Deficiency of apoA-IV in Female 129X1/SvJ Mice Leads to Diet-Induced Obesity, Insulin Resistance, and Decreased Energy Expenditure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Body Composition
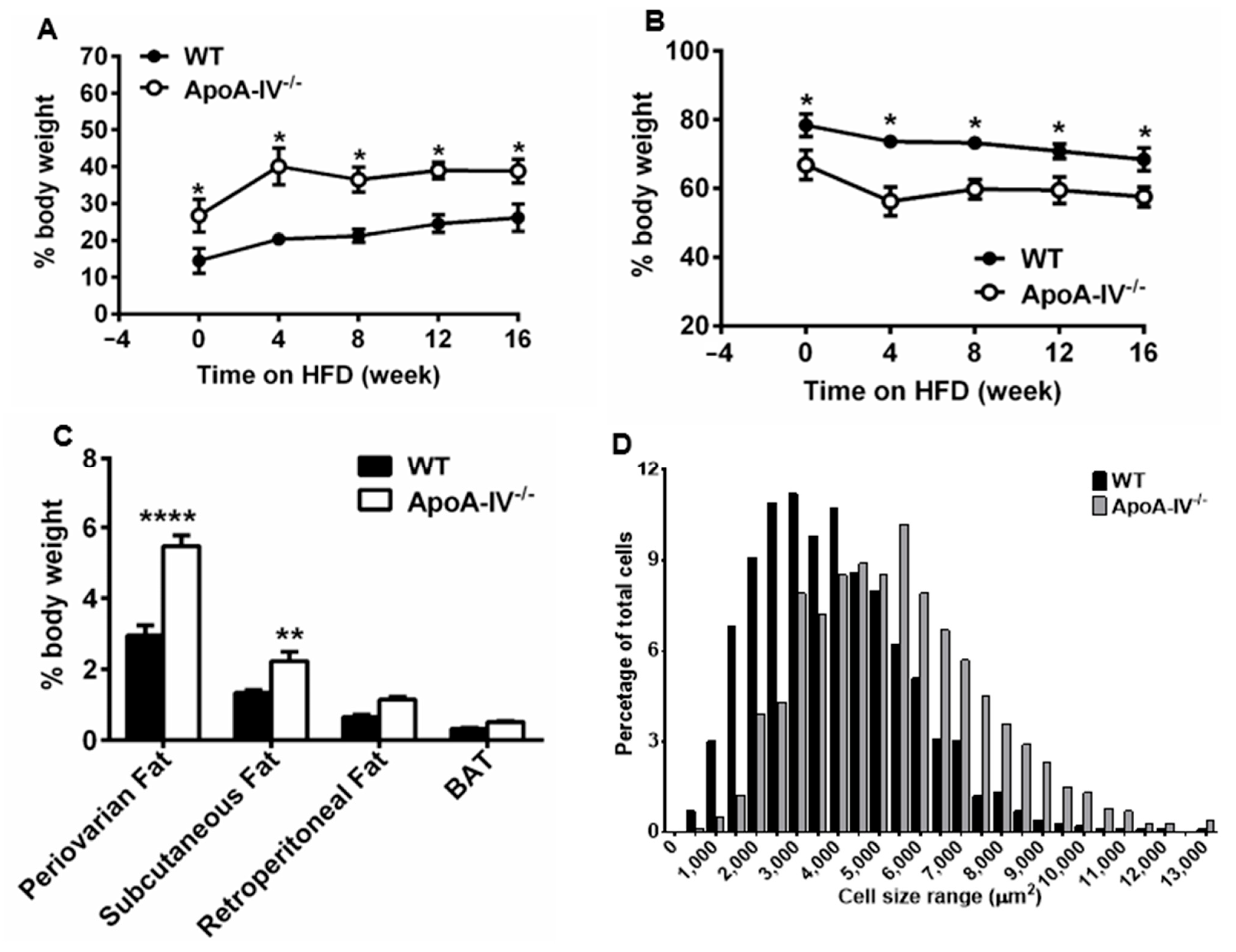
2.3. Quantification of Adipocyte Size
2.4. Measurement of Leptin and Adiponectin Levels
2.5. Measurement of Plasma Lipid Contents
2.6. Analysis of Indirect Calorimetry and Physical Activity
2.7. Glucose Tolerance Assay
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
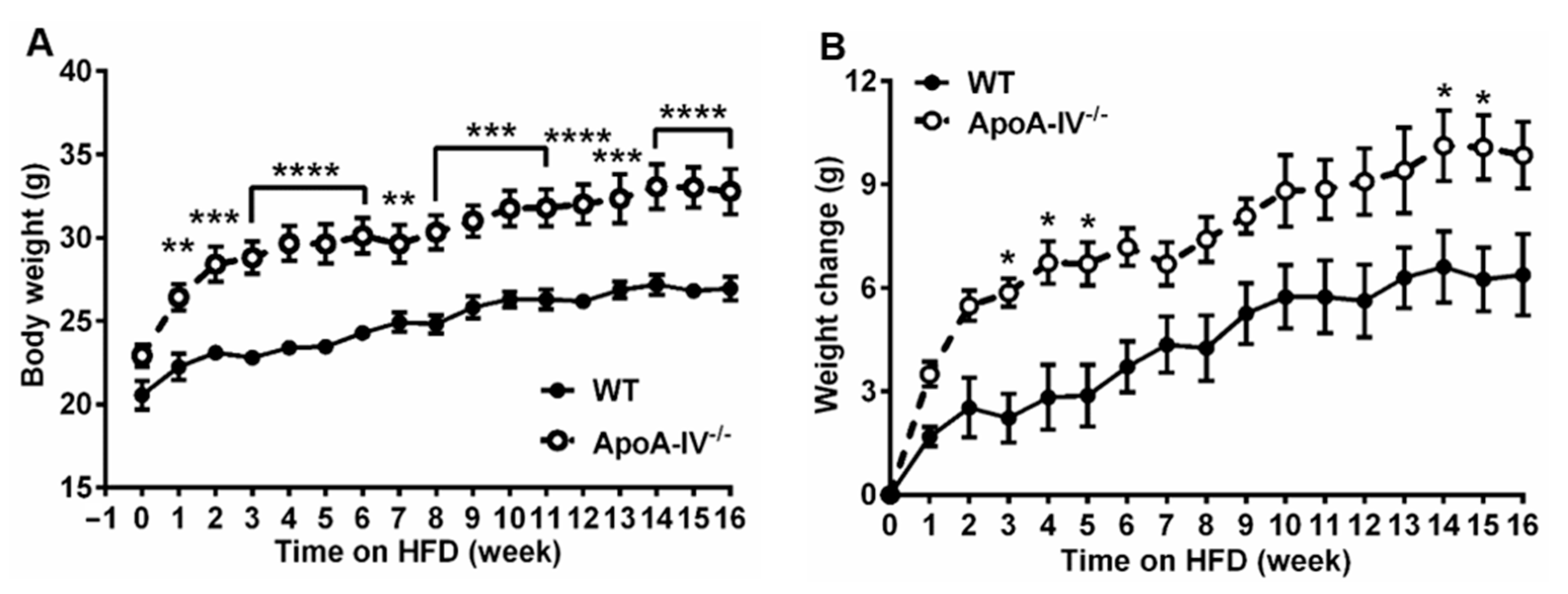
3.1. ApoA-IV−/− Mice Develop Obesity under High Fat Diet
3.2. ApoA-IV−/− Mice Contain Higher Leptin but Lower Cholesterol and Phospholipid Levels
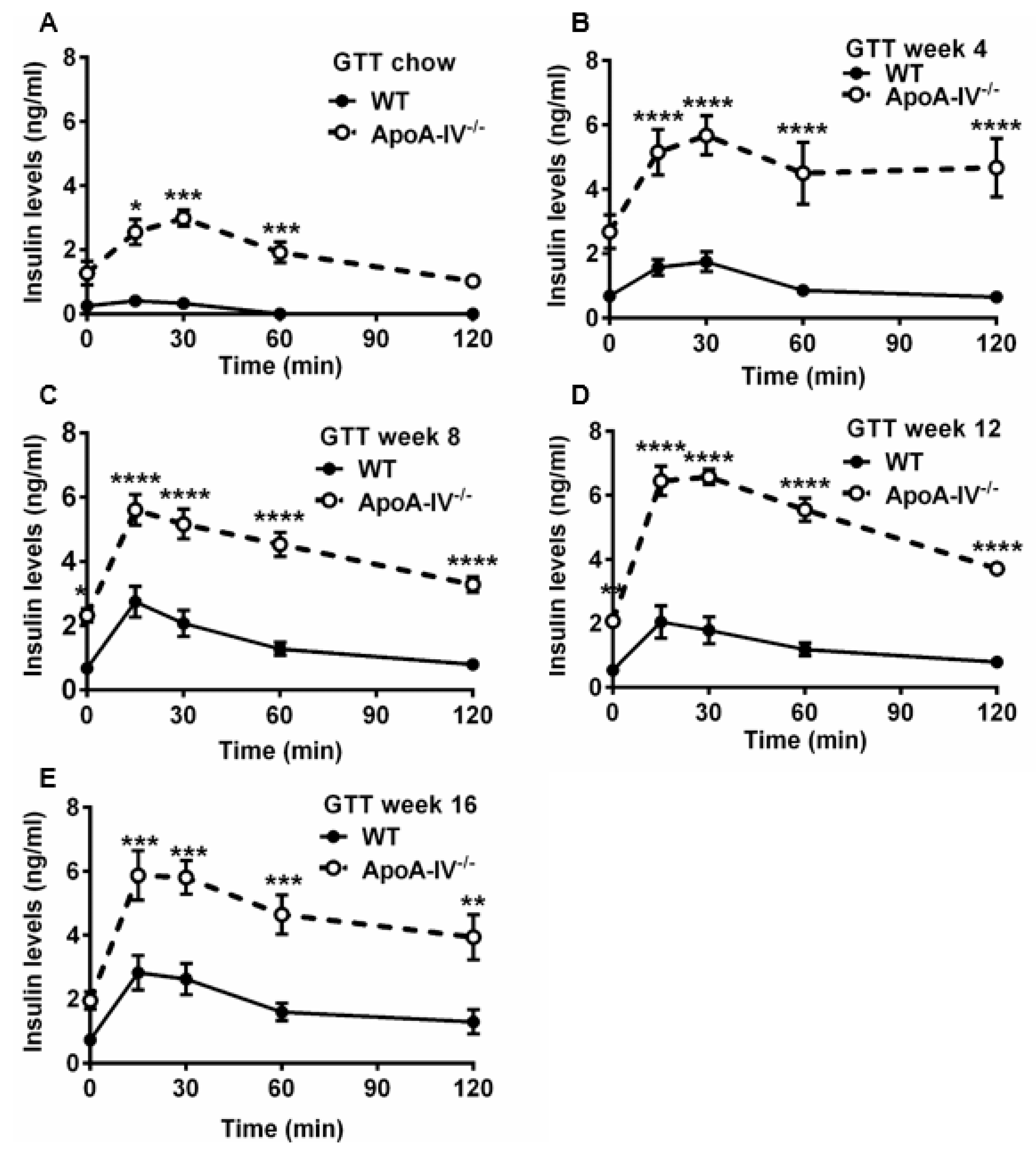
3.3. Obese ApoA-IV−/− Mice Are Glucose Intolerant and Insulin Resistant
3.4. Energy Expenditure but Not Food Intake Is Reduced in ApoA-IV−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Global Obesity Epidemic Worsening. JAMA 2017, 318, 603. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B. The Effect of Obesity on Health Outcomes. Mol. Cell. Endocrinol. 2010, 316, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Travieso, J.L.; Hopeman, M.M. Weighing the Impact of Obesity on Female Reproductive Function and Fertility. Nutr. Rev. 2013, 71 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Metwally, M.; Li, T.C.; Ledger, W.L. The Impact of Obesity on Female Reproductive Function. Obes. Rev. 2007, 8, 515–523. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen Modulates Abdominal Adiposity and Protects Female Mice from Obesity and Impaired Glucose Tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Boston, B.A.; Cone, R.D. Characterization of Melanocortin Receptor Subtype Expression in Murine Adipose Tissues and in the 3T3-L1 Cell Line. Endocrinology 1996, 137, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Chen, A.S.; Marsh, D.J.; Trumbauer, M.E.; Frazier, E.G.; Guan, X.M.; Yu, H.; Rosenblum, C.I.; Vongs, A.; Feng, Y.; Cao, L.; et al. Inactivation of the Mouse Melanocortin-3 Receptor Results in Increased Fat Mass and Reduced Lean Body Mass. Nat. Genet. 2000, 26, 97–102. [Google Scholar] [CrossRef]
