The Effects of Oral Probiotics on Type 2 Diabetes Mellitus (T2DM): A Clinical Trial Systematic Literature Review
Abstract
:1. Introduction
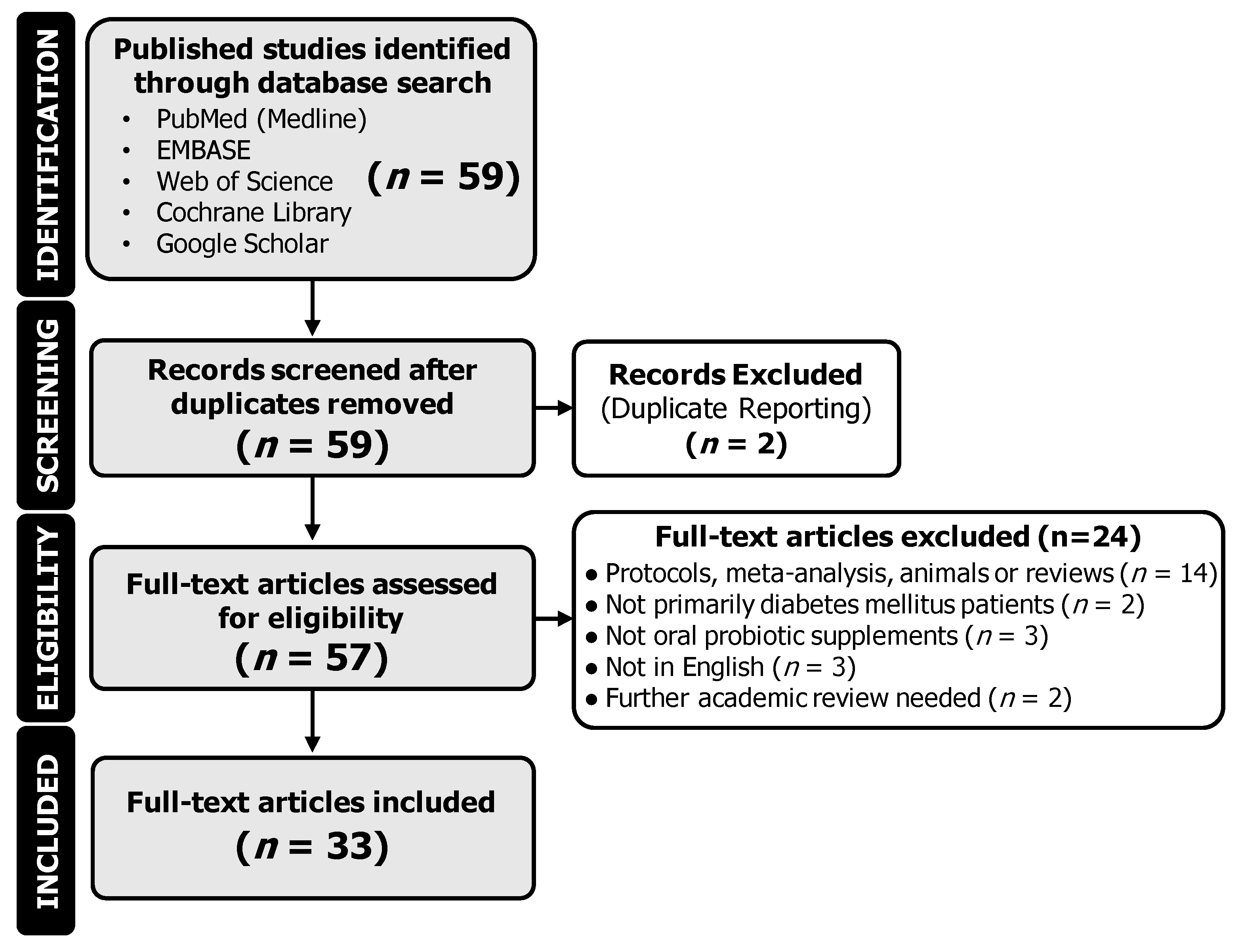
2. Methods
2.1. Data Sources, Search Strategy, and Selection Process
2.2. Data Collection and Interpretation
3. Results
3.1. Study Characteristics
| Article Identification | Trial Characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, Year of Publication | Citation | DOI (https://doi.org/) | Geography | CT Type | Number of True Participants | Number of Completed Participants | Control Group | Interest Group | ||||||
| Placebo or None | Number of Participants | Mean Age | SD (±) of Age | Probiotic | Number of Participants | Mean Age | SD (±) of Age | |||||||
| Chen et al., 2023 | [20] | 10.1128/msystems.01300-22 | China | Two-phase, R, DB | 58 | 48 | Placebo | 29 | 46.9 | 11.25 | Probiotic | 29 | 48.7 | 11.11 |
| García et al., 2023 | [21] | 10.26502/jbb.2642-91280090 | Cuba | R, DB | 64 | 57 | Placebo | 30 | 53.2 | 7.6 | Sugar shift (SS) cohort | 30 | 56.3 | 6.7 |
| Hasanpour et al., 2023 | [22] | 10.1186/s12902-023-01290-w | Iran | R, DB | 100 | 92 | Soymilk + placebo | 25 | 54.24 | 6.58 | Soymilk + Probiotic | 25 | 51.16 | 7.16 |
| Milk + placebo | 25 | 52.06 | 11.42 | Probiotic | 25 | 54.4 | 8.72 | |||||||
| Mirjalili et al., 2023 | [23] | 10.1016/j.clnesp.2023.01.014 | Iran | R, DB | 72 | 60 | Conventional yogurt | 36 | 58.1 | 9.8 | Probiotic yogurt | 36 | 54.5 | 8 |
| Ahmadian et al., 2022 | [24] | 10.1186/s13098-022-00822-z | Iran | R, DB | 68 | 60 | Placebo | 30 | 61 | Range: 57 to 65 | Probiotic | 30 | 58.5 | Range: 52 to 64 |
| Gupta et al., 2022 | [25] | 10.4103/jod.jod_106_21 | India | Before-and-after, NB | 308 | 308 | N/A | N/A | N/A | N/A | Participants | 308 | 54.2 | 10.9 |
| Hata et al., 2022 | [26] | 10.1111/jdi.13698 | Japan | Open-label, single-arm, exploratory research | 40 | 36 | N/A | N/A | N/A | N/A | Participants | 40 | 64 | 9.4 |
| Kumar et al., 2022 | [27] | N/A | India | R, DB | 150 | 150 | Metformin only | 75 | 51.1 | 5.4 | Metformin + probiotic | 75 | 50.9 | 6.2 |
