Therapeutic Effect on Swallowing Function and on Hydration Status of a New Liquid Gum-Based Thickener in Independently-Living Older Patients with Oropharyngeal Dysphagia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Product and Sample Preparation
2.2. Rheological Characterization
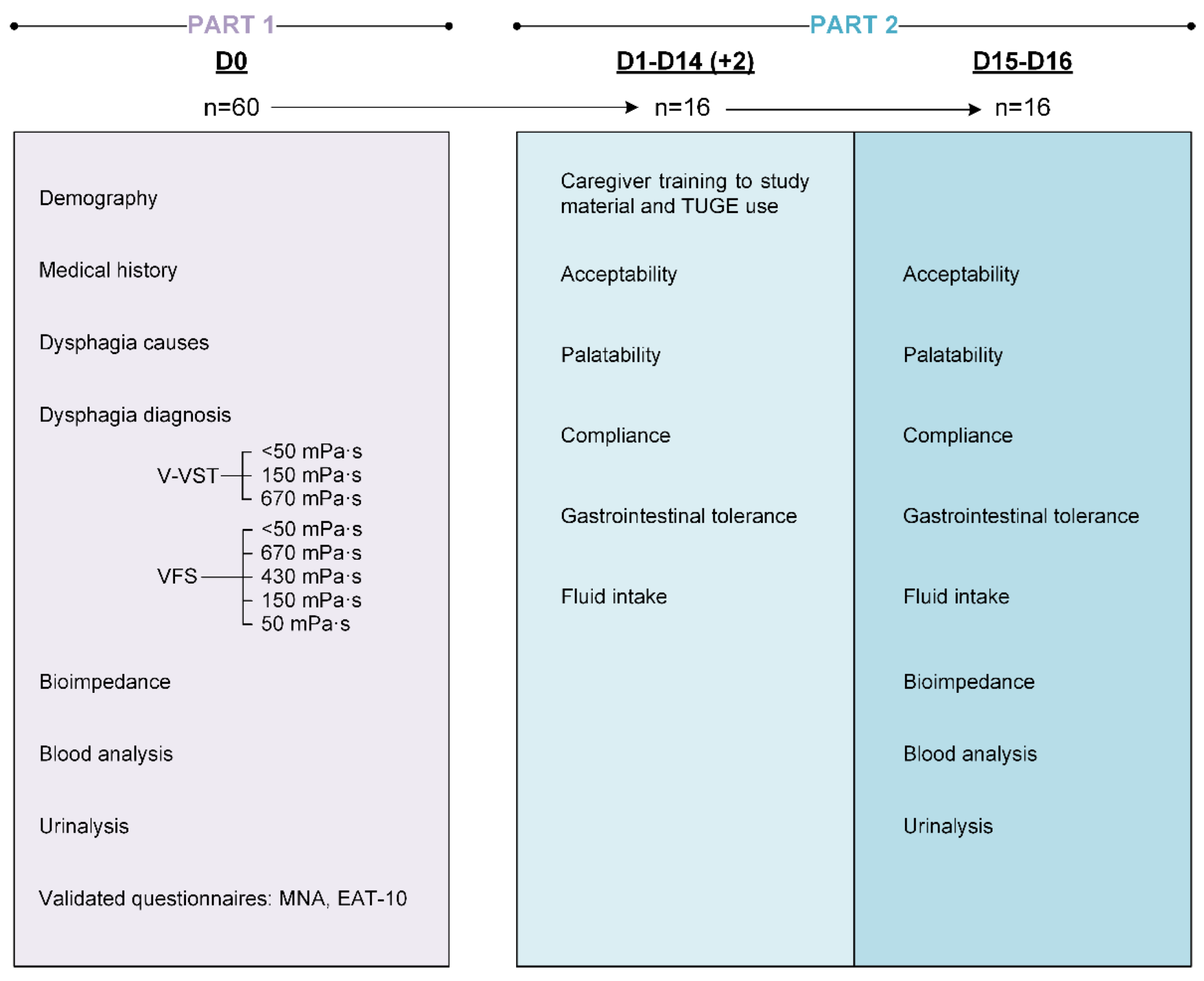
2.3. Clinical Study Design
2.4. Study Population
2.5. Swallowing Evaluation
2.5.1. Volume–Viscosity Swallowing Test (V-VST)
2.5.2. Videofluoroscopy (VFS)
2.6. Hydration Status and Analytical Parameters
2.6.1. Serum and Urine Biochemical Analysis
2.6.2. Bioelectrical Impedance Analysis (BIA)
2.7. Fluid Intake, Compliance and Acceptability
2.7.1. Fluid Intake and Compliance with the Intervention
2.7.2. Acceptability
2.7.3. Gastrointestinal Tolerance
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
3.1. Rheological Characterization of TUGE
3.2. Part 1: VFS Studies
3.2.1. Study Population
3.2.2. Hydration and Nutritional Status
3.2.3. Oropharyngeal Dysphagia Status
3.2.4. Therapeutic Effect of TUGE on PAS Score (Primary Endpoint)
3.2.5. Therapeutic Effect of TUGE on VFS Signs of Impaired Safety
3.2.6. Therapeutic Effect of TUGE on VFS Signs of Impaired Efficacy
3.2.7. Effect of Fluid Thickened with TUGE on Timing and Kinematics of OSR
3.3. Part 2: Effect of TUGE on Hydration Status, Acceptability and GI Tolerance
3.3.1. Study Population
3.3.2. Compliance and Therapeutic Effect on Hydration Status
3.3.3. Acceptability and Gastrointestinal Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rommel, N.; Hamdy, S. Oropharyngeal Dysphagia: Manifestations and Diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Baijens, L.W.J.; Clavé, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.-C.; Masiero, S.; Mateos-Nozal, J.; Ortega, O.; et al. European Society for Swallowing Disorders-European Union Geriatric Medicine Society White Paper: Oropharyngeal Dysphagia as a Geriatric Syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef] [PubMed]
- ICD-11. Available online: https://icd.who.int/en (accessed on 9 March 2023).
- Rajati, F.; Ahmadi, N.; Naghibzadeh, Z.A.; Kazeminia, M. The Global Prevalence of Oropharyngeal Dysphagia in Different Populations: A Systematic Review and Meta-Analysis. J. Transl. Med. 2022, 20, 175. [Google Scholar] [CrossRef]
