Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting of Trial
2.2. Participants
2.3. Inclusion and Exclusion Criteria
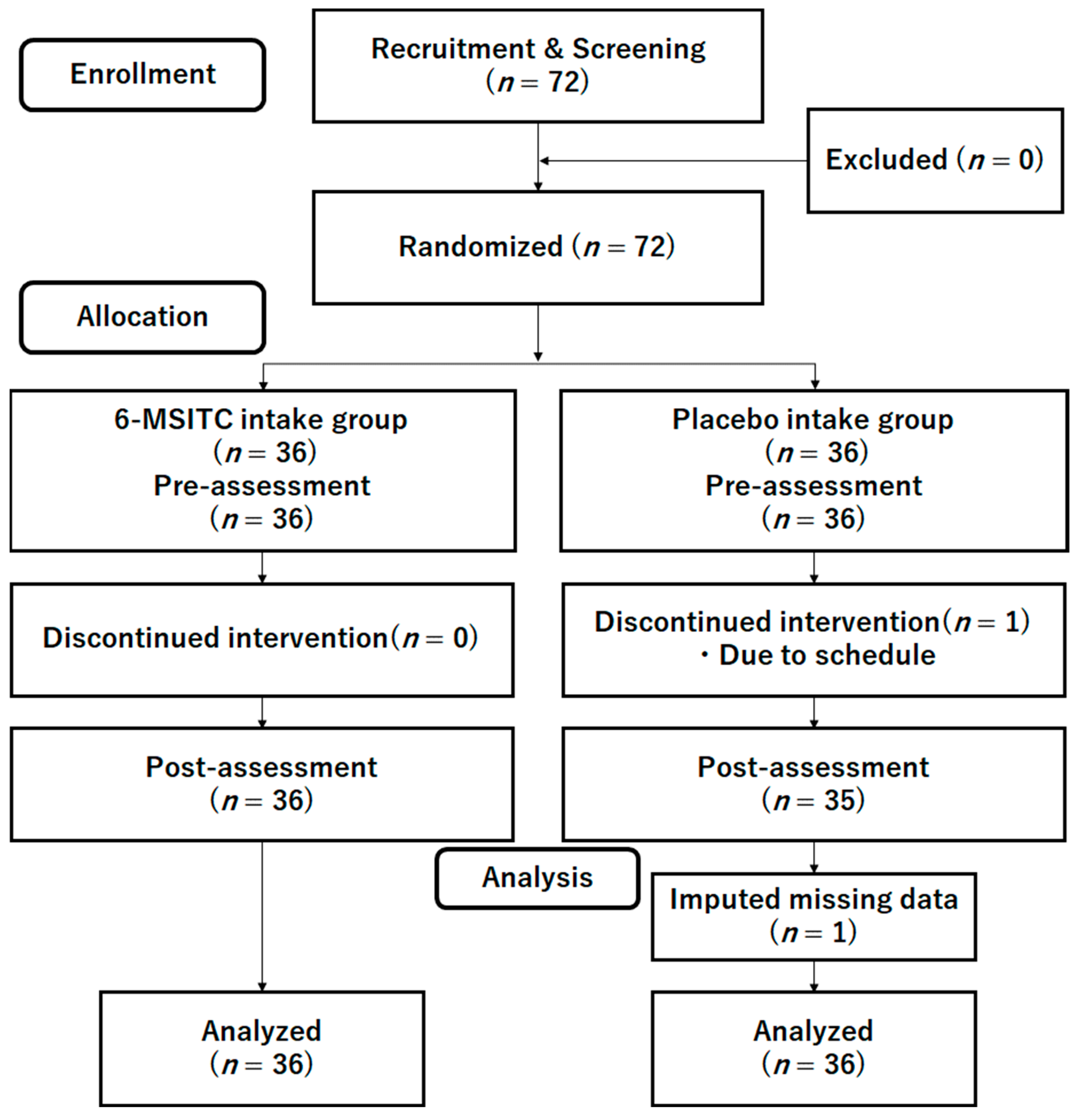
2.4. Sample Size Estimation
2.5. Randomization
2.6. General Procedure of the Intervention
2.7. 6-MSITC and Placebo Supplements
2.8. Cognitive Functions
2.9. Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
References
- Wilson, R.S.; Wang, T.; Yu, L.; Bennett, D.A.; Boyle, P.A. Normative Cognitive Decline in Old Age. Ann. Neurol. 2020, 87, 816–829. [Google Scholar] [CrossRef]
- Njegovan, V.; Man-Son-Hing, M.; Mitchell, S.L.; Molnar, F.J. The Hierarchy of Functional Loss Associated with Cognitive Decline in Older Persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M638–M643. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Agosti, P.; Lozupone, M.; Custodero, C.; Schilardi, A.; Valiani, V.; Sardone, R.; Dibello, V.; di Lena, L.; Lamanna, A.; et al. Nutritional Intervention as a Preventive Approach for Cognitive-Related Outcomes in Cognitively Healthy Older Adults: A Systematic Review. J. Alzheimer’s Dis. 2018, 64, S229–S254. [Google Scholar] [CrossRef]
- Gardener, S.L.; Rainey-Smith, S.R. The Role of Nutrition in Cognitive Function and Brain Ageing in the Elderly. Curr. Nutr. Rep. 2018, 7, 139–149. [Google Scholar] [CrossRef]
- Nouchi, R.; Suiko, T.; Kimura, E.; Takenaka, H.; Murakoshi, M.; Uchiyama, A.; Aono, M.; Kawashima, R. Effects of Lutein and Astaxanthin Intake on the Improvement of Cognitive Functions among Healthy Adults: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 617. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective Potency of Some Spice Herbs, a Literature Review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef]
- Jiang, T.A. Health Benefits of Culinary Herbs and Spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Panickar, K.S. Beneficial Effects of Herbs, Spices and Medicinal Plants on the Metabolic Syndrome, Brain and Cognitive Function. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 13–29. [Google Scholar] [CrossRef]
- Mirmosayyeb, O.; Tanhaei, A.; Sohrabi, H.; Martins, R.; Tanhaei, M.; Najafi, M.; Safaei, A.; Meamar, R. Possible Role of Common Spices as a Preventive and Therapeutic Agent for Alzheimer′s Disease. Int. J. Prev. Med. 2017, 8, 5. [Google Scholar] [CrossRef]
- Isbill, J.; Kandiah, J.; Khubchandani, J. Use of Ethnic Spices by Adults in the United States: An Exploratory Study. Health Promot. Perspect. 2017, 8, 33–40. [Google Scholar] [CrossRef]