- Ste Marie, L.; Miura, G.I.; Marsh, D.J.; Yagaloff, K.; Palmiter, R.D. A Metabolic Defect Promotes Obesity in Mice Lacking Melanocortin-4 Receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 12339–12344. [Google Scholar] [CrossRef]
- Cohen, P.; Zhao, C.; Cai, X.; Montez, J.M.; Rohani, S.C.; Feinstein, P.; Mombaerts, P.; Friedman, J.M. Selective Deletion of Leptin Receptor in Neurons Leads to Obesity. J. Clin. Investig. 2001, 108, 1113–1121. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased Adipose Tissue in Male and Female Estrogen Receptor-Alpha Knockout Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Habibi, J.; Mugerfeld, I.; Garro, M.; Sowers, J.R. Loss of Estrogen Receptor α Signaling Leads to Insulin Resistance and Obesity in Young and Adult Female Mice. Cardiorenal Med. 2012, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Utermann, G.; Beisiegel, U. Apolipoprotein A-IV: A Protein Occurring in Human Mesenteric Lymph Chylomicrons and Free in Plasma. Isolation and Quantification. Eur. J. Biochem. 1979, 99, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Cook, V.R.; Rao, A.; Weinberg, R.B. Impact of Murine Intestinal Apolipoprotein A-IV Expression on Regional Lipid Absorption, Gene Expression, and Growth. J. Lipid Res. 2011, 52, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Glickman, R.M.; Riley, J.W.; Quinet, E. Human Apolipoprotein A-IV. Intestinal Origin and Distribution in Plasma. J. Clin. Investig. 1980, 65, 911–919. [Google Scholar] [CrossRef]
- Ohta, T.; Fidge, N.H.; Nestel, P.J. Studies on the In Vivo and In Vitro Distribution of Apolipoprotein A-IV in Human Plasma and Lymph. J. Clin. Investig. 1985, 76, 1252–1260. [Google Scholar] [CrossRef]
- Qu, J.; Ko, C.-W.; Tso, P.; Bhargava, A. Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes. Cells 2019, 8, 319. [Google Scholar] [CrossRef]
