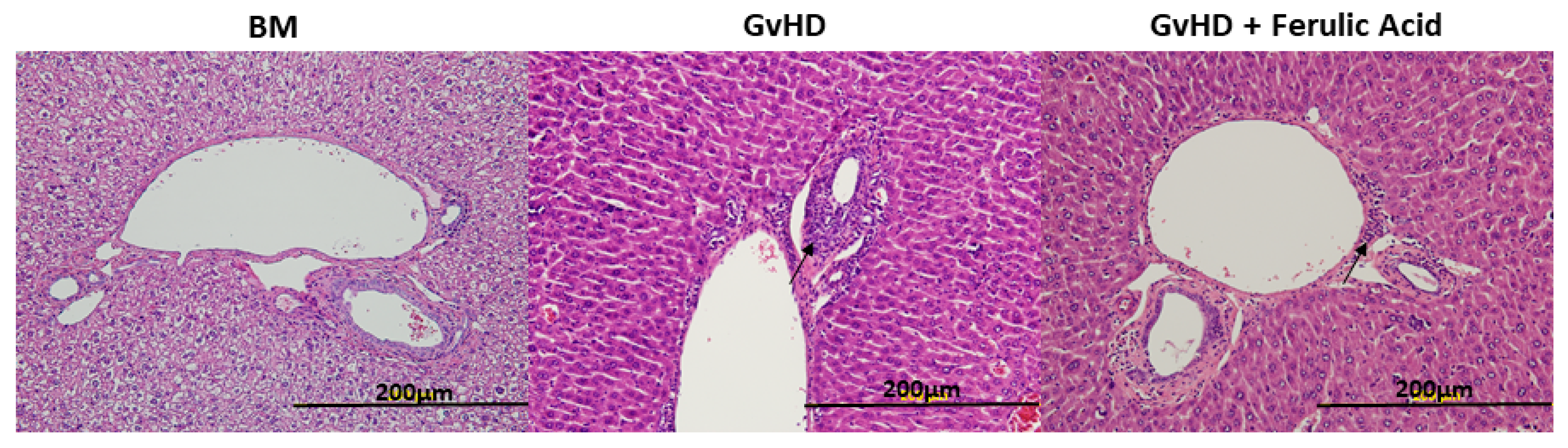
3.2. Histological Evaluation of Murine GvHD
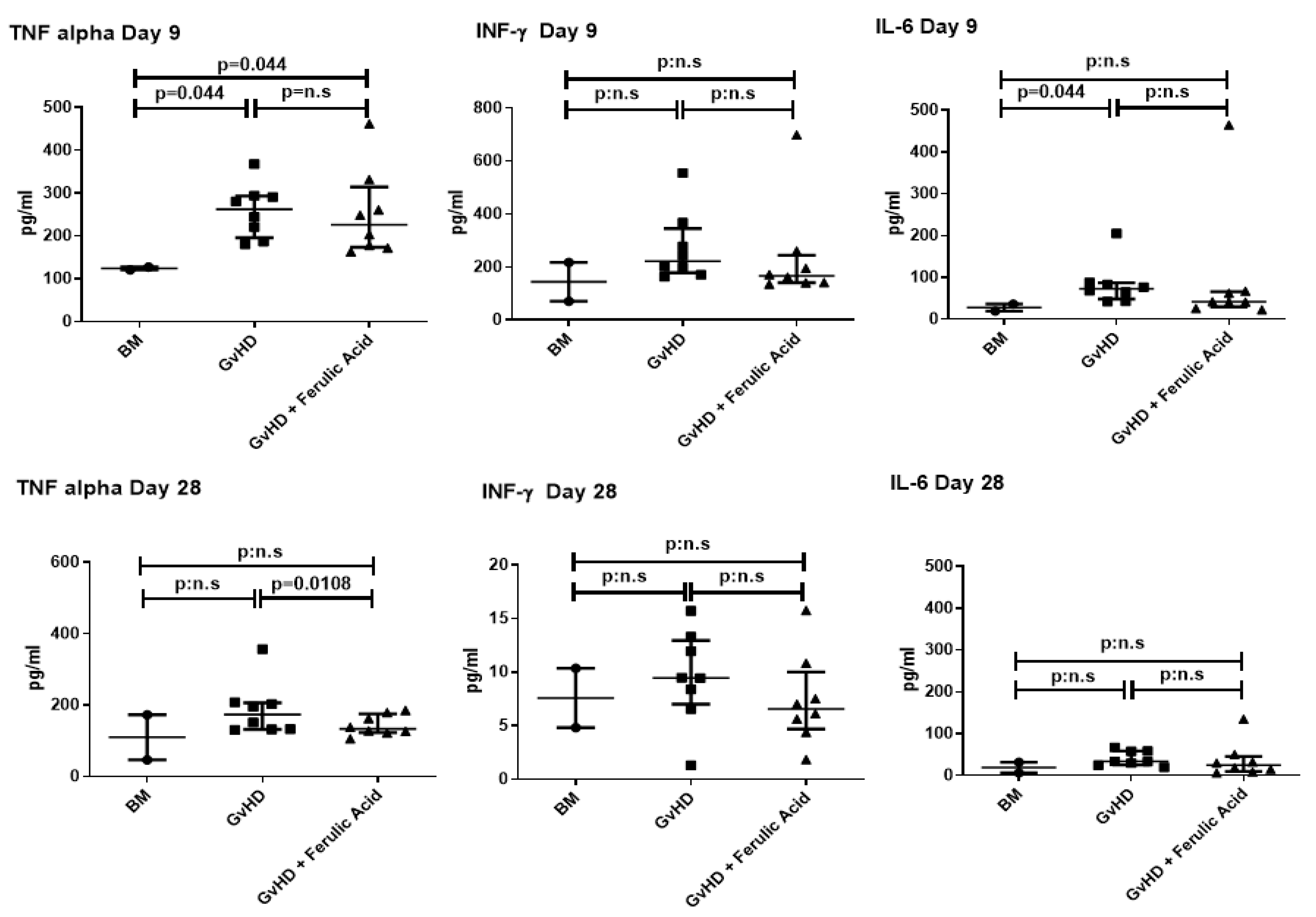
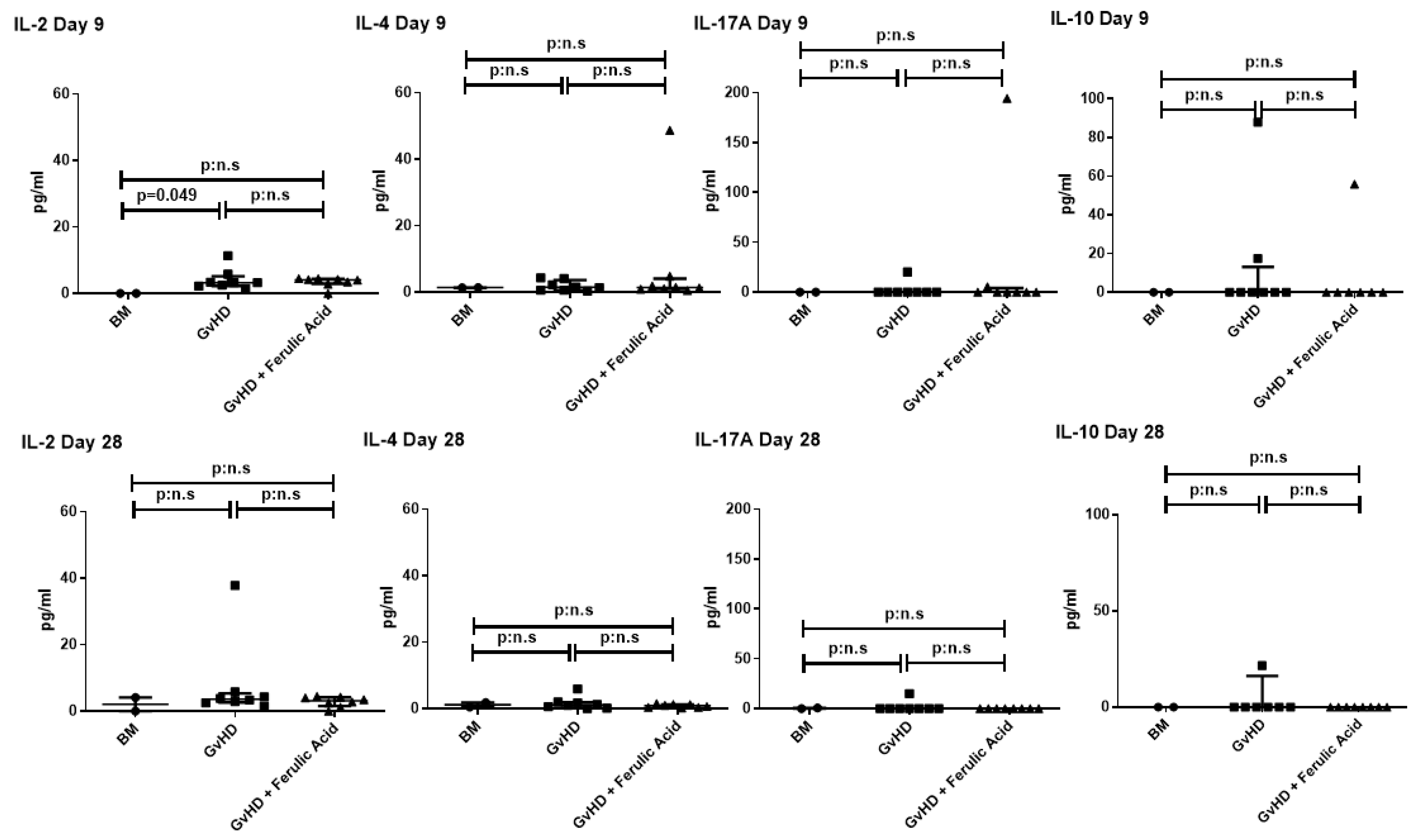
3.3. Evaluation of Murine Serum Cytokine Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrara, J.L.M.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.E.; Sullivan, K.M. Chronic graft-versus-host disease. Blood Rev. 2006, 20, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Couriel, D.; Caldera, H.; Champlin, R.; Komanduri, K. Acute graft-versus-host disease: Pathophysiology, clinical manifestations, and management. Cancer 2004, 101, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.; Alousi, A.; Kebriaei, P.; Mehta, R.; Rezvani, K.; Shpall, E. New and emerging therapies for acute and chronic graft versus host disease. Ther. Adv. Hematol. 2018, 9, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guijo, F.; Caballero-Velázquez, T.; López-Villar, O.; Redondo, A.; Parody, R.; Martínez, C.; Olavarría, E.; Andreu, E.; Prósper, F.; Díez-Campelo, M.; et al. Sequential Third-Party Mesenchymal Stromal Cell Therapy for Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 1580–1585. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Tomé-Sánchez, I.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Rico, D. A novel strategy to produce a soluble and bioactive wheat bran ingredient rich in ferulic acid. Antioxidants 2021, 10, 969. [Google Scholar] [CrossRef]
- Bautista-expósito, S.; Tomé-sánchez, I.; Martín-diana, A.B.; Frias, J.; Peñas, E.; Rico, D.; Casas, M.J.G.; Martínez-villaluenga, C. Enzyme selection and hydrolysis under optimal conditions improved phenolic acid solubility, and antioxidant and anti-inflammatory activities of wheat bran. Antioxidants 2020, 9, 984. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed Wheat Ingredients: Characterization, Bioaccessibility of Phenolic Compounds, and Bioactivity During in vitro Digestion. Front. Plant Sci. 2021, 12, 790898. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martínez-Villaluenga, C.; Martín-Diana, A.B.; Rico, D.; Jiménez-Pulido, I.; Frias, J.; Dia, V.P. Antioxidant, Immunostimulatory, and Anticancer Properties of Hydrolyzed Wheat Bran Mediated through Macrophages Stimulation. Int. J. Mol. Sci. 2023, 24, 7436. [Google Scholar] [CrossRef] [PubMed]
- Palani Swamy, S.K.; Govindaswamy, V. Therapeutical properties of ferulic acid and bioavailability enhancement through feruloyl esterase. J. Funct. Foods 2015, 17, 657–666. [Google Scholar] [CrossRef]
- Lee, C.C.; Wang, C.C.; Huang, H.M.; Lin, C.L.; Leu, S.J.; Lee, Y.L. Ferulic Acid Induces Th1 Responses by Modulating the Function of Dendritic Cells and Ameliorates Th2-Mediated Allergic Airway Inflammation in Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 678487. [Google Scholar] [CrossRef]
- Lin, C.M.; Chiu, J.H.; Wu, I.H.; Wang, B.W.; Pan, C.M.; Chen, Y.H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1α. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Li, Z.; Fang, X.; Cao, T.; Xu, Y. Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting microRNA-340 to induce β-catenin expression through hypoxia. Eur. J. Cell Biol. 2017, 96, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, P.; Zhao, P.; Wang, D.; Zhang, Y.; Wang, J.; Chen, L.; Guo, W.; Gao, H.; Jiao, Y. Pretreatment of ferulic acid attenuates inflammation and oxidative stress in a rat model of lipopolysaccharide-induced acute respiratory distress syndrome. Int. J. Immunopathol. Pharmacol. 2018, 32. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Qiu, W.; Shi, Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol. Cell. Toxicol. 2022, 18, 509–519. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.J.; Lim, S.T.; Kim, H.K.; Heo, H.J.; Kim, E.K.; Jun, W.J.; Cho, H.Y.; Kim, Y.J.; Shin, D.H. Ferulic acid supplementation prevents trimethyltin-induced cognitive deficits in mice. Biosci. Biotechnol. Biochem. 