| Ziegler et al., 2022 | [28] | 10.1016/j.clnesp.2022.04.002 | Brazil | Before-and-after, NB | 20 | 17 | N/A | N/A | N/A | N/A | Participants | 20 | 62.5 | N/A |
| Aron et al., 2021 | [29] | 10.31688/ABMU.2021.56.2.09 | Romania | Patient choice, prospective 3-month comparative study, NB | 41 | 41 | Control | 22 | 58.14 | 11.17 | Study group | 19 | 60.74 | 5.84 |
| Ismail et al., 2021 | [30] | 10.5603/DK.a2021.0037 | Egypt | Pilot, NB | 150 | 150 | Diet only | 50 | 46.4 | 13.2 | Yogurt and diet | 50 | 48.3 | 12.9 |
| Yeast and diet | 50 | 48.6 | 11.5 | |||||||||||
| Jiang et al., 2021 | [31] | 10.1002/jcla.23650 | China | R, DB, PG | 101 | 76 | Placebo | 34 | 56.12 | 8.23 | Probiotic | 42 | 55.96 | 8.45 |
| Kanazawa et al., 2021 | [32] | 10.3390/nu13020558 | Japan | R, NB | 88 | 80 | Control | 42 | 55.9 | 10.7 | Synbiotic | 44 | 61.1 | 11 |
| Toejing et al., 2021 | [33] | 10.3390/foods10071455 | Thailand | R, DB | 50 | 36 | Placebo | 18 | 61.8 | 7.7 | Probiotic | 18 | 63.5 | 5.9 |
| Palacios et al., 2020 | [19] | 10.3390/nu12072041 | Australia | R, DB | 60 | 53 | Placebo | 30 | 56.1 | 12.3 | Probiotic | 30 | 61.4 | 8.9 |
| Khalili et al., 2019 | [34] | 10.29252/.23.1.68. | Iran | R, DB, PG | 40 | NS | Placebo | 20 | 45 | 5.37 | Intervention group | 20 | 43.95 | 8.14 |
| Lestari et al., 2019 | [35] | 10.2478/rjdnmd-2019-0041 | Indonesia | R, DB | 38 | 32 | Control | 19 | 53 | 10 | Intervention | 19 | 56 | 7 |
| Madempudi et al., 2019 | [36] | 10.1371/journal.pone.0225168 | India | R, DB | 79 | 74 | Placebo | 39 | 50.6 | N/A | UB0316 | 40 | 54.1 | N/A |
| Sabico et al., 2019 Sabico et al., 2017 | [37,38] | 10.1016/j.clnu.2018.08.009 10.1186/s12967-017-1354-x. | Saudi Arabia | R, DB | 96 | 61 | Placebo | 39 | 46.6 | 5.9 | Probiotic | 39 | 48 | 8.3 |
| Hsieh et al., 2018 | [39] | 10.1038/s41598-018-35014-1 | USA | R, DB | 74 | 68 | Placebo | 24 | 55.77 | 8.55 | ADR-1 | 25 | 52.32 | 10.2 |
| ADR-3 | 25 | 53.88 | 7.78 | |||||||||||
| Raygan et al., 2018 | [40] | 10.1016/j.pnpbp.2018.02.007 | Iran | R, DB | 60 | 52 | Placebo | 30 | 67.3 | 11 | Vitamin plus probiotic group | 30 | 71.5 | 10.9 |
| Feizollahzadeh et al., 2017 | [41] | 10.1007/s12602-016-9233-y | Iran | R, DB, PG | 48 | 40 | Placebo | 20 | 53.6 | 1.6 | Intervention | 20 | 56.9 | 1.81 |