- Peter, K.V.; Babu, K.N. Introduction to Herbs and Spices: Medicinal Uses and Sustainable Production. In Handbook of Herbs and Spices; Peter, K., Ed.; Woodhead Publishing: Oxford, UK, 2012; Volume 2, pp. 1–16. [Google Scholar]
- Watanabe, M.; Ohata, M.; Hayakawa, S.; Isemura, M.; Kumazawa, S.; Nakayama, T.; Furugori, M.; Kinae, N. Identification of 6-Methylsulfinylhexyl Isothiocyanate as an Apoptosis-Inducing Component in Wasabi. Phytochemistry 2003, 62, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yang, J.H.; Bae, M.J.; Yoo, W.K.; Ye, S.; Xue, C.C.L.; Li, C.G. Anti-Oxidant and Anti-Hypercholesterolemic Activities of Wasabia Japonica. Evid. Based Complement. Altern. Med. 2010, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Kato, T.; Nagai, M.; Ohnishi, M.; Yoshida, K. Inhibitory Effects of Wasabi Isothiocyanates on Chemical Mediator Release in RBL-2H3 Rat Basophilic Leukemia Cells. J. Nutr. Sci. Vitaminol. 2012, 58, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Hayek, S.S.; Goldstein, F.C.; Martin, G.; Jones, D.P.; Quyyumi, A. Oxidative Stress Predicts Cognitive Decline with Aging in Healthy Adults: An Observational Study. J. Neuroinflammation 2018, 15, 17. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef]
- Custodero, C.; Ciavarella, A.; Panza, F.; Gnocchi, D.; Lenato, G.M.; Lee, J.; Mazzocca, A.; Sabbà, C.; Solfrizzi, V. Role of Inflammatory Markers in the Diagnosis of Vascular Contributions to Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis. Geroscience 2022, 44, 1373–1392. [Google Scholar] [CrossRef]
- Meydani, M. Antioxidants and Cognitive Function. Nutr. Rev. 2009, 59, S75–S82. [Google Scholar] [CrossRef]
- Oka, T.; Yamada, Y.; Lkhagvasuren, B.; Nakao, M.; Nakajima, R.; Kanou, M.; Hiramatsu, R.; Nabeshima, Y. Clinical Effects of Wasabi Extract Containing 6-MSITC on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Open-Label Trial. Biopsychosoc. Med. 2022, 16, 26. [Google Scholar] [CrossRef]
- Okunishi, I.; Yamada-Kato, T.; Saito, J. The Effects of Wasabi Root-Derived 6-(Methylsulfinyl) Hexyl Isothiocyanate on Neurocognitive Functions in Cognitively Intact Middle-Aged and Older Adults: A Randomized, Double Blind, Placebo Controlled Trial. Jpn. Pharmacol. Ther. 2019, 47, 275–286. [Google Scholar]
- McWhirter, L.; Smyth, H.; Hoeritzauer, I.; Couturier, A.; Stone, J.; Carson, A.J. What Is Brain Fog? J. Neurol. Neurosurg. Psychiatry 2023, 94, 321–325. [Google Scholar] [CrossRef]