2007, 71, 1063–1068. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, Q.H.; Xiong, X.G.; Chen, J.; Wu, D.; Wang, Y.; Yang, B.; Zhang, Y.; Zhang, Y.; Huang, X. Anti-inflammatory effects of the bioactive compound ferulic acid contained in oldenlandia diffusa on collagen-induced arthritis in rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 573801. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Sannigrahi, S.; Rahaman, N.; Ahmed, F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: Effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine 2013, 44, 369–379. [Google Scholar] [CrossRef]
- Baskaran, N.; Manoharan, S.; Balakrishnan, S.; Pugalendhi, P. Chemopreventive potential of ferulic acid in 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in Sprague-Dawley rats. Eur. J. Pharmacol. 2010, 637, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Sheng, Y.C.; Ji, L.L.; Wang, Z.T. Ferulic acid prevents liver injury and increases the anti-tumor effect of diosbulbin B in vivo. J. Zhejiang Univ. Sci. B 2014, 15, 540–547. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, R.; Sánchez-Abarca, L.I.; Nieto-Gómez, C.; Martín García, E.; Sánchez-Guijo, F.; Argüeso, P.; Aijón, J.; Hernández-Galilea, E.; Velasco, A. Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul. Surf. 2019, 17, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; DiPersio, J.F. Mouse models of graft-versus-host disease: Advances and limitations. Dis. Model. Mech. 2011, 4, 318–333. [Google Scholar] [CrossRef]
- Cooke, K.R.; Kobzik, L.; Martin, T.R.; Brewer, J.; Delmonte, J.; Crawford, J.M.; Ferrara, J.L.M. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: 1. The roles of minor H antigens and endotoxin. Blood 1996, 88, 3230–3239. [Google Scholar] [CrossRef]