- Xu, X.R.; Wang, Y.; Adili, R.; Ju, L.; Spring, C.M.; Jin, J.W.; Yang, H.; Neves, M.A.D.; Chen, P.; Yang, Y.; et al. Apolipoprotein A-IV Binds AIIbβ3 Integrin and Inhibits Thrombosis. Nat. Commun. 2018, 9, 3608. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Kindel, T.L.; Corbin, K.L.; Nunemaker, C.S.; Obici, S.; Woods, S.C.; Davidson, W.S.; Tso, P. Apolipoprotein A-IV Improves Glucose Homeostasis by Enhancing Insulin Secretion. Proc. Natl. Acad. Sci. USA 2012, 109, 9641–9646. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Wang, F.; Ji, Y.; DavidsoN, W.S.; Li, Z.; Tso, P. Interaction of ApoA-IV with NR4A1 and NR1D1 Represses G6Pase and PEPCK Transcription: Nuclear Receptor-Mediated Downregulation of Hepatic Gluconeogenesis in Mice and a Human Hepatocyte Cell Line. PLoS ONE 2015, 10, e0142098. [Google Scholar] [CrossRef]
- Weinstock, P.H.; Bisgaier, C.L.; Hayek, T.; Aalto-Setala, K.; Sehayek, E.; Wu, L.; Sheiffele, P.; Merkel, M.; Essenburg, A.D.; Breslow, J.L. Decreased HDL Cholesterol Levels but Normal Lipid Absorption, Growth, and Feeding Behavior in Apolipoprotein A-IV Knockout Mice. J. Lipid Res. 1997, 38, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying Size and Number of Adipocytes in Adipose Tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Tritos, N.A.; Mantzoros, C.S. Leptin: Its Role in Obesity and Beyond. Diabetologia 1997, 40, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin Levels and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef]
- Franssen, R.; Monajemi, H.; Stroes, E.S.G.; Kastelein, J.J.P. Obesity and Dyslipidemia. Med. Clin. N. Am. 2011, 95, 893–902. [Google Scholar] [CrossRef]
- Abranches, M.V.; Oliveira, F.C.; Conceicao, L.L.; Peluzio, M.D. Obesity and Diabetes: The Link between Adipose Tissue Dysfunction and Glucose Homeostasis. Nutr. Res. Rev. 2015, 28, 121–132. [Google Scholar] [CrossRef]
- Davis, B.H.; Poon, A.F.Y.; Whitlock, M.C. Compensatory Mutations Are Repeatable and Clustered within Proteins. Proc. Biol. Sci. 2009, 276, 1823–1827. [Google Scholar] [CrossRef]