| Firouzi et al., 2017 | [42] | 10.1007/s00394-016-1199-8 | Malaysia | R, DB, PG | 136 | 101 | Placebo | 68 | 54.2 | 8.3 | Probiotic | 68 | 52.9 | 9.2 |
| Tonucci et al., 2017 | [43] | 10.1016/j.clnu.2015.11.011 | Brazil | R, DB, PG | 50 | 45 | Placebo | 22 | 50.95 | 7.2 | Probiotic | 23 | 51.83 | 6.64 |
| Sato et al., 2017 | [44] | 10.1038/s41598-017-12535-9 | Japan | R, NB | 69 | 68 | control | 34 | 65 | 8.3 | Probiotic | 34 | 64 | 9.2 |
| Asemi et al., 2016 | [45] | 10.1016/j.clnu.2015.07.009 | Iran | R, DB, CO | 51 | 48 | Placebo | N/A | N/A | N/A | Beta-carotene fortified synbiotic food group | 51 | 52.9 | 8.1 |
| Bahmani et al., 2016 | [46] | 10.1080/07315724.2015.1032443 | Iran | R, DB | 81 | 76 | Control bread | 27 | 53.4 | 7.5 | synbiotic bread | 27 | 51.3 | 10.4 |
| Tofighiyan et al., 2016 | [47] | 10.12691/jfnr-4-12-5 | Iran | R, DB | 44 | 42 | Placebo | 22 | 54.5 | 11.1 | Probiotic | 22 | 53.45 | 10.8 |
| Ogawa et al., 2014 | [48] | 10.1186/1476-511X-13-36 | Japan | SB, CO | 20 | 20 | Placebo | N/A | N/A | N/A | LG2055 treatment | 20 | 51.1 | 6.6 |
| Shakeri et al., 2014 | [49] | 10.1007/s11745-014-3901-z | NS | NS | 78 | 72 | Control bread | 26 | 53.1 | 7.5 | Synbiotic bread | 26 | 52.3 | 10.8 |
| Mohamadshahi et al., 2014 | [50] | PMID: 25197295 | Iran | R, DB | 44 | 44 | Conventional yogurt | 22 | 51 | NS | Probiotic yogurt | 22 | 51 | NS |
| Ejtahed et al., 2012 Ejtahed et al., 2011 | [51,52] | 10.1016/j.nut.2011.08.013 10.3168/jds.2010-4128 | Iran | R, DB | 64 | 60 | Conventional yogurt | 30 | 51 | 7.32 | Probiotic yogurt | 30 | 50.87 | 7.68 |
| Moroti et al., 2012 | [53] | 10.1186/1476-511X-11-29 | Brazil | R, DB | 50 | 18 | GP (group placebo shake) | 9 | 56.89 | 1.7 | GS (group symbiotic shake) | 9 | 55.47 | 2 |
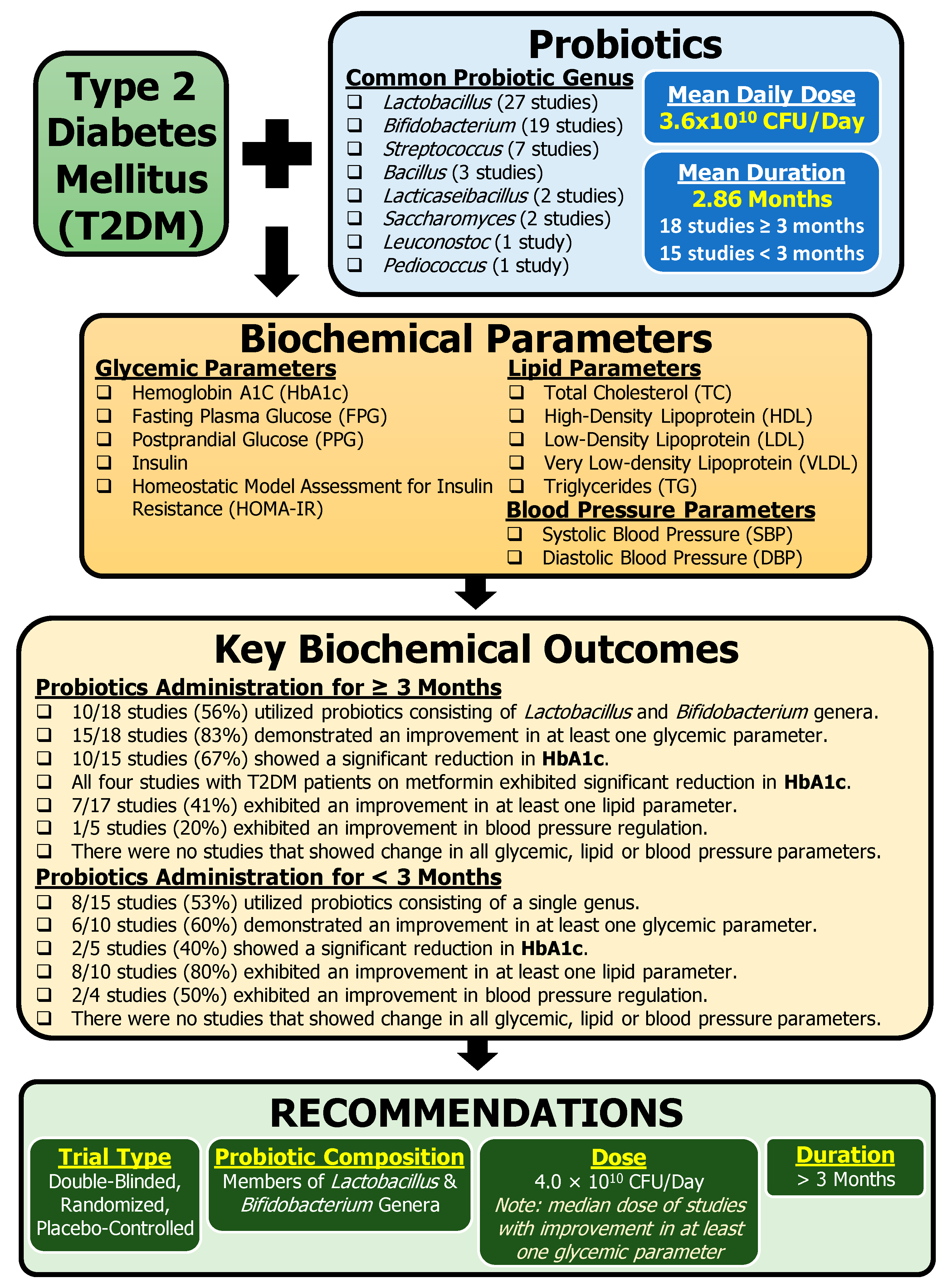
3.2. Probiotic Composition
3.3. Biochemical Parameters
3.4. Fecal Analysis
4. Discussion
4.1. Glycemic Profile
4.2. Lipid Profile
4.3. Cardiovascular and Kidney Parameters
4.4. Inflammation
4.5. Metformin and Probiotics as Adjunctive Therapy
4.6. Limitations and Issues of the Studies
4.7. Next Steps and Recommendations
- 1.
