Abstract
The objective of this study was to examine the correlation between gut microbiota and both age-related macular degeneration (AMD) and glaucoma. Mendelian randomization studies were conducted utilizing the data sourced from the genome-wide association study (GWAS) database for the gut microbiome, AMD, and glaucoma. Single nucleotide polymorphism (SNP) estimates were summarized through five Mendelian randomization (MR) methods. We utilized Cochran’s Q statistic to evaluate the heterogeneity of the instrumental variables (IVs). Additionally, we employed a “leave-one-out” approach to verify the stability of our findings. Inverse variance weighted (IVW) suggests that Eubacterium (oxidoreducens group) and Parabacteroides had a protective effect on AMD. Both weighted median and IVW suggest that Lachnospiraceae (NK4A136 group) and Ruminococcaceae (UCG009) had a protective effect on AMD. However, both weighted median and IVW suggest that Dorea had a risk effect on AMD. Similarly, The IVW of Eubacterium (ventriosum group) showed a risk effect on AMD. The weighted median of Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), and Roseburia had a risk effect on glaucoma. IVW suggested that Ruminococcaceae (UCG004) had a risk effect on glaucoma. Reverse MR analysis found a causal link between Eubacterium (nodatum group) and glaucoma. No causal relationships were found between AMD or glaucoma and the other mentioned bacterial groups. No significant heterogeneity or evidence of horizontal pleiotropy was detected. This study found that certain gut bacteria had protective effects on AMD, while others may be risk factors for AMD or glaucoma. Likewise, reverse MR found that glaucoma led to an increased abundance of certain gut bacteria. Further trials are needed to clarify the specific mechanisms involved.
1. Introduction
Age-related macular degeneration (AMD) and glaucoma are prevalent eye diseases that can lead to blindness on a global scale [1,2]. AMD, characterized by the gradual loss of central vision due to progressive damage to the macula, is a primary cause of blindness in the elderly. Clinically, AMD is divided into dry and wet types according to the nature of the lesion. Dry AMD is marked by deterioration of the macula, causing blurred or reduced central vision. Wet AMD is a more severe form of the disease, characterized by the growth of abnormal blood vessels under the macula, leading to rapid and severe vision loss. The development of AMD involves a complex interplay of genetic polymorphism, immune, metabolic, light damage, nutrition, and other factors. During the progression of AMD, microglia and macrophages migrate to the subretinal and choroidal regions, causing a local imbalance in the immune microenvironment of the retinal pigment epithelial cell layer, but the exact mechanism is still unclear [3]. For wet AMD, intraocular injection of anti-VEGF drugs is commonly used for treatment. However, dry AMD, which constitutes 80% of all AMD cases, currently lacks an effective treatment. Glaucoma is a progressive neurodegenerative disorder that is characterized by the gradual deterioration of retinal ganglion cells and their axons [4]. The pathogenesis of glaucoma is still under investigation. Although lowering intraocular pressure (IOP) is the key treatment method, it is difficult to prevent the progression of glaucoma. Current treatment strategies for both AMD and glaucoma still have significant limitations.
The gut microbiota, a diverse microbial community residing in the human intestine, plays a pivotal role in host metabolism, immune defense, and immune tolerance. The concept of the gut-eye axis, first introduced by Vujkovic-Cvijin et al. [5], highlights the significance of the gut microbiota in the development of various eye diseases, such as dry eye syndrome, glaucoma, AMD, uveitis, and diabetic retinopathy (DR). Zinkernagel et al. [6] conducted a study in which they sequenced the gut microbiota of both patients with wet AMD and a control group. The results revealed substantial variations in the abundance of specific gut microbiota between the two groups. Another clinical study on AMD analyzed the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and found 72 metabolic pathways with notable variations in gut microbiota between individuals with AMD and those in the control group, revealing the pathogenesis of gut microbiota involvement in AMD [7]. Gong et al. [8] sequenced the bacterial genomes in fecal samples of patients with primary open-angle glaucoma (POAG) using 16S rRNA V4 gene sequencing and found that the abundance of Prevotella and Escherichia coli was notably higher in POAG patients than in healthy individuals. They also discovered that the development of angle-closure glaucoma is influenced by the gut microbiota. In a comparison of the distribution of gut microbiota between patients with POAG and primary angle-closure glaucoma (PACG), distinct differences were observed [9]. However, most previous studies have struggled to confirm exposure duration and outcomes. Moreover, the correlation between gut microbiota and AMD or glaucoma may be influenced by various confounding factors, including the environment, age, lifestyle, and dietary habits. Therefore, the causal relationship between gut microbiota and both AMD and glaucoma is limited by these confounding factors.