- Berglund, E.D.; Li, C.Y.; Poffenberger, G.; Ayala, J.E.; Fueger, P.T.; Willis, S.E.; Jewell, M.M.; Powers, A.C.; Wasserman, D.H. Glucose Metabolism In Vivo in Four Commonly Used Inbred Mouse Strains. Diabetes 2008, 57, 1790–1799. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy Balance and Obesity: What Are the Main Drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Brown, L.M.; Clegg, D.J. Central Effects of Estradiol in the Regulation of Food Intake, Body Weight, and Adiposity. J. Steroid Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Eckel, L.A. The Ovarian Hormone Estradiol Plays a Crucial Role in the Control of Food Intake in Females. Physiol. Behav. 2011, 104, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Musatov, S.; Chen, W.; Pfaff, D.W.; Mobbs, C.V.; Yang, X.-J.; Clegg, D.J.; Kaplitt, M.G.; Ogawa, S. Silencing of Estrogen Receptor Alpha in the Ventromedial Nucleus of Hypothalamus Leads to Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Stubbins, R.E.; Smith, R.R.; Harvey, A.E.; Núñez, N.P. Differential Susceptibility to Obesity between Male, Female and Ovariectomized Female Mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Naaz, A. Role of Estrogens in Adipocyte Development and Function. Exp. Biol. Med. 2004, 229, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Wade, G.N.; Gray, J.M.; Bartness, T.J. Gonadal Influences on Adiposity. Int. J. Obes. 1985, 9 (Suppl. 1), 83–92. [Google Scholar]
- Shen, L.; Wang, D.Q.; Lo, C.M.; Tso, P.; Davidson, W.S.; Woods, S.C.; Liu, M. Estradiol Increases the Anorectic Effect of Central Apolipoprotein A-IV. Endocrinology 2010, 151, 3163–3168. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen Receptor Alpha and Beta in Health and Disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Pence, S.; Zhu, Q.; Binne, E.; Liu, M.; Shi, H.; Lo, C.C. Reduced Diet-Induced Thermogenesis in Apolipoprotein A-IV Deficient Mice. Int. J. Mol. Sci. 2019, 20, 3176. [Google Scholar] [CrossRef]
- Thiriet, M. Vasculopathies: Behavioral, Chemical, Environmental, and Genetic Factors; Springer: Berlin/Heidelberg, Germany, 2019; Volume 8, ISBN 3319893157. [Google Scholar]