- Nouchi, R.; Hu, Q.; Saito, T.; dos Santos Kawata, N.Y.; Nouchi, H.; Kawashima, R. Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients 2021, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Hu, Q.; Ushida, Y.; Suganuma, H.; Kawashima, R. Effects of Sulforaphane Intake on Processing Speed and Negative Moods in Healthy Older Adults: Evidence from a Randomized Controlled Trial. Front. Aging Neurosci. 2022, 14, 929628. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at Bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, K.; Sugishita, M.; Hemmi, I.; Asada, T.; Tanigawa, T. A Validity and Reliability Study of the Japanese Version of the Geriatric Depression Scale 15 (GDS-15-J). Clin. Gerontol. 2017, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G* Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- McCarrey, A.C.; An, Y.; Kitner-Triolo, M.H.; Ferrucci, L.; Resnick, S.M. Sex Differences in Cognitive Trajectories in Clinically Normal Older Adults. Psychol. Aging 2016, 31, 166–175. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing Dentate Gyrus Function with Dietary Flavanols Improves Cognition in Older Adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.L.R.; Fulford, J. Enhanced Task-Related Brain Activation and Resting Perfusion in Healthy Older Adults after Chronic Blueberry Supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- Whyte, A.; Cheng, N.; Fromentin, E.; Williams, C. A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlueTM) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef]
- Nakajima, R.; Kanou, M.; Tokushima, M.; Iwama, Y.; Yamana, K. Oral Administration of 6-Methylsulfinylhexyl Isothiocyanate Extracted from Wasabi Is Safe and Improves the Fatigue and Sleep of Healthy Volunteers. Biopsychosoc. Med. 2023, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, I.; Yamada-Kato, T.; Saito, J.; Hou, D.-X. Safety Evaluation of 6-(Methylsulfinyl) Hexyl Isothiocyanate (6-MSITC) and Wasabi Sulfinyl, a 6-MSITC-Containing Supplement. Food Sci. Technol. Res. 2020, 26, 813–824. [Google Scholar] [CrossRef]
- Matsuoka, K.; Uno, M.; Kasai, K.; Koyama, K.; Kim, Y. Estimation of Premorbid IQ in Individuals with Alzheimer’s Disease Using Japanese Ideographic Script (Kanji) Compound Words: Japanese Version of National Adult Reading Test. Psychiatry Clin. Neurosci. 2006, 60, 332–339. [Google Scholar] [CrossRef]
- Wechsler, D.A. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Hatta, T.; Ito, Y.; Yoshizaki, K. D-CAT Manual (Screening Test for Attention); Union Press: Osaka, Japan, 2000. [Google Scholar]
- Hakoda, Y.; Watanabe, M. Manual for New Stroop Test II; Toyo Physical: Fukuoka, Japan, 2004. [Google Scholar]
- Smits, C.H.M.; Smit, J.H.; van den Heuvel, N.; Jonker, C. Norms for an Abbreviated Raven’s Coloured Progressive Matrices in an Older Sample. J. Clin. Psychol. 1997, 53, 687–697. [Google Scholar] [CrossRef]