Mendelian randomization (MR) is an analytical technique that utilizes genetic variability as a randomized tool to investigate the exposure of interest, providing valuable insights into the establishment of causality [10]. This innovative approach offers an opportunity to explore the potential causal connections between the gut microbiota and the incidence of AMD or glaucoma. Given that the transmission of genotypes from parents to offspring occurs randomly, the association between genetic variation and outcome remains unaffected by common confounding factors, thus rendering the causal pathway biologically plausible [11]. The application of MR has been widely employed to establish the causal links between the gut microbiota and ocular diseases [12,13,14]. Genome-Wide Association Studies (GWAS) represent a research technique used to identify associations between specific genetic variations and particular diseases across the entire genome. This approach is instrumental in facilitating a greater understanding of the genetic foundations of diseases and uncovering potential targets for treatment. In the current study, the MR analysis was performed using GWAS data, aiming to estimate the causal relationships between the gut microbiota and both AMD and glaucoma.
2. Materials and Methods
2.1. Data Sources
2.1.1. Gut Microbiota
Genetic variations for gut microbiota are available from the MRC Integrative Epidemiology Unit (IEU) Open GWAS database (http://gwas.mrcieu.ac.uk). The research involved 18,340 participants from 24 cohorts, primarily of European descent (n = 13,266). The study employed direct taxonomic binning to screen and categorize microbiota composition based on variable regions V4, V3–V4, and V1–V2 of the 16S rRNA gene. The researchers performed microbiota quantitative trait loci (mbQTL) mapping analysis in order to detect genetic variations in the host and determine their location on genetic loci linked to the abundance levels of bacterial taxa in the gut microbiota. The study identified a total of 131 genera with an average abundance greater than 1% at the lowest taxonomic level, including 12 unknown genera [15]. Therefore, the analysis included 119 genus-level taxonomic units.
2.1.2. AMD
Genetic variations of AMD are also available from the IEU Open GWAS database. A total of 105,248 individuals from 11 different data sources, such as the International AMD Genomics Consortium (IAMDGC) and UK Biobank (UKBB), were involved in the research. The participants were of European descent and consisted of 14,034 cases and 91,214 controls. The study utilized both GWAS and a candidate approach based on 14 early AMD variants to identify early AMD loci [16]. This study merged significant genome-wide mutations (p < 5 × 10−8) into independent loci. The genes that overlapped with the specified loci were utilized for further biological investigation. In addition, this study also used GCTA for approximate conditional analysis based on meta-analysis to find independent secondary signals in new AMD loci.
2.1.3. Glaucoma
Genetic variations of glaucoma are also obtained from the IEU Open GWAS database. The study performed GWAS on glaucoma and its key endophenotypes (including vertical cup-disc ratio and intraocular pressure) and included 351,696 individuals, these participants were of European descent, including 133,492 cases and 90,939 controls [17]. This study first conducted a GWAS analysis on glaucoma and its main phenotypes, including vertical cup-disc ratio (VCDR) and intraocular pressure. The data was combined through multiple trait analysis of GWAS (MTAG) to identify new loci. The reliability of the new loci was validated by two independent POAG cohorts. In addition, this study created a polygenic risk score (PRS) based on MTAG summary data and further confirmed its clinical significance in early and late glaucoma cohorts.
2.1.4. Instrumental Variables (IVs)
Ivs were selected based on the following selection criteria: (1) For the forward MR analysis, potential Ivs for each genus were identified as single nucleotide polymorphisms (SNPs) within the locus range, with a significant threshold of p < 5.0 × 10−6. For the reversed MR analysis, SNPs associated with each genus were selected as potential Ivs at a significant threshold (p < 5.0 × 10−8) within the locus range. (2) In order to determine the linkage disequilibrium (LD) between SNPs, the reference panel used was the European sample data from the 1000 Genomes project. SNPs with an R2 < 0.001 and a cluster window size of 10,000 kb were retained, with only the SNP having the smallest p-value. (3) Remove palindromic SNPs with intermediate allele frequencies.