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D.; Xiong, S.; Zhang, J. Resveratrol Inhibits CD4+ T Cell Activation by Enhancing the Expression and Activity of Sirt1. PLoS ONE 2013, 8, e0075139. [Google Scholar] [CrossRef]
- Alvarez-Laderas, I.; Ramos, T.L.; Medrano, M.; Caracuel-García, R.; Barbado, M.V.; Sánchez-Hidalgo, M.; Zamora, R.; Alarcón-de-la-Lastra, C.; Hidalgo, F.J.; Piruat, J.I.; et al. Polyphenolic Extract (PE) from Olive Oil Exerts a Potent Immunomodulatory Effect and Prevents Graft-versus-Host Disease in a Mouse Model. Biol. Blood Marrow Transplant. 2020, 26, 615–624. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Afaq, F.; Ahmad, N.; Mukhtar, H. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J. Cell. Biochem. 2004, 91, 232–242. [Google Scholar] [CrossRef]
- Beevers, C.S.; Li, F.; Liu, L.; Huang, S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int. J. Cancer 2006, 119, 757–764. [Google Scholar] [CrossRef]
- Kanamune, J.; Iwanaga, Y.; Kina, T.; Noguchi, H.; Matsumura, K.; Uemoto, S.; Hyon, S.H. Attenuation of murine graft-versus-host disease by a tea polyphenol. Cell Transplant. 2012, 21, 909–918. [Google Scholar] [CrossRef]
- Westphal, S.; McGeary, A.; Rudloff, S.; Wilke, A.; Penack, O. The green tea catechin epigallocatechin gallate ameliorates graft-versus-host disease. PLoS ONE 2017, 12, e0169630. [Google Scholar] [CrossRef]
- Fang, H.Y.; Chen, Y.K.; Chen, H.H.; Lin, S.Y.; Fang, Y.T. Immunomodulatory effects of feruloylated oligosaccharides from rice bran. Food Chem. 2012, 134, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Shu, G.; Du, H.; Zheng, Y.; Fu, H.; Zhang, W.; Lv, C.; Xu, F.; Li, H.; Ouyang, P.; et al. Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide. Molecules 2023, 28, 1720. [Google Scholar] [CrossRef]