- Trial type and reporting: Double-blind, randomized, placebo-controlled study with statistical significance considered between the groups is mandatory. It is important that trials have different participants in groups, as crossover designs and washout periods are not sufficient for microbiome studies. Trials should provide all probiotic information including the duration of probiotic administration, the CFU, the frequency, the mechanism/vector, the composition, the placebo substitute, and the specific probiotic strains. Pharmacotherapy should also be detailed. Studies need to report all data, even if it is through supplementary information. All parameters collected should be made accessible as selective reporting leads to bias.
- 2.
- Probiotic composition: Multi-genus probiotics (≥two genera) rather than single-genus probiotics are predominantly used across the 33 clinical studies presented in this review. Based on the overall studies, we recommend probiotics consisting of Lactobacillus and Bifidobacterium, the most common combination of the genera studied across the studies (16 CTs).
- 3.
- Probiotic dose: We recommend a probiotic dose of 4.0 × 1010 CFU/Day. This value is based on the median dose of studies with improvement in at least one glycemic parameter.
- 4.
- Use proper placebo: The studies should utilize placebos, not just control groups. The placebos should be the modification of a single variable, the probiotic. Moreover, the placebo composition should be sugar-free as sugar affects T2DM and may interfere with the results. It may be prudent to use the same mechanism/vector as that of the probiotic but simply omit the probiotic aspect without noticeability.
- 5.
- Trial length: We recommend a minimum of 3 months of probiotic administration to achieve clinically successful glycemic improvement with probiotic intervention for T2DM management. Rationale: ≥3 months of probiotic intervention resulted in higher frequency of studies (83%) demonstrating improved glycemic parameters compared to < 3 months of probiotic intervention (60%).
- 6.
- Assessment of biochemical parameters: Studies need to collect and publish key biochemical parameters as outlined here: glycemic parameters (HbA1c, FPG, PPG, insulin, and HOMA-IR), lipid parameters (TC, HDL, LDL, VLDL, and TG), and blood pressure parameters (SBP and DBP). In addition, anthropometric measurements should be treated as outcomes, not just group baseline characteristics.
- 7.
- Significance: Interpretation of clinically significant results should be performed using a between-group comparison and an analysis of respective differences from baseline. Studies should consider factoring covariates, if appropriate.
- 8.
- Removal of bias: Multiple biases are identified throughout this review, with reporting bias being acknowledged the most. Future studies need to use standardized tools to limit bias.
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CFU | colony forming units |
| CTs | clinical trials |
| DBP | diastolic blood pressure |
| FPG | fasting plasma glucose |
| GI | gastrointestinal |
| HbA1c | glycosylated hemoglobin A1C |
| HDL | high-density lipoprotein |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| LDL | low-density lipoprotein |
| NGP | next-generation probiotics |
| PPG | postprandial glucose |
| RCT | randomized control trial |
| SBP | systolic blood pressure |
| SCFA | short-chain fatty acids |
| TC | total cholesterol |
| TG | triglycerides |
| T2DM | type 2 diabetes mellitus |
| WHR | waist-to-hip ratio |
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Asif, M. The Prevention and Control the Type-2 Diabetes by Changing Lifestyle and Dietary Pattern. J. Educ. Health Promot. 2014, 3, 1. [Google Scholar] [CrossRef]
- Gérard, C.; Vidal, H. Impact of Gut Microbiota on Host Glycemic Control. Front. Endocrinol. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota Medicine: Towards Clinical Revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Appanna, V.D. Microbiomes and Their Functions; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781003166481. [Google Scholar]
- Bosco, N.; Noti, M. The Aging Gut Microbiome and Its Impact on Host Immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Appanna, V.D. Human Microbes—The Power Within Health, Healing and Beyond, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9789811076848. [Google Scholar]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role of the Gut Microbiome and Its Metabolites in Metabolic Diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut Microbiota and Metabolic Syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.A.; Gavin, J.R.; Aguilar, R.B. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016, 39, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics—A Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Appanna, V.D. Human Microbes—The Power Within; Springer Singapore: Singapore, 2018; ISBN 978-981-10-7683-1. [Google Scholar]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Chapter Nine—Interactions of Probiotics and Prebiotics with the Gut Microbiota. In The Microbiome in Health and Disease; Sun, J., Ed.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2020; Volume 171, pp. 265–300. [Google Scholar]