- Ekberg, O.; Hamdy, S.; Woisard, V.; Wuttge-Hannig, A.; Ortega, P. Social and Psychological Burden of Dysphagia: Its Impact on Diagnosis and Treatment. Dysphagia 2002, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of Oropharyngeal Dysphagia on Healthcare Cost and Length of Stay in Hospital: A Systematic Review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Labeit, B.; Kremer, A.; Muhle, P.; Claus, I.; Warnecke, T.; Dziewas, R.; Suntrup-Krueger, S. Costs of Post-Stroke Dysphagia during Acute Hospitalization from a Health-Insurance Perspective. Eur. Stroke J. 2023, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yébenes, J.C. Total Body Water and Intracellular Water Relationships with Muscle Strength, Frailty and Functional Performance in an Elderly Population. A Cross-Sectional Study. J. Nutr. Health Aging 2019, 23, 96–101. [Google Scholar] [CrossRef]
- Lorenzo, I.; Serra-Prat, M.; Carlos Yébenes, J. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef]
- Viñas, P.; Bolivar-Prados, M.; Tomsen, N.; Costa, A.; Marin, S.; Riera, S.A.; Barcons, N.; Clavé, P. The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews. Nutrients 2022, 14, 2497. [Google Scholar] [CrossRef]
- Crary, M.A.; Carnaby, G.D.; Shabbir, Y.; Miller, L.; Silliman, S. Clinical Variables Associated with Hydration Status in Acute Ischemic Stroke Patients with Dysphagia. Dysphagia 2016, 31, 60–65. [Google Scholar] [CrossRef]
- Goldberg, L.R.; Heiss, C.J.; Parsons, S.D.; Foley, A.S.; Mefferd, A.S.; Hollinger, D.; Parham, D.F.; Patterson, J. Hydration in Older Adults: The Contribution of Bioelectrical Impedance Analysis. Int. J. Speech Lang Pathol. 2014, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vázquez, A.G.; Reyes-Torres, C.A.; Castillo-Martínez, L.; Serralde Zúñiga, A.E. Body Composition by Bioelectrical Impedance, Muscle Strength, and Nutritional Risk in Oropharyngeal Dysphagia Patients. Nutr. Hosp. 2021, 38, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Hamad, A.; Sasegbon, A.; Hamdy, S. Advances in the Treatment of Dysphagia in Neurological Disorders: A Review of Current Evidence and Future Considerations. Neuropsychiatr. Dis. Treat. 2022, 18, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Barbon, C.E.A.; Chen, J.; Cichero, J.A.Y.; Coutts, K.; Dantas, R.O.; Duivestein, J.; Giosa, L.; et al. The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef]
- Hadde, E.K.; Chen, J. Food Texture and Texture Modification for Dysphagia Management. J. Texture Stud. 2021, 52, 538–539. [Google Scholar] [CrossRef]
- Flynn, E.; Smith, C.H.; Walsh, C.D.; Walshe, M. Modifying the Consistency of Food and Fluids for Swallowing Difficulties in Dementia. Cochrane Database Syst. Rev. 2018, 9, CD011077. [Google Scholar] [CrossRef]