2.2. Statistical Analysis
This study used five methods, including MR Egger, weighted median, inverse variance weighted (IVW), simple mode, and weighted mode to test the causal relationship between gut microbiota and AMD and glaucoma. The MR-Egger method is one of the commonly used randomization patterns in Mendelian randomization, which evaluates the impact of a factor on a disease based on a linear regression model. Egger regression is used to estimate bias and correct the results, resulting in more accurate causal estimates [18]. The Weighted median method is mainly used to handle biased samples, effectively reducing sample bias and improving the reliability and accuracy of randomized experiments [19]. The IVW merges the Wald estimates of each SNP using meta-analysis to provide a comprehensive estimation of the influence of gut microbiota on AMD and glaucoma. Its advantage is that it can simultaneously consider the effects of multiple genotypes on the study factor, thereby improving the accuracy of causal inference. The IVW result will be unbiased if there is no horizontal pleiotropy [20]. Both the simple mode and weighted mode are frequently implemented randomization patterns that eliminate interfering factors in experimental results by randomly grouping [21]. In this study, Cochran’s IVW Q statistic was utilized to quantify the heterogeneity of Ivs. The MR-PRESSO analysis was employed to identify and mitigate the effects of horizontal pleiotropy by eliminating notable outliers. Furthermore, we conducted the “leave-one-out” analysis to detect potential heterogeneous SNPs by sequentially excluding each instrumental SNP. To evaluate the potential causal relationship between gut microbiota and both AMD and glaucoma, a reverse MR analysis of the two eye diseases with gut microbiota was performed. The method is consistent with the forward MR, and the threshold for significant gene locus selection is p < 5 × 10−8. All statistical analyses were performed using R version 4.2.2. The MR analyses were conducted using TwosampleMR [22] and MR-PRESSO [23]. The flowchart is presented in Figure 1.
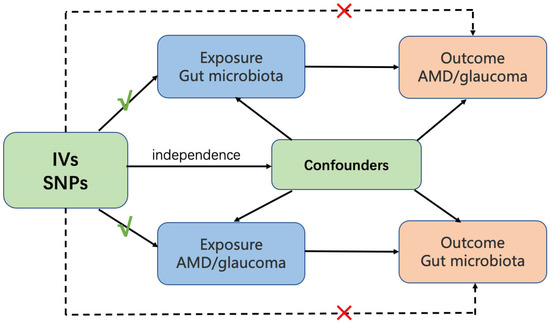
Figure 1.
Overview of MR analysis process and major assumptions. AMD, age-related macular degeneration. Ivs, instrumental variables. SNPs, single nucleotide polymorphisms.
3. Results
119 bacterial genera were analyzed using 774 SNPs as Ivs based on the specified selection criteria. Detailed information about the selected Ivs is provided in Tables S1 and S2. Tables S3 and S4 present the MR estimates of the impact of bacteria on AMD and glaucoma, respectively. Table 1 outlines the five MR methods used to investigate the relationship between gut microbiota and AMD, while Table 2 exhibits the five MR methods applied to explore the connection between six gut microbiota and glaucoma. Figure 2 illustrates scatter plots depicting the causal associations between gut microbiota (specifically, Dorea, Eubacterium (oxidoreducens group), Eubacterium (ventriosum group), Lachnospiraceae (NK4A136 group), Parabacteroides and Ruminococcaceae (UCG009)) and AMD. Similarly, Figure 3 displayed scatter plots illustrating the casual relationship between gut microbiota and glaucoma, focusing on specific bacterial genera such as Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), Roseburia and Ruminococcaceae (UCG004). The IVW estimate indicates that Eubacterium (oxidoreducens group) (OR = 0.84, 95% CI, 0.70–1.00, p = 0.049) and Parabacteroides (OR = 0.70, 95% CI, 0.51–0.96, p = 0.025) may have a protective effect against AMD. Similarly, both the weighted median and IVW estimates suggest that Lachnospiraceae (NK4A136 group) (weighted median OR = 0.81, 95% CI, 0.66–0.99, p = 0.041; IVW OR = 0.84, 95% CI, 0.71–0.98, p = 0.031) and Ruminococcaceae (UCG009) (weighted median OR = 0.76, 95% CI, 0.62–0.94, p = 0.011; IVW OR = 0.83, 95% CI, 0.70–0.99, p = 0.036) may also provide protection against AMD. Conversely, both the weighted median and IVW estimates suggest that Dorea may increase the risk of AMD (weighted median OR = 1.50, 95% CI, 1.08–2.08, p = 0.02; IVW OR = 1.46, 95% CI, 1.15–1.85, p = 0.002). The IVW estimate also indicates that Eubacterium (ventriosum group) (OR = 1.23, 95% CI, 1.01–1.50, p = 0.038) may increase the risk of AMD. In terms of glaucoma, the weighted median estimates suggest that Eubacterium (nodatum group) (OR = 1.16, 95% CI, 1.01–1.35, p = 0.041), Lachnospiraceae (NC2004 group) (OR = 1.24, 95% CI, 1.03–1.51, p = 0.026), and Roseburia (OR = 1.28, 95% CI, 1.03–1.59, p = 0.028) may increase the risk. The IVW estimate also suggests that Ruminococcaceae (UCG004) may increase the risk of glaucoma (OR = 1.21, 95% CI, 1.02–1.43, p = 0.029).

Table 1.
MR estimate for the association between gut microbiota and AMD.

Table 2.
MR estimate for the association between gut microbiota and glaucoma.