- Marques, A.M.; Sarandy, M.M.; Novaes, R.D.; Gonçalves, R.V.; Freitas, M.B. Preclinical Relevance of Probiotics in Type 2 Diabetes: A Systematic Review. Int. J. Exp. Pathol. 2020, 101, 68–79. [Google Scholar] [CrossRef]
- Noël de Tilly, A.; Tharmalingam, S. Review of Treatments for Oropharyngeal Fungal Infections in HIV/AIDS Patients. Microbiol. Res. 2022, 13, 219–234. [Google Scholar] [CrossRef]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Lam, Y.Y.; Manuel, R.; Briskey, D.; Hendy, C.; Kim, J.N.; Ishoey, T.; et al. Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study. Nutrients 2020, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, X.; Ma, T.; Yu, X.; Kwok, L.-Y.; Li, Y.; Sun, Z.; Li, D.; Zhang, H. Adjunctive Probio-X Treatment Enhances the Therapeutic Effect of a Conventional Drug in Managing Type 2 Diabetes Mellitus by Promoting Short-Chain Fatty Acid-Producing Bacteria and Bile Acid Pathways. mSystems 2023, 8, e0130022. [Google Scholar] [CrossRef]
- García, G.; Soto, J.; Rodríguez, L.; Nuez, M.; Domínguez, N.; Buchaca, E.F.; Martínez, D.; Gómez, R.J.; Ávila, Y.; Carlin, M.R.; et al. Metabolic Shifting Probiotic in Type 2 Diabetes Mellitus Management: Randomized Clinical Trial. J. Biotechnol. Biomed. 2023, 6, 270–280. [Google Scholar] [CrossRef]
- Hasanpour, A.; Babajafari, S.; Mazloomi, S.M.; Shams, M. The Effects of Soymilk plus Probiotics Supplementation on Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. BMC Endocr. Disord. 2023, 23, 36. [Google Scholar] [CrossRef]
- Mirjalili, M.; Salari Sharif, A.; Sangouni, A.A.; Emtiazi, H.; Mozaffari-Khosravi, H. Effect of Probiotic Yogurt Consumption on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Clin. Nutr. ESPEN 2023, 54, 144–149. [Google Scholar] [CrossRef]
- Ahmadian, F.; Razmpoosh, E.; Ejtahed, H.S.; Javadi, M.; Mirmiran, P.; Azizi, F. Effects of Probiotic Supplementation on Major Cardiovascular-Related Parameters in Patients with Type-2 Diabetes Mellitus: A Secondary-Data Analysis of a Randomized Double-Blind Controlled Trial. Diabetol. Metab. Syndr. 2022, 14, 52. [Google Scholar] [CrossRef]
- Gupta, S.; Kamat, T.; Chawla, R.; Abhyankar, M.; Revankar, S.; Walia, S. Probiotic Supplementation (Vibact DS) in Patients with Type 2 Diabetes Mellitus: Real-World Experience from India. J. Diabetol. 2022, 13, 101. [Google Scholar] [CrossRef]
- Hata, S.; Nakajima, H.; Hashimoto, Y.; Miyoshi, T.; Hosomi, Y.; Okamura, T.; Majima, S.; Nakanishi, N.; Senmaru, T.; Osaka, T.; et al. Effects of Probiotic Bifidobacterium Bifidum G9-1 on the Gastrointestinal Symptoms of Patients with Type 2 Diabetes Mellitus Treated with Metformin: An Open-Label, Single-Arm, Exploratory Research Trial. J. Diabetes Investig. 2022, 13, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Manoj Kumar, V.; Ahmed, Z.; Atiq, S.; Rahman, U. Probiotics Efficacy and Safety as Add-on Therapy to Metformin in Type 2 Diabetes Mellitus. Indian J. Public Health Res. Dev. 2022, 13, 317–321. [Google Scholar]
- Ziegler, M.C.; Garbim Junior, E.E.; Jahnke, V.S.; Lisbôa Moura, J.G.; Brasil, C.S.; Schimitt da Cunha, P.H.; Lora, P.S.; Gemelli, T. Impact of Probiotic Supplementation in a Patient with Type 2 Diabetes on Glycemic and Lipid Profile. Clin. Nutr. ESPEN 2022, 49, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Aron, R.A.C.; Tit, D.M.; Purza, A.L.; Abid, A.; Vesa, C.M.; Angelescu, G.; Bungau, S. Effects of Probiotic Supplementation on Metabolic Syndrome Components in Type 2 Diabetes Mellitus Patients a Case-Control Study. Arch. Balk. Med. Union 2021, 56, 201–212. [Google Scholar] [CrossRef]
- Ismail, A.A.; Darwish, O.A.; Tayel, D.I.; Elneily, D.A.; Elshaarawy, G.H. Impact of Probiotic Intake on the Glycemic Control, Lipid Profile and Inflammatory Markers among Patients with Type 2 Diabetes Mellitus. Clin. Diabetol. 2021, 10, 468–475. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xu, D.; Wang, Q. Probiotics Ameliorates Glycemic Control of Patients with Diabetic Nephropathy: A Randomized Clinical Study. J. Clin. Lab. Anal. 2021, 35, e23650. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef]
- Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus Paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. [Google Scholar] [CrossRef]
- Khalili, L.; Alipour, B.; Asghari Jafar-Abadi, M.; Faraji, I.; Hassanalilou, T.; Abbasi, M.M.; Vaghef-Mehrabany, E.; Sani, M.A. The Effects of Lactobacillus Casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Biomed. J. 2019, 23, 68–77. [Google Scholar] [CrossRef]