- Bolivar-Prados, M.; Rofes, L.; Arreola, V.; Guida, S.; Nascimento, W.V.; Martin, A.; Vilardell, N.; Ortega Fernández, O.; Ripken, D.; Lansink, M.; et al. Effect of a Gum-Based Thickener on the Safety of Swallowing in Patients with Poststroke Oropharyngeal Dysphagia. Neurogastroenterol. Motil. 2019, 31, e13695. [Google Scholar] [CrossRef]
- Brito-de La Fuente, E.; Turcanu, M.; Ekberg, O.; Gallegos, C. Rheological Aspects of Swallowing and Dysphagia: Shear and Elongational Flows. In Dysphagia; Med Radiol Diagn Imaging; Springer: Cham, Switzerland, 2017; Volume 49, pp. 557–561. [Google Scholar] [CrossRef]
- Gallegos, C.; Turcanu, M.; Assegehegn, G.; Brito-de la Fuente, E. Rheological Issues on Oropharyngeal Dysphagia. Dysphagia 2023, 38, 558–585. [Google Scholar] [CrossRef]
- Goroff, H.; Herzog, L.; Cardi, R.; Reding, M. Use of Oral Hydration Protocols for Dysphagic Patients Following Stroke. Rehabil. Nurs. J. 2018, 43, 290. [Google Scholar] [CrossRef]
- Sezgin, B.; Durusoy, D.; Demirci, M.S.; Ozturk, K.; Kaya, I.; Eyigor, S.; Gode, S. The Effect of “Xanthan Gum-Based Fluid Thickener” on Hydration, Swallowing Functions and Nutritional Status in Total Maxillectomy Patients. Eur. Arch Otorhinolaryngol. 2018, 275, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Prados, M.; Tomsen, N.; Arenas, C.; Ibáñez, L.; Clave, P. A Bit Thick: Hidden Risks in Thickening Products’ Labelling for Dysphagia Treatment. Food Hydrocoll. 2021, 123, 106960. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The Effects of a Xanthan Gum-Based Thickener on the Swallowing Function of Patients with Dysphagia. Aliment. Pharmacol. Ther. 2014, 39, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.J.; White, C.; McKenzie, S.; Belafsky, P.C. Effects of Bolus Rheology on Aspiration in Patients with Dysphagia. J. Acad. Nutr. Diet. 2014, 114, 590–594. [Google Scholar] [CrossRef]
- Steele, C.M.; Peladeau-Pigeon, M.; Barbon, C.A.E.; Guida, B.T.; Tapson, M.S.; Valenzano, T.J.; Wolkin, T.S.; Waito, A.A.; Hanson, B.; Ong, J.J.X.; et al. Modulation of Tongue Pressure According to Liquid Flow Properties in Healthy Swallowing. J. Speech Lang. Hear. Res. 2019, 62, 22–33. [Google Scholar] [CrossRef]
- Ortega, O.; Bolívar-Prados, M.; Arreola, V.; Nascimento, W.; Tomsen, N.; Gallegos, C.; Brito-de La Fuente, E.; Clavé, P. Therapeutic Effect, Rheological Properties and Xanthan Gum Thickener on Four Different. Nutrients 2020, 12, 1873. [Google Scholar] [CrossRef]