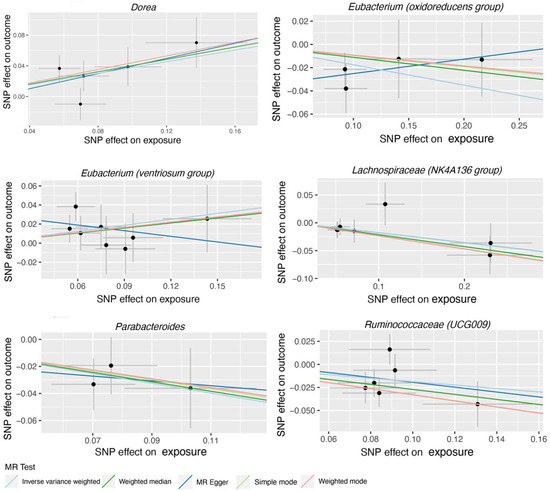
Figure 2.
Scatter plots for the casual association between gut microbiota (Dorea, Eubacterium (oxidoreducens group), Eubacterium (ventriosum group), Lachnospiraceae (NK4A136 group), Parabacteroides and Ruminococcaceae (UCG009), and AMD. Each point in the scatter plot represents an SNP. The effect of the same SNP on exposure is placed on the horizontal axis, and the effect on outcome is placed on the vertical axis. The vertical and horizontal lines show the 95% confidence interval (CI) for each SNP. At this point, the slope of the solid line in the plot is each MR estimate. The light blue, light green, dark blue, green, and pink lines correspond to the Inverse Variance Weighted, Simple Mode, MR-Egger, Weighted Median, and Weighted Model methods, respectively. AMD, age-related macular degeneration. SNP, single nucleotide polymorphism.
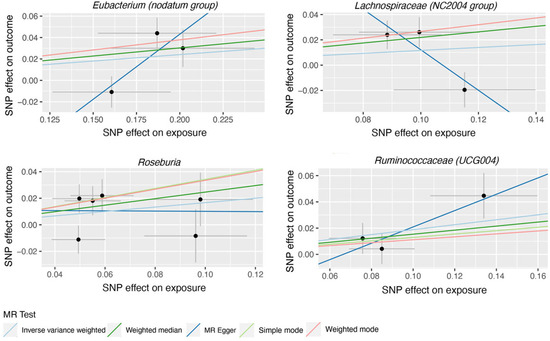
Figure 3.
Scatter plots for the casual association between gut microbiota (Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), Roseburia and Ruminococcaceae (UCG004)) and glaucoma. Each point in the scatter plot represents an SNP. The effect of the same SNP on exposure is placed on the horizontal axis, and the effect on outcome is placed on the vertical axis. The vertical and horizontal lines show the 95% CI for each SNP. At this point, the slope of the solid line in the plot is each MR estimate. The light blue, light green, dark blue, green, and pink lines correspond to the Inverse Variance Weighted, Simple Mode, MR-Egger, Weighted Median, and Weighted Model methods, respectively. SNP, single nucleotide polymorphism.
Based on the data provided in Table S5, no significant heterogeneity was detected by Cochran’s IVW Q test. Additionally, the MR-Egger regression intercept analysis, as displayed in Table S6, did not reveal any obvious directional horizontal pleiotropy. The MR-PRESSO method also did not detect any noteworthy exceptional values. Thus, there was insufficient evidence to support the presence of horizontal pleiotropy in the association between these gut microbiota compositions and the two diseases under investigation. To validate the influence of each SNP on the overall causal estimate, a leave-one-out method was employed. Each SNP was systematically removed, followed by the repetition of MR analysis on the remaining SNPs. Figure 4 depicts the leave-one-out plots, illustrating the causal association between gut microbiota (Dorea, Eubacterium (oxidoreducens group), Eubacterium (ventriosum group), Lachnospiraceae (NK4A136 group), Parabacteroides and Ruminococcaceae (UCG009), and AMD. Similarly, Figure 5 illustrates the leave-one-out plots for the causal association between gut microbiota (Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), Roseburia and Ruminococcaceae (UCG004)) and glaucoma. These findings consistently support a significant causal connection among the computed results of all SNPs.
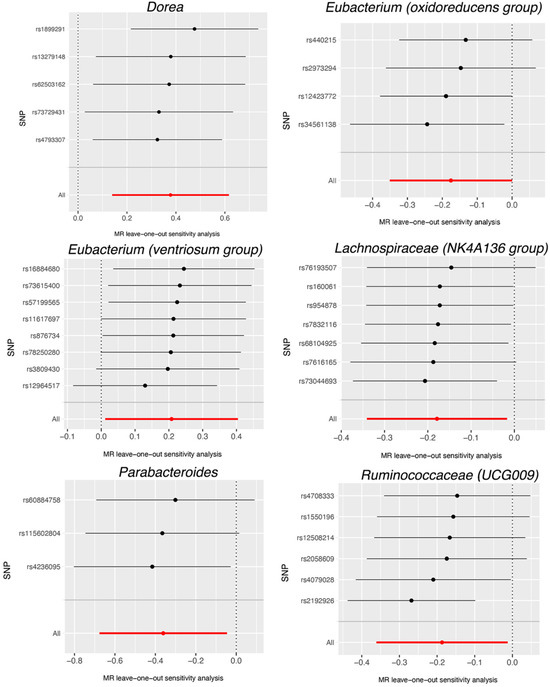
Figure 4.