- Lestari, L.A.; Ratnasari, D.; Azizah, E.F.; Farida, I.N.; Nuriannisa, F.; Yuliani, K.; Kusuma, R.J.; Huriyati, E.; Kertia, N. Short-Term Consumption of Probiotic Yogurt Improved HDL-C of Type 2 Diabetes Mellitus Patients: A Double-Blind Randomized Controlled Trial. Rom. J. Diabetes Nutr. Metab. Dis. 2019, 26, 381–392. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Efficacy of UB0316, a Multi-Strain Probiotic Formulation in Patients with Type 2 Diabetes Mellitus: A Double Blind, Randomized, Placebo Controlled Study. PLoS ONE 2019, 14, e0225168. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-Month Multi-Strain Probiotics Supplementation in Endotoxemic, Inflammatory and Cardiometabolic Status of T2DM Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a Multi-Strain Probiotic Supplement for 12 Weeks in Circulating Endotoxin Levels and Cardiometabolic Profiles of Medication Naïve T2DM Patients: A Randomized Clinical Trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Tsai, W.H.; Jheng, Y.P.; Su, S.L.; Wang, S.Y.; Lin, C.C.; Chen, Y.H.; Chang, W.W. The Beneficial Effects of Lactobacillus Reuteri ADR-1 or ADR-3 Consumption on Type 2 Diabetes Mellitus: A Randomized, Double-Blinded, Placebo-Controlled Trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The Effects of Vitamin D and Probiotic Co-Supplementation on Mental Health Parameters and Metabolic Status in Type 2 Diabetic Patients with Coronary Heart Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef]
- Feizollahzadeh, S.; Ghiasvand, R.; Rezaei, A.; Khanahmad, H.; Sadeghi, A.; Hariri, M. Effect of Probiotic Soy Milk on Serum Levels of Adiponectin, Inflammatory Mediators, Lipid Profile, and Fasting Blood Glucose Among Patients with Type II Diabetes Mellitus. Probiotics Antimicrob. Proteins 2017, 9, 41–47. [Google Scholar] [CrossRef]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.-Y. Effect of Multi-Strain Probiotics (Multi-Strain Microbial Cell Preparation) on Glycemic Control and Other Diabetes-Related Outcomes in People with Type 2 Diabetes: A Randomized Controlled Trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical Application of Probiotics in Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic Reduces Bacterial Translocation in Type 2 Diabetes Mellitus: A Randomised Controlled Study. Sci. Rep. 2017, 7, 12115. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.-A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of Beta-Carotene Fortified Synbiotic Food on Metabolic Control of Patients with Type 2 Diabetes Mellitus: A Double-Blind Randomized Cross-over Controlled Clinical Trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Tajadadi-Ebrahimi, M.; Kolahdooz, F.; Mazouchi, M.; Hadaegh, H.; Jamal, A.-S.; Mazroii, N.; Asemi, S.; Asemi, Z. The Consumption of Synbiotic Bread Containing Lactobacillus Sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll Nutr. 2016, 35, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Tofighiyan, T.; Kooshki, A.; Hoseini, B.L.; Mohammadi, M. The Effects of Probiotic on Serum Lipid Profiles in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. J. Food Nutr. Res. 2016, 4, 795–798. [Google Scholar]
- Ogawa, A.; Kadooka, Y.; Kato, K.; Shirouchi, B.; Sato, M. Lactobacillus Gasseri SBT2055 Reduces Postprandial and Fasting Serum Non-Esterified Fatty Acid Levels in Japanese Hypertriacylglycerolemic Subjects. Lipids Health Dis 2014, 13, 36. [Google Scholar] [CrossRef]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of Synbiotic Bread Decreases Triacylglycerol and VLDL Levels While Increasing HDL Levels in Serum from Patients with Type-2 Diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Javid, A.Z.; Mohammadi, F.; Shirbeigi, E. Effects of Probiotic Yogurt Consumption on Lipid Profile in Type 2 Diabetic Patients: A Randomized Controlled Clinical Trial. J. Res. Med. Sci. 2014, 19, 531–536. [Google Scholar]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic Yogurt Improves Antioxidant Status in Type 2 Diabetic Patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V.; Akbarian-Moghari, A. Effect of Probiotic Yogurt Containing Lactobacillus Acidophilus and Bifidobacterium Lactis on Lipid Profile in Individuals with Type 2 Diabetes Mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the Consumption of a New Symbiotic Shake on Glycemia and Cholesterol Levels in Elderly People with Type 2 Diabetes Mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Wang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Meta-Analysis of Randomized Controlled Trials of the Effects of Probiotics on Type 2 Diabetes in Adults. Clin. Nutr. 2022, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.N.; Ding, W.Y.; Ning, J.; Wang, Y.; Yan, Y.; Wang, Z. Bin Effects of Probiotic Supplementation on Inflammatory Markers and Glucose Homeostasis in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 770861. [Google Scholar] [CrossRef]