- Riera, S.A.; Marin, S.; Serra-Prat, M.; Tomsen, N.; Arreola, V.; Ortega, O.; Walshe, M.; Clavé, P. A Systematic and a Scoping Review on the Psychometrics and Clinical Utility of the Volume-Viscosity Swallow Test (V-vst) in the Clinical Screening and Assessment of Oropharyngeal Dysphagia. Foods 2021, 10, 1900. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ekberg, O.; Wiklund, J.; Mansoor, R.; Stading, M. Simultaneous X-Ray Video-Fluoroscopy and Pulsed Ultrasound Velocimetry Analyses of the Pharyngeal Phase of Swallowing of Boluses with Different Rheological Properties. Dysphagia 2020, 35, 898–906. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): A Practical Tool for Identification of Nutritional Status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and Reliability of the Eating Assessment Tool (EAT10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef]
- Uppsala Monitoring Centre The Use of the WHO-UMC System for Standardised Case Causality Assessment. Upps. Monit. Cent. 2018, 2–7.
- Karch, F.E.; Lasagna, L. Toward the Operational Identification of Adverse Drug Reactions. Clin. Pharmacol. Ther. 1977, 21, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A Penetration-Aspiration Scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Bayley, M.T.; Bohn, M.K.; Higgins, V.; Peladeau-Pigeon, M.; Kulasingam, V. Reference Values for Videofluoroscopic Measures of Swallowing: An Update. J. Speech Lang Hear Res. 2023, 66, 3804–3824. [Google Scholar] [CrossRef]
- Catàleg Laboratori de Referència de Catalunya. Available online: http://central.lrc.cat/cgi-bin/nph-mgwcgi.exe?App=CATALEG (accessed on 9 March 2023).
- Guidelines for Submitting a Nutritional Product to the ACBS-GOV.UK. Available online: https://www.gov.uk/government/publications/guidelines-for-submitting-a-nutritional-product-to-the-acbs (accessed on 9 March 2023).
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN Practical Guideline: Clinical Nutrition and Hydration in Geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Palomera, M.; Gomez, C.; Sar-Shalom, D.; Saiz, A.; Montoya, J.G.; Navajas, M.; Palomera, E.; Clavé, P. Oropharyngeal Dysphagia as a Risk Factor for Malnutrition and Lower Respiratory Tract Infection in Independently Living Older Persons: A Population-Based Prospective Study. Age Ageing 2012, 41, 376–381. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef]
- Bolivar-Prados, M.; Tomsen, N.; Hayakawa, Y.; Kawakami, S.; Miyaji, K.; Kayashita, J.; Clavé, P. Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia. Nutrients 2022, 14, 5028. [Google Scholar] [CrossRef]
- National Dysphagia Diet Task Force. National Dysphagia Diet: Standarization for Optimal Care; American Dietetic Association: Chicago, IL, USA, 2002. [Google Scholar]