- Wechsler, D.A. Wechsler Memory Scale Revised; The Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Wilson, B.A.; Cockburn, J.; Baddeley, A.D. The Rivermead Behavioral Memory Test; Thamas Valley Test Company: Reading, UK, 1985. [Google Scholar]
- Peters, M.; Laeng, B.; Latham, K.; Jackson, M.; Zaiyouna, R.; Richardson, C. A Redrawn Vandenberg and Kuse Mental Rotations Test—Different Versions and Factors That Affect Performance. Brain Cogn. 1995, 28, 39–58. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Nouchi, R.; Kobayashi, A.; Nouchi, H.; Kawashima, R. Newly Developed TV-Based Cognitive Training Games Improve Car Driving Skills, Cognitive Functions, and Mood in Healthy Older Adults: Evidence From a Randomized Controlled Trial. Front. Aging Neurosci. 2019, 11, 99. [Google Scholar] [CrossRef]
- Bob, W.; Marco, T. LmPerm: Permutation Tests for Linear Models; R Package Version 2.1.0; GitHub, Inc.: San Francisco, CA, USA, 2016; Available online: https://github.com/mtorchiano/lmPerm (accessed on 27 October 2023).
- Fujita, T.; Notoya, M.; Sunahara, N.; Nakatani, K.; Kimura, D. Risk Factors for Impaired Instrumental Activities of Daily Living in Alzheimer’s Disease. Asian J. Occup. Ther. 2018, 14, 9–16. [Google Scholar] [CrossRef]
- Bolla, K.I.; Lindgren, K.N.; Bonaccorsy, C.; Bleecker, M.L. Memory Complaints in Older Adults. Fact or Fiction? Arch. Neurol. 1991, 48, 61–64. [Google Scholar] [CrossRef]
- Leirer, V.O.; Morrow, D.G.; Sheikh, J.I.; Pariante, G.M. Memory Skills Elders Want to Improve. Exp. Aging Res. 1990, 16, 155–158. [Google Scholar] [CrossRef]
- Yonelinas, A.P. The Hippocampus Supports High-Resolution Binding in the Service of Perception, Working Memory and Long-Term Memory. Behav. Brain Res. 2013, 254, 34–44. [Google Scholar] [CrossRef]
| 6-MSITC (Female: N = 9, Male: N = 27) | Placebo (Female: N = 10, Male: N = 26) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | d | |
| Age | 65.92 | 3.94 | 64.94 | 3.61 | 0.28 | 0.26 |
| Education (years) | 12.5 | 0.8 | 12.9 | 1.1 | 0.87 | 0.04 |
| MMSE | 28.5 | 1.48 | 28.75 | 1.57 | 0.49 | 0.16 |
| FAB | 15.39 | 1.86 | 15.37 | 1.93 | 0.79 | 0.06 |
| JART | 20.33 | 3.85 | 20.78 | 3.62 | 0.62 | 0.12 |
| GDS | 2.44 | 1.36 | 2.07 | 1.07 | 0.18 | 0.31 |
| Main Ingredients | 6-MSITC Capsule | Placebo Capsule |
|---|---|---|
| wasabi extract powder (contains 0.8 mg of 6-MSITC) | 22.40% | 0% |
| α cyclodextrin | 77.60% | 100% |
| 6-MSITC | Placebo | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | d | |
| Processing speed | ||||||
| Cd | 75.89 | 10.16 | 76.42 | 9.19 | 0.82 | 0.05 |