- Quigley, E.M.M.; Gajula, P. Recent Advances in Modulating the Microbiome. F1000Res 2020, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Naseri, K.; Saadati, S.; Ghaemi, F.; Ashtary-Larky, D.; Asbaghi, O.; Sadeghi, A.; Afrisham, R.; de Courten, B. The Effects of Probiotic and Synbiotic Supplementation on Inflammation, Oxidative Stress, and Circulating Adiponectin and Leptin Concentration in Subjects with Prediabetes and Type 2 Diabetes Mellitus: A GRADE-Assessed Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Clinical Trials. Eur. J. Nutr. 2023, 62, 543–561. [Google Scholar]
- Naseri, K.; Saadati, S.; Yari, Z.; Asbaghi, O.; Hezaveh, Z.S.; Mafi, D.; Hoseinian, P.; Ashtary-Larky, D.; Hekmatdoost, A.; de Courten, B. Beneficial Effects of Probiotic and Synbiotic Supplementation on Some Cardiovascular Risk Factors among Individuals with Prediabetes and Type 2 Diabetes Mellitus: A Grade-Assessed Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Clinical Trials. Pharmacol. Res. 2022, 182, 106288. [Google Scholar]
- Dempsey, E.; Corr, S.C. Lactobacillus Spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Probiotic Supplementation on Dyslipidemia in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Foods 2020, 9, 1540. [Google Scholar] [CrossRef]
- Bosi, E. Metformin—The Gold Standard in Type 2 Diabetes: What Does the Evidence Tell Us? Diabetes Obes. Metab. 2009, 11, 3–8. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella Spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Hiel, S.; Delzenne, N.M. Metformin: Old Friend, New Ways of Action–Implication of the Gut Microbiome? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 294–301. [Google Scholar] [CrossRef]
- Li, W.-Z.; Stirling, K.; Yang, J.-J.; Zhang, L. Gut Microbiota and Diabetes: From Correlation to Causality and Mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Comparisons against Baseline within Randomised Groups Are Often Used and Can Be Highly Misleading. Trials 2011, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Luo, J.; Liu, Y.; Li, H.; Jin, R.; Li, S.; Wei, J.; Wei, H.; Chen, T. Improvement effect of a next-generation probiotic L. plantarum-pMG36e-GLP-1 on type 2 diabetes mellitus via the gut-pancreas-liver axis. Food Funct. 2023, 14, 3179–3195. [Google Scholar] [CrossRef] [PubMed]
| Article ID | Probiotic Administration Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors, Year of Publication | Time | Delivery Method | Probiotic | |||||||
| Duration of Administration (Months) | Average CFU/Dose | Frequency/Day (Assumed 1 If Not Indicated) | Average Total CFU /Day | Mechanism/ Vector | Composition | Placebo Substitute | Probiotic Genus | Number of Different Strains | Specific Probiotic Strains | |
| [20] | 3 | 3.00 × 1010 | 1 | 3.00 × 1010 | Sachet | NS | Maltodextrin | Lactobacillus, Bifidobacterium | 5 | L. casei Zhang, B. lactis V9, L. plantarum P-8, L. rhamnosus Probio-M9, and B. lactis Probio-M8 (Probio-X; Jinhua Yinhe Biotechnology Co., Ltd.; Beijing, China) |
| [21] | 3 | 1.80 × 1010 | 2 | 3.60 × 1010 | Capsules | 370 mg of prebiotics and fillers such as inulin, microcrystalline cellulose, D-mannitol, and stearic acid | Lacking the bacterial consortium | Lactobacillus, Bifidobacterium, Bacillus, Leuconostoc, Pediococcus | 8 | Ba. subtilis De111TM, B. bifidum, B. longum, L. paracasei, L. plantarum TBC0036, L. reuteri, Le. mesenteroides TBC0037, P. acidilactici (BlisterPak Pro, LLC in Lafayette, CO, USA) |
| [22] | 1.5 | NS | 1 | NS | Capsule | Fructooligosaccharides as prebiotic | Starch | Lactobacillus, Bifidobacterium, Streptococcus | 7 | L. rhamnosus, L. casei, L. bulgaricus, L. acidophilus, B. breve, B. longum, S. thermophilus (FamiLact; Zist Takhmir Pharmaceutical Co., Tehran, Iran) |
| [23] | 3 | 4.65 × 108 | 2 | 9.30 × 108 | Yogurt | 100 g yogurt | Lacking the probiotics | Lactobacillus, Bifidobacterium | 2 | L. acidophilus, B. lactis |
| [24] | 1.5 | 2.40 × 1011 | 2 | 4.80 × 1011 | Capsule | 100 mg of fructo-oligosaccharide with lactose as carrier substances | Magnesium stearate | Lactobacillus, Bifidobacterium, Streptococcus | 7 | L. acidophilus, L. casei, L. bulgaricus, L. rhamnosus, B. breve, B. longum, S. thermophilus (Familact; Zist Takhmir Pharmaceutical Co., Tehran, Iran) |
| [25] | 3 | 1.66 × 108 | 1 | 1.66 × 108 | Capsule | NS | NA | Lactobacillus Streptococcus Clostridium | 3 | S. Faecalis, C. butyricum, B. mesentricus |
| [26] | 2.5 | NS | 3 | NA | Tablet | 12 mg | NA | Bifidobacterium | 1 | B. bifidum G9-1 (Biofermin; Taisho Pharmaceutical, Tokyo, Japan) |
| [27] | 3 | NS | 2 | NS | Capsule | NS | NS | NS | NS | NS |
| [28] | 3 | 4.00 × 109 | 1 | 4.00 × 109 | Liquid | NS | NA | Bacillus (spores) | 1 | B. clause spores (Enterogermina Plus; Sanofi, Paris, France) |
| [29] | 3 | Varied, NS | 1/day first 2 weeks; 2 capsules/day rest of trial | Varied, NS | Capsule | NS | NA | Bacillus (spores) | 5 | B. licheniformis, B. indicus, B. subtilis, B. clausii, B. coagulans |