- Kayashita, J.; Fujishima, I.; Fujitani, J.; Hironaka, S.; Kojo, A.; Mizukami, M.; Senda, N.; Moriwaki, M.; Watanabe, E. The Japanese Dysphagia Diet of 2021 by the Japanese Society of Dysphagia Rehabilitation. JJCRS 2022, 13, 64–77. [Google Scholar] [CrossRef]
- O’Keeffe, S.T. Use of Modified Diets to Prevent Aspiration in Oropharyngeal Dysphagia: Is Current Practice Justified? BMC Geriatr. 2018, 18, 167. [Google Scholar] [CrossRef]











| g TUGE /150 mL Water | Viscosity (mPa·s) at 50 s−1 | Viscosity (mPa·s) at 50 s−1 after Oral Incubation | α-Amylase Effect (%) | Viscosity (mPa·s) at 300 s−1 | Shear Thinning Effect (%) |
|---|---|---|---|---|---|
| 5 | 56.22 ± 4.23 | 56.10 ± 5.09 | −0.29 | 16.22 ± 0.88 | −71.15 |
| 10 | 154.20 ± 0 | 155.12 ± 18.81 | 0.59 | 37.69 ± 2.03 | −75.56 |
| 20 | 407.23 ± 11.66 | 374.10 ± 34.20 | −8.14 | 94.61 ± 0.87 | −76.77 |
| 30 | 614.20 ± 11.42 | 624.03 ± 36.43 | 1.60 | 143.8 ± 0.57 | −76.60 |
| g TUGE/75 g Water + 101.2 g Omnipaque | Viscosity (mPa·s) at 50 s−1 | Viscosity (mPa·s) at 50 s−1 after Oral Incubation | α-Amylase Effect (%) | Viscosity (mPa·s) at 300 s−1 | Shear Thinning Effect (%) |
|---|---|---|---|---|---|
| 5 | 49.41 ± 2.38 | 58.62 ± 3.48 | 18.64 | 18.74 ± 0.11 | −71.89 |
| 10 | 154.83 ± 10.22 | 152.29 ± 5.11 | −1.64 | 43.53 ± 3.95 | −71.89 |
| 20 | 439.33 ± 11.72 | 408.81 ± 9.29 | −6.95 | 131.30 ± 31.27 | −70.11 |
| 30 | 672.5 ± 35.62 | 596.86 ± 17.94 | −11.25 | 166.2 ± 1.70 | −75.29 |
| Impaired efficacy (%) | 100 |
| Impaired labial seal | 5.0 |
| Piecemeal deglutition | 88.3 |
| Oral residue | 71.7 |
| Pharyngeal residue | 76.3 |
| Impaired safety (%) | 100 |
| Penetrations PAS 2–5 (%) | 96.7 |
| Aspirations PAS 6–8 (%) | 25.0 |
| Mean max. PAS score | 4.1 ± 2.2 |
| Time to LVC (ms) | 294.5 ± 129.4 |
| Time to UESO (ms) | 174.0 ± 86.5 |
| Time to LVO (ms) | 859.9 ± 180.4 |
| Pre-Intervention | Post-Intervention | p-Value | |
|---|---|---|---|
| Serum: hematology | |||
| Hemoglobin (g/dL) | 14.3 ± 1.5 | 13.9 ± 1.6 | 0.016 |
| Hematocrit (%) | 42.8 ± 4.2 | 41.8 ± 4.3 | 0.040 |
| Mean red blood cell volume (fL) | 91.0 ± 4.8 | 86.3 ± 22.0 | 0.623 |
| White blood cell count (u/µL) | 6.7 ± 1.7 | 6.6 ± 1.9 | 0.831 |
| Platelets (u/µL) | 224.1 ± 53.6 | 216.6 ± 52.1 | 0.374 |
| Serum: biochemistry | |||
| Sodium (mmol/L) | 141.0 ± 2.9 | 140.9 ± 3.3 | 0.899 |
| Potassium (mmol/L) | 4.4 ± 0.4 | 4.3 ± 0.4 | 0.283 |
| Calcium (mg/dL) | 9.7 ± 0.6 | 9.4 ± 0.3 | 0.028 |
| Magnesium (mg/dL) | 2.1 ± 0.2 | 2.0 ± 0.2 | 0.006 |
| Chloride (mmol/L) | 102.0 ± 3.2 | 102.4 ± 4.0 | 0.387 |
| Bicarbonate (mmol/L) | 27.1 ± 3.3 | 27.4 ± 3.8 | 0.264 |