- He, S.; Liu, F.; Xu, L.; Yin, P.; Li, D.; Mei, C.; Jiang, L.; Ma, Y.; Xu, J. Protective effects of ferulic acid against heat stress-induced intestinal epithelial barrier dysfunction in vitro and in vivo. PLoS ONE 2016, 11, e0145236. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.S.; Cook, D.P.; Ferreira, G.B.; Gysemans, C.; Mathieu, C. Vitamin D-modulated dendritic cells delay lethal graft-versus-host disease through induction of regulatory T cells. J. Steroid Biochem. Mol. Biol. 2019, 188, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cooper, M.L.; Ziga, E.D.; Ritchey, J.; DiPersio, J.F. Effect of epigallocatechin-3-gallate on graft-versus-host disease. Cell Transplant. 2014, 23, 1163–1166. [Google Scholar] [CrossRef]
- Riesner, K.; Kalupa, M.; Shi, Y.; Elezkurtaj, S.; Penack, O. A preclinical acute GVHD mouse model based on chemotherapy conditioning and MHC-matched transplantation. Bone Marrow Transplant. 2016, 51, 410–417. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preciado, S.; Martínez-Villaluenga, C.; Rico, D.; Muntión, S.; García-Macías, M.-C.; Navarro-Bailón, A.; Martín-Diana, A.B.; Sánchez-Guijo, F. Effects of Dietary Supplementation with a Ferulic Acid-Rich Bioactive Component of Wheat Bran in a Murine Model of Graft-Versus-Host Disease. Nutrients 2023, 15, 4582. https://doi.org/10.3390/nu15214582
Preciado S, Martínez-Villaluenga C, Rico D, Muntión S, García-Macías M-C, Navarro-Bailón A, Martín-Diana AB, Sánchez-Guijo F. Effects of Dietary Supplementation with a Ferulic Acid-Rich Bioactive Component of Wheat Bran in a Murine Model of Graft-Versus-Host Disease. Nutrients. 2023; 15(21):4582. https://doi.org/10.3390/nu15214582
Chicago/Turabian StylePreciado, Silvia, Cristina Martínez-Villaluenga, Daniel Rico, Sandra Muntión, María-Carmen García-Macías, Almudena Navarro-Bailón, Ana Belén Martín-Diana, and Fermín Sánchez-Guijo. 2023. "Effects of Dietary Supplementation with a Ferulic Acid-Rich Bioactive Component of Wheat Bran in a Murine Model of Graft-Versus-Host Disease" Nutrients 15, no. 21: 4582. https://doi.org/10.3390/nu15214582