| SS | 38.58 | 5.96 | 37.17 | 4.99 | 0.28 | 0.26 |
| Attention | ||||||
| D-CAT | 48.69 | 7.55 | 51.19 | 8.08 | 0.18 | 0.32 |
| Executive functions | ||||||
| ST | 34.75 | 6.33 | 36.17 | 5.82 | 0.33 | 0.23 |
| rST | 48.97 | 6.06 | 48.69 | 6.71 | 0.85 | 0.04 |
| Reasoning | ||||||
| CPM | 34.5 | 1.65 | 33.86 | 3.55 | 0.33 | 0.23 |
| Short-term memory | ||||||
| DS-F | 8.36 | 1.84 | 8.97 | 2.34 | 0.22 | 0.29 |
| Working memory | ||||||
| DS-B | 6.42 | 1.96 | 7.06 | 2.76 | 0.26 | 0.27 |
| Episodic memory | ||||||
| Immediate LM | 12.03 | 3.85 | 12.47 | 3.18 | 0.6 | 0.13 |
| Delayed LM | 10.89 | 4.16 | 11.61 | 3.87 | ||
| FSN | 4.39 | 2.31 | 4.67 | 1.67 | 0.56 | 0.14 |
| Visual–spatial performance | ||||||
| MR | 19.56 | 5.46 | 18.78 | 4.61 | 0.52 | 0.15 |
| 6-MSITC | Placebo | Adjusted p Value 1 | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | Eta2 | ||
| Processing speed | |||||||
| Cd | 1.72 | 5.19 | 2.37 | 7.15 | 0.40 | 1.00 | 0.01 |
| SS | 0.86 | 3.71 | 0.86 | 3.6 | 0.80 | 1.00 | 0.00 |
| Attention | |||||||
| D-CAT | 1.22 | 6.83 | 1.11 | 7.44 | 0.65 | 1.00 | 0.00 |
| Executive functions | |||||||
| T | 1.28 | 3.27 | 0.69 | 4.93 | 0.62 | 1.00 | 0.00 |
| rST | 0.61 | 4.15 | 0.23 | 3.99 | 0.32 | 1.00 | 0.01 |
| Reasoning | |||||||
| CPM | 0.31 | 1.43 | 0.31 | 1.71 | 0.69 | 1.00 | 0.00 |
| Short-term memory | |||||||
| DS-F | 0.14 | 1.46 | 0.29 | 1.49 | 0.80 | 1.00 | 0.00 |
| Working memory | |||||||
| DS-B | 1.14 | 1.84 | −0.03 | 1.98 | 0.00 | 0.00 | 0.11 |
| Episodic memory | |||||||
| Immediate LM | 1.53 | 2.66 | −0.11 | 2.68 | 0.00 | 0.00 | 0.19 |
| Delayed LM | 1.97 | 3.28 | 0.34 | 3.03 | 0.00 | 0.03 | 0.14 |
| FSN | 1.78 | 2.03 | 0.69 | 1.95 | 0.00 | 0.00 | 0.16 |
| Visual–spatial performance | |||||||
| MR | −0.28 | 6.97 | 0.37 | 4.38 | 0.76 | 1.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouchi, R.; Kawata, N.Y.S.; Saito, T.; Nouchi, H.; Kawashima, R. Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial. Nutrients 2023, 15, 4608. https://doi.org/10.3390/nu15214608
Nouchi R, Kawata NYS, Saito T, Nouchi H, Kawashima R. Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial. Nutrients. 2023; 15(21):4608. https://doi.org/10.3390/nu15214608
Chicago/Turabian StyleNouchi, Rui, Natasha Y. S. Kawata, Toshiki Saito, Haruka Nouchi, and Ryuta Kawashima. 2023. "Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial" Nutrients 15, no. 21: 4608. https://doi.org/10.3390/nu15214608
APA StyleNouchi, R., Kawata, N. Y. S., Saito, T., Nouchi, H., & Kawashima, R. (2023). Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial. Nutrients, 15(21), 4608. https://doi.org/10.3390/nu15214608