Leave-one-out plots for the causal association between gut microbiota (Dorea, Eubacterium (oxidoreducens group), Eubacterium (ventriosum group), Lachnospiraceae (NK4A136 group), Parabacteroides and Ruminococcaceae (UCG009), and AMD. The leave-one-out plot presents how the causal estimates (point with horizontal circle) for the effect of gut on AMD were influenced by the removal of a single variant. The bars indicate the confidence interval of MR estimates. AMD, age-related macular degeneration. SNP, single nucleotide polymorphism.
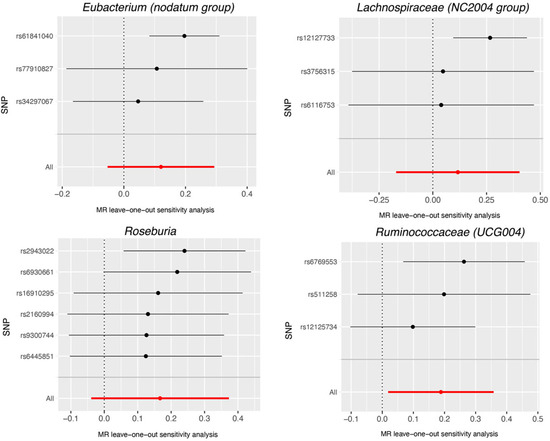
Figure 5.
Leave-one-out plots for the causal association between gut microbiota (Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), Roseburia and Ruminococcaceae (UCG004), and glaucoma. The leave-one-out plot presents how the causal estimates (point with horizontal circle) for the effect of gut on glaucoma were influenced by the removal of a single variant. The bars indicate the confidence interval of MR estimates. SNP, single nucleotide polymorphism.
Reverse MR analysis was conducted on the gut microbiota compositions that were identified to have a causal link with AMD and glaucoma in the forward MR analysis. Table 3 lists five MR Methods used to analyze the relationship between AMD and the above gut microbiota. Table 4 lists five MR Methods used to analyze the relationship between glaucoma and the above gut microbiota. The results indicated a correlation between Eubacterium (nodatum group) and glaucoma. As shown in Table 4, the weighted median estimate suggested that glaucoma had a risk effect on Eubacterium (nodatum group) (OR = 1.15, 95% CI, 1.04–1.28, p = 0.006). However, we did not find any significant causal association between the two eye diseases and the other mentioned gut microbiota. The Cochran’s IVW Q test results indicated no substantial heterogeneity of these IVs. Furthermore, the results of the MR-Egger regression intercept analysis did not reveal any significant evidence of directional horizontal pleiotropy (Tables S7 and S8).

Table 3.
MR estimate for the association between AMD and the above gut microbiota.

Table 4.
MR estimate for the association between glaucoma and the above gut microbiota.
4. Discussion
An MR analysis was conducted in this study using summary statistics data extracted from the IEU Open GWAS Project on gut microbiome, AMD, and glaucoma. The objective was to identify the causal relationship between gut microbiome and AMD/glaucoma. It was found some gut microbiotas (including Eubacterium (oxidoreducens group), Parabacteroides, Lachnospiraceae (NK4A136 group), and Ruminococcaceae (UCG009) that had a significant protective effect on AMD, while Dorea and Eubacterium (ventriosum group) had a risk effect on AMD. Gut microbiotas (including Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), Roseburia, and Ruminococcaceae (UCG004) were all found to have a risk effect on glaucoma. In recent times, mounting evidence has bolstered the understanding of the “gut-eye axis” in the development of several eye disorders [5]. Various cells within the eye, including microglial cells, perivascular macrophages, dendritic cells, and retinal pigment epithelium (RPE) cells express pattern recognition receptors (PRRs) that can be activated by gut-derived cells, thereby provoking ocular inflammation [24]. In addition, microbial byproducts have the potential to trigger autoimmune responses localized within the eye by activating retina-specific signaling pathways [25]. The inseparable link between the gut microbiota and AMD has been reviewed by Lima-Fontes et al. [7]. As for glaucoma, it is a neurodegenerative disease with multifactorial origins, involving inflammation and immune responses in its pathogenesis. Zhang et al. conducted a metagenomic analysis of the gut microbiota in individuals with glaucoma, further confirming the crucial role of the gut microbiota and its derivatives in the initiation and progression of glaucoma [26].