- de Ferranti, S.; Mozaffarian, D. The Perfect Storm: Obesity, Adipocyte Dysfunction, and Metabolic Consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Roberts, R.; Hodson, L.; Dennis, A.L.; Neville, M.J.; Humphreys, S.M.; Harnden, K.E.; Micklem, K.J.; Frayn, K.N. Markers of de Novo Lipogenesis in Adipose Tissue: Associations with Small Adipocytes and Insulin Sensitivity in Humans. Diabetologia 2009, 52, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Reue, K.; Purcell-Huynh, D.A.; Leete, T.H.; Doolittle, M.H.; Durstenfeld, A.; Lusis, A.J. Genetic Variation in Mouse Apolipoprotein A-IV Expression Is Determined Pre- and Post-Transcriptionally. J. Lipid Res. 1993, 34, 893–903. [Google Scholar]
- Reue, K.; Leete, T.H. Genetic Variation in Mouse Apolipoprotein A-IV Due to Insertion and Deletion in a Region of Tandem Repeats. J. Biol. Chem. 1991, 266, 12715–12721. [Google Scholar] [PubMed]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse Strain-Dependent Variation in Obesity and Glucose Homeostasis in Response to High-Fat Feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.B. Apolipoprotein A-IV Polymorphisms and Diet-Gene Interactions. Curr. Opin. Lipidol. 2002, 13, 125–134. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Wu, D.; Ko, C.-W.; Zhu, Q.; Liu, M.; Tso, P. Deficiency of apoA-IV in Female 129X1/SvJ Mice Leads to Diet-Induced Obesity, Insulin Resistance, and Decreased Energy Expenditure. Nutrients 2023, 15, 4655. https://doi.org/10.3390/nu15214655
Qu J, Wu D, Ko C-W, Zhu Q, Liu M, Tso P. Deficiency of apoA-IV in Female 129X1/SvJ Mice Leads to Diet-Induced Obesity, Insulin Resistance, and Decreased Energy Expenditure. Nutrients. 2023; 15(21):4655. https://doi.org/10.3390/nu15214655
Chicago/Turabian StyleQu, Jie, Dong Wu, Chih-Wei Ko, Qi Zhu, Min Liu, and Patrick Tso. 2023. "Deficiency of apoA-IV in Female 129X1/SvJ Mice Leads to Diet-Induced Obesity, Insulin Resistance, and Decreased Energy Expenditure" Nutrients 15, no. 21: 4655. https://doi.org/10.3390/nu15214655
APA StyleQu, J., Wu, D., Ko, C.-W., Zhu, Q., Liu, M., & Tso, P. (2023). Deficiency of apoA-IV in Female 129X1/SvJ Mice Leads to Diet-Induced Obesity, Insulin Resistance, and Decreased Energy Expenditure. Nutrients, 15(21), 4655. https://doi.org/10.3390/nu15214655