| ALT (U/L) | 21.5 ± 17.3 | 22.2 ± 20.7 | 0.969 |
| AST (U/L) | 21.8 ± 13.2 | 20.9 ± 9.0 | 0.989 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.591 |
| CRP (mg/dL) | 0.7 ± 1.1 | 0.3 ± 0.3 | 0.208 |
| Citrulline (µmol/L) | 43.8 ± 21.9 | 44.7 ± 13.9 | 0.867 |
| Urea (mg/dL) | 40.6 ± 13.1 | 36.9 ± 11.3 | 0.039 |
| Urea/creatinine ratio (mg/dL) | 46.1 ± 12.1 | 43.2 ± 13.2 | 0.144 |
| % Dehydrated * | 81.3 | 43.8 | 0.066 |
| Osmolarity (mmol/L) | 298.8 ± 5.3 | 298.4 ± 6.7 | 0.599 |
| % Dehydrated ** | 86.7 | 73.3 | 0.651 |
| Urine: Urinalysis | |||
| Urine osmolarity (mOsm/Kg) | 530.1 ± 215.4 | 501.9 ± 188.4 | 0.401 |
| Urine specific gravity (g/mL) | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.215 |
| Sodium (mmol/L) | 95.0 ± 50.9 | 106.8 ± 42.8 | 0.348 |
| BIA | |||
| Free fat mass (%) | 49.0 ± 7.3 | 49.2 ± 7.7 | 0.813 |
| Body fat (%) | 29.0 ± 6.4 | 27.6 ± 8.0 | 0.563 |
| Body cell mass (%) | 26.5 ± 4.7 | 31.6 ± 4.9 | >0.999 |
| TBW (%) | 36.3 ± 5.5 | 36.5 ± 5.7 | 0.844 |
| ECW (%) | 14.3 ± 2.3 | 14.4 ± 2.3 | 0.781 |
| ICW (%) | 21.9 ± 3.3 | 22.1 ± 3.4 | 0.844 |
| Texture | Taste | Smell | Appearance | |
|---|---|---|---|---|
Like a lot  | 12% | 31% | 37% | 13% |
Liked  | 44% | 25% | 19% | 25% |
In between  | 25% | 38% | 44% | 56% |
Dislike  | 19% | 6% | 0% | 6% |
Dislike a lot  | 0% | 0% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomsen, N.; Bolívar-Prados, M.; Ortega, O.; Clavé, P. Therapeutic Effect on Swallowing Function and on Hydration Status of a New Liquid Gum-Based Thickener in Independently-Living Older Patients with Oropharyngeal Dysphagia. Nutrients 2023, 15, 4621. https://doi.org/10.3390/nu15214621
Tomsen N, Bolívar-Prados M, Ortega O, Clavé P. Therapeutic Effect on Swallowing Function and on Hydration Status of a New Liquid Gum-Based Thickener in Independently-Living Older Patients with Oropharyngeal Dysphagia. Nutrients. 2023; 15(21):4621. https://doi.org/10.3390/nu15214621
Chicago/Turabian StyleTomsen, Noemí, Mireia Bolívar-Prados, Omar Ortega, and Pere Clavé. 2023. "Therapeutic Effect on Swallowing Function and on Hydration Status of a New Liquid Gum-Based Thickener in Independently-Living Older Patients with Oropharyngeal Dysphagia" Nutrients 15, no. 21: 4621. https://doi.org/10.3390/nu15214621
APA StyleTomsen, N., Bolívar-Prados, M., Ortega, O., & Clavé, P. (2023). Therapeutic Effect on Swallowing Function and on Hydration Status of a New Liquid Gum-Based Thickener in Independently-Living Older Patients with Oropharyngeal Dysphagia. Nutrients, 15(21), 4621. https://doi.org/10.3390/nu15214621