Dorea is the main gas-producing bacterium in the gut. Previous metabolomics analysis has shown that Dorea is involved in the formation of the gut barrier, affects innate immunity, participates in the regulation of the malignant tumor cell cycle, and hosts adaptive immunity [27]. Dorea has been found to be associated with fungal keratitis (FK) [28], Sjögren’s syndrome (SS), and dry eye syndrome (DES) [29]. However, its effects on AMD and glaucoma are rarely reported. Eubacterium, another important genus in the human gut microbiota, is involved in nutrient metabolism and the maintenance of gut homeostasis. It specifically produces short-chain fatty acids (SCFAs), including butyrate, which serves as a vital source of nutrients and energy for the intestinal epithelium. Previous studies have found causal relationships between Eubacterium and diabetic retinopathy (DR) and optic neuritis (ON) [12,14]. In addition, there have been reports of interactions between Eubacterium and AMD, but its correlation with glaucoma has not been reported. Lachnospiraceae and Ruminococcaceae are both core genera of human gut microbiota and may be potentially beneficial bacteria involved in carbohydrate metabolism, producing butyrate as the main source of energy for the host. The increase in their abundance is related to aging [30]. In eye diseases, Lachnospiraceae and Ruminococcaceae are related to bacterial keratitis and uveitis [31,32] and Lachnospiraceae is also related to fungal keratitis and mucous membrane pemphigoid [27,33]. In addition, studies have reported interactions between Lachnospiraceae, Ruminococcaceae, and AMD, but their causal relationship with AMD is still unknown. Their interaction with glaucoma has not been reported yet. Parabacteroides has been less studied in eye diseases, and its effects on AMD and glaucoma are rarely reported. Zysset-Burri et al. [6] compared 57 patients with neovascular AMD to 58 healthy controls in a study investigating the correlation between gut microbiota and AMD. The results indicated that non-AMD patients had significantly higher levels of Oscillibacter and Bacteroides. However, it should be noted that the study did not establish a causal relationship between Oscillibacter or Bacteroides and AMD, possibly due to different types of AMD. Zinkernagel et al. [34] analyzed the gut microbiome of both AMD patients and healthy controls and discovered an increased abundance of Oscillibacter, Anaerotruncus, Eubacterium ventriosum, and Ruminococcus torques in AMD patients. On the other hand, Bacteroides eggerthii was found to be more prevalent in the control group. The study further revealed that Eubacterium ventriosum had a risk effect on AMD. Another study found decreased levels of Oscillospira, Blautia, and Dorea in AMD patients [35]. Notably, this study also found that Dorea had a risk effect on AMD. It was reported that the abundance of Bacteroides and Prevotella is associated with POAG. Gong et al. [8] used 16S rRNA sequencing to detect the fecal microbiota of 30 POAG patients and 30 healthy individuals. Their findings revealed a notable increase in Escherichia coli, as well as unidentified Enterobacteriaceae and Prevotellaceae in POAG patients. Conversely, Bacteroides plebeius and Megamonas were notably reduced. Another study demonstrated a noteworthy reduction in the distribution of Blautia and Fusicatenibacter in fecal samples of patients with primary angle-closure glaucoma (PACG) [9]. However, this study did not establish a significant causal relationship between these bacterial genera and glaucoma, potentially due to variances in the ethnicity of the study participants and disease classification. The above studies mostly focused on Chinese people, while the present study mainly included Europeans.
There are several advantages to this study. By analyzing MR, causal connections between gut microbiota and both AMD and glaucoma can be determined, which eliminates confounding factors and reverses causal inference. We obtained genetic variation data of gut microbiota from the largest GWAS meta-analysis to ensure the reliability of the MR analysis instrument. To detect and eliminate horizontal pleiotropy, we utilized MR-PRESSO and MR-Egger regression interval trial tests. Additionally, we used an MR design and non-overlapping exposure and outcome summary-level data to avoid bias [36]. Nonetheless, this study also has some limitations. Since summary statistics data were used instead of raw data, it is not possible to conduct subgroup analyses, such as distinguishing between early AMD and late AMD, and different types of glaucoma cannot be performed. As the exposure dataset only provides genus-level classification, we are unable to investigate the potential causal link between gut microbiota and AMD/glaucoma at the species level. In order to perform sensitivity analysis and horizontal pleiotropy testing, additional genetic variations must be incorporated as IVs. The sample size for gut microbiota is limited, and instrument bias may weakly influence the results of reverse MR analysis, making it difficult to completely exclude reverse causality. Although most participants in the GWAS gut microbiota data meta-analysis were of European descent, population stratification may still pose a potential confounding factor, rendering the study results not entirely generalizable to non-European populations. To enhance the applicability of deeper investigations into the causal relationships between gut microbiota and AMD/glaucoma, it is recommended to conduct studies in diverse European and non-European populations.
From a clinical practice perspective, the results of this study may contribute to the development of new prevention and treatment strategies for AMD and glaucoma. For example, by adjusting the balance of the gut microbiota, it may be possible to reduce the risk of developing AMD or glaucoma or to improve the severity of these diseases. This can be achieved through changes in diet, supplementation with probiotics, and other methods. However, research in this field is still in its early stages, and more studies are needed to confirm these preliminary findings and to determine the most effective intervention strategies. In addition, the complexity and individual variability of the gut microbiota presents a challenge, which may require personalized treatment plans.
5. Conclusions
In conclusion, the present study identified causal relationships between certain gut microbiota including Dorea, Eubacterium (oxidoreducens group), Eubacterium (ventriosum group), Lachnospiraceae (NK4A136 group), Parabacteroides, and Ruminococcaceae (UCG009) with AMD. It also identified a connection between Eubacterium (nodatum group), Lachnospiraceae (NC2004 group), Roseburia, and Ruminococcaceae (UCG004) with glaucoma. The exact protective or risk mechanisms these gut microbiota have on AMD and glaucoma necessitate further Randomized Controlled Trial (RCT) studies. In addition, a reverse MR revealed a causal relationship between glaucoma and Eubacterium (nodatum group) but found no such links between AMD or glaucoma and the other bacterial groups mentioned. Nonetheless, the possibility that AMD or glaucoma could influence the gut microbiota cannot be dismissed, and further research is needed to validate these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15214646/s1, Table S1: SNPs information of bacteria with AMD; Table S2: SNPs information of bacteria with glaucoma; Table S3: MR estimates of bacteria on AMD; Table S4: MR estimates of bacteria on glaucoma. Table S5: Heterogeneity tests of bacteria on AMD and glaucoma; Table S6: Horizontal pleiotropy tests of bacteria on AMD and glaucoma; Table S7: Heterogeneity and horizontal pleiotropy tests of AMD on bacteria. Table S8: Heterogeneity and horizontal pleiotropy tests of glaucoma on bacteria.
Author Contributions
Conceptualization C.L. and P.L.; methodology, C.L.; software, C.L.; validation, C.L. and P.L.; resources, C.L.; data curation, C.L.; writing—original draft preparation, C.L. and P.L.; writing—review and editing, C.L. and P.L.; visualization, C.L.; supervision, P.L.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiangsu Provincial Medical Innovation Team (grant No. CXTDA2017039) and the National Natural Science Foundation in China (grant No. 81671641, 82271113) to P.L.
Institutional Review Board Statement
This article does not contain any studies involving human participants performed by any of the authors.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data are available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.; Xie, Y.; Yuan, Y.; Xiong, R.; Hu, Y.; Ning, K.; Ha, J.; Wang, W.; Han, X.; He, M. The Mediterranean Diet and Age-Related Eye Diseases: A Systematic Review. Nutrients 2023, 15, 2043. [Google Scholar] [PubMed]
- Yao, X.; Yang, H.; Han, H.; Kou, X.; Jiang, Y.; Luo, M.; Zhou, Y.; Wang, J.; Fan, X.; Wang, X.; et al. Genome-wide analysis of genetic pleiotropy and causal genes across three age-related ocular disorders. Hum. Genet. 2023, 142, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.C.; Ma, J.Y.W.; Jobling, A.I.; Brandli, A.; Greferath, U.; Fletcher, E.L.; Vessey, K.A. Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef] [PubMed]
- Prokosch, V.; Li, P.; Shi, X. Glaucoma as a Neurodegenerative and Inflammatory Disease. Das Glaukom ist eine neurodegenerative und neuroinflammatorische Erkrankung. Klin. Monatsblätter Augenheilkd. 2023, 240, 125–129. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Sklar, J.; Jiang, L.; Natarajan, L.; Knight, R.; Belkaid, Y. Host variables confound gut microbiota studies of human disease. Nature 2020, 587, 448–454. [Google Scholar] [CrossRef]
- Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Largiadèr, C.R.; Wittwer, M.; Wolf, S.; Zinkernagel, M.S. Associations of the intestinal microbiome with the complement system in neovascular age-related macular degeneration. NPJ Genom. Med. 2020, 5, 34. [Google Scholar] [CrossRef]
- Lima-Fontes, M.; Meira, L.; Barata, P.; Falcão, M.; Carneiro, Â. Gut microbiota and age-related macular degeneration: A growing partnership. Surv. Ophthalmol. 2022, 67, 883–891. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, S.; Li, Q.; Zuo, C.; Gao, X.; Zheng, B.; Lin, M. Gut microbiota compositional profile and serum metabolic phenotype in patients with primary open-angle glaucoma. Exp. Eye Res. 2020, 191, 107921. [Google Scholar] [CrossRef]
- Gong, H.; Zeng, R.; Li, Q.; Liu, Y.; Zuo, C.; Ren, J.; Zhao, L.; Lin, M. The profile of gut microbiota and central carbon-related metabolites in primary angle-closure glaucoma patients. Int. Ophthalmol. 2022, 42, 1927–1938. [Google Scholar] [CrossRef]
- Greenland, S. An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 2000, 29, 722–729. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Mendelian Randomization: Methods for Causal Inference Using Genetic Variants; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Liu, K.; Zou, J.; Fan, H.; Hu, H.; You, Z. Causal effects of gut microbiota on diabetic retinopathy: A Mendelian randomization study. Front. Immunol. 2022, 13, 930318. [Google Scholar] [CrossRef] [PubMed]
- Nusinovici, S.; Li, H.; Thakur, S.; Baskaran, M.; Tham, Y.-C.; Zhou, L.; Sabanayagam, C.; Aung, T.; Silver, D.; Fan, Q.; et al. High-Density Lipoprotein 3 Cholesterol and Primary Open-Angle Glaucoma: Metabolomics and Mendelian Randomization Analyses. Ophthalmology 2022, 129, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wu, P.; Zou, J.; Fan, H.; Hu, H.; Cheng, Y.; He, F.; Liu, J.; You, Z. Mendelian randomization analysis reveals causal relationships between gut microbiome and optic neuritis. Hum. Genet. 2022, 28, 1139–1148. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Garay, J.A.R.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.W.; Grassmann, F.; Brandl, C.; Kiel, C.; Günther, F.; Strunz, T.; Weidner, L.; Zimmermann, M.E.; Korb, C.A.; Poplawski, A.; et al. Genome-wide association meta-analysis for early age-related macular degeneration highlights novel loci and insights for advanced disease. BMC Med. Genom. 2020, 13, 120. [Google Scholar] [CrossRef]
- Craig, J.E.; Han, X.; Qassim, A.; Hassall, M.; Bailey, J.N.C.; Kinzy, T.G.; Khawaja, A.P.; An, J.; Marshall, H.; Gharahkhani, P.; et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat. Genet. 2020, 52, 160–166. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Pocock, S.J.; Simon, R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975, 31, 103–115. [Google Scholar] [CrossRef]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef]
- Scott, N.W.; McPherson, G.C.; Ramsay, C.R.; Campbell, M.K. The method of minimization for allocation to clinical trials. A review. Control. Clin. Trials 2002, 23, 662–674. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.-C.; Yamane, H.; Honda, K.; et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 2015, 43, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Lu, Y. Gut microbiota and derived metabolomic profiling in glaucoma with progressive neurodegeneration. Front. Cell. Infect. Microbiol. 2022, 12, 968992. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Ranjith, K.; Dutta, A.; Pinna, N.K.; Mande, S.S.; Sharma, S.; Garg, P.; Murthy, S.I.; Shivaji, S. Alterations in the gut bacterial microbiome in fungal Keratitis patients. PLoS ONE 2018, 13, e0199640. [Google Scholar] [CrossRef]
- Moon, J.; Choi, S.H.; Yoon, C.H.; Kim, M.K. Gut dysbiosis is prevailing in Sjögren’s syndrome and is related to dry eye severity. PLoS ONE 2020, 15, e0229029. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging—Relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef]
- Jayasudha, R.; Chakravarthy, S.K.; Prashanthi, G.S.; Sharma, S.; Garg, P.; I Murthy, S.; Shivaji, S. Alterations in gut bacterial and fungal microbiomes are associated with bacterial Keratitis, an inflammatory disease of the human eye. J. Biosci. 2018, 43, 835–856. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef]
- Low, L.; Suleiman, K.; Shamdas, M.; Bassilious, K.; Poonit, N.; Rossiter, A.E.; Acharjee, A.; Loman, N.; Murray, P.I.; Wallace, G.R.; et al. Gut Dysbiosis in Ocular Mucous Membrane Pemphigoid. Front. Cell. Infect. Microbiol. 2022, 12, 780354. [Google Scholar] [CrossRef]
- Zinkernagel, M.S.; Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Leichtle, A.B.; Largiadèr, C.R.; Fiedler, G.M.; Wolf, S. Association of the Intestinal Microbiome with the Development of Neovascular Age-Related Macular Degeneration. Sci. Rep. 2017, 7, 40826. [Google Scholar] [CrossRef] [PubMed]
- Lin, P. Importance of the intestinal microbiota in ocular inflammatory diseases: A review. Clin. Exp. Ophthalmol. 2019, 47, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).