| [30] | 4 | NS | NS | NA | Yogurt | 2 cups fortified yogurt | NA | Bifidobacterium | 1 | B. animalis dn-173 010 |
| 4 | NS | 1 | NA | Solid | 1 teaspoonful of natural baking yeast | NA | Saccharomyces | 1 | S. cerevisiae | |
| [31] | 3 | 3.20 × 109 | 1 | 3.20 × 109 | Capsule | NS | Starch (Tian San Qi Company, Xiamen, China) | Lactobacillus, Bifidobacterium, Streptococcus | 3 | B. bifidum, L. acidophilus, S. thermophilus (LactoCare, Zist Takhmir Pharmaceutical Co., Tehran, Iran) |
| [32] | 6 | 9.00 × 108 | 2 | 1.80 × 109 | Powder | 3.0 g/day probiotic and 7.5 g/day galacto-oligosccharides | NA | Lacticaseibacillus, Bifidobacterium | 2 | La. paracasei YIT 9029 (strain Shirota: LcS), B. breve YIT 12272 (BbrY) (Yakult Honsha Co., Ltd., Tokyo, Japan) |
| [33] | 3 | 5.00 × 1010 | 1 | 5.00 × 1010 | Capsule | NS | 10 mg/day corn starch | Lactobacillus | 1 | L. paracasei HII01 |
| [19] | 3 | 1.00 × 1010 | 2 | 2.00 × 1010 | Capsule | Probiotics, 40 mg microcrystalline cellulose, 5 mg silica, and 10 mg magnesium stearate | 200 mg microcrystalline cellulose, 10 mg silica, and 10 mg magnesium stearate per capsule | Lactobacillus, Bifidobacterium, Streptococcus, Saccharomyces | 8 | L. plantarum Lp-115,L. bulgaricus Lb-64, L. gasseri Lg-36, B. breve Bb-03, B. animalis sbsp. lactis Bi-07, B. bifidum Bb-06, S. thermophilus St-21 and S. boulardii DBVPG 6763 |
| [34] | 2 | 1.00 × 108 | 1 | 1.00 × 108 | Capsule | Maltodextrin | Lacking the probiotics | Lactobacillus | 1 | L. casei (Chr. Hansen, Hoersholm, Denmark) |
| [35] | 1 | 1.01 × 1010 | 1 | 1.01 × 1010 | Yogurt | 100 mL/day conventional yogurt (L. bulgaricus, S. thermophilus) | NA | Lactobacillus, Bifidobacterium, Streptococcus | 2 | L. acidophilus La-5, B. lactis BB-12 |
| [36] | 3 | 3.00 × 1010 | 2 | 6.00 × 1010 | Capsule | 100 mg fructo-oligosaccharide | Maltodextrin | Lactobacillus, Bifidobacterium, Streptococcus, Bacillus | 6 | L. salivarius UBLS22, L. casei UBLC42, L. plantarum UBLP40, L. acidophilus UBLA34, B. breve UBBr01, B. coagulans Unique IS2 (Unique Biotech Limited, Kolthur, Hyderabad, India) |
| [37,38] | 6 | 5.00 × 109 | 2 | 1.00 × 1010 | Sachet | 2 g freeze-dried maize starch and maltodextrins | Lacking the probiotics | Lactobacillus, Bifidobacterium | 8 | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, L. lactis W19, L. lactis W58 (Winclove probiotics, Amsterdam, The Netherlands) |
| [39] | 6 | 4.00 × 109 | 1 | 4.00 × 109 | Capsule | NS | NS | Lactobacillus | 1 | L. reuteri (GenMont Biotech Inc., Tainan City, Taiwan) |
| 6 | 2.00 × 109 | 1 | 2.00 × 109 | Capsule | NS | NS | Lactobacillus | 1 | L. reuteri (heat killed) (GenMont Biotech Inc., Tainan City, Taiwan) | |
| [40] | 3 | 8.00 × 109 | 1 | 8.00 × 109 | Capsule | NA | Starch (Barij Essence Pharmaceutical Company, Kashan, Iran) | Lactobacillus, Bifidobacterium | 4 | L. acidophilus, B. bifidum, L. reuteri, L. fermentum (Lactocare, Zist Takhmir Pharmaceutical Co., Tehran, Iran) |
| [41] | 2 | 2.00 × 109 | 1 | 2.00 × 109 | Milk | Soy milk | NA | Lactobacillus | 1 | L. plantarum A7 |
| [42] | 3 | 3.00 × 1010 | 2 | 6.00 × 1010 | Sachet | NS | NS | Lactobacillus, Bifidobacterium | 6 | L. acidophilus, L. casei, L. lactis, B. bifidum, B. longum, B. infantis (Hexbio® B-Crobes Laboratory Sdn. Bhd. (Ipoh, Malaysia) |
| [43] | 4 | 4.00 × 1010 | 1 | 4.00 × 1010 | Milk | Yakult 400LT (Yakult Honsha Co., Ltd., Tokyo, Japan) | NA | Lactobacillus | 1 | L. casei |
| [44] | 1.5 | 2.00 × 109 | 1 | 2.00 × 109 | Milk | 120 g/d of conventional fermented goat milk (Embrapa Goat and Sheep, Ceara, Brazil) | Streptococcus thermophilus TA-40 (Danisco, Sassenage, France) | Lacticaseibacillus, Bifidobacterium | 2 | L. acidophilus La-5, B. animalis subsp. lactis BB-12 (Chr. Hansen, Hoersholm,, Denmark) |
| [45] | 1.5 | 9.00 × 107 | 3 | 2.70 × 108 | Food | Probiotic, 0.1 g inulin (HPX) as prebiotic, 0.05 g beta-carotene with 0.38 g isomalt, 0.36 g sorbitol, and 0.05 g stevia per 1 g | Same substance without probiotic, inulin and beta-carotene | Lactobacillus | 1 | L. sporogenes |
| [46] | 2 | 4.00 × 109 | 3 | 1.20 × 1010 | Bread | Bread 120 g/day (Probiotic + 0.07 g inulin per 1 g) (Sahar Bread Company, Tehran, Iran) | Lacking the probiotics and inulin | Lactobacillus | 1 | L. sporogenes (heat-resistant) (Tak Gen Zist Company, Tehran, Iran) |
| [47] | 2 | NS | 1 | NS | Tablet | Fructooligosaccharides | Farina (Pharmaceutics Department of Mashhad School of Pharmacy) | Lactobacillus | 1 | L. coagulans (Bioplus Company, Bangalore, India) |
| [48] | 1 | 5.00 × 1010 | 2 | 1.00 × 1011 | Milk | Starter culture (Streptococcus thermophilus and Lactobacillus delbrueckii spp. Bulgaricus) 11% skim milk powder, flavoring, agar, and sucralose | Lacking the probiotics | Lactobacillus | 1 | L. gasseri SBT2055 (LG2055) |
| [49] | 2 | 1.11 × 109 | 1 | 1.11 × 109 | Yogurt | Conventional yogurt (L. bulgaricus, S. thermophilus) | N/A | Lactobacillus, Bifidobacterium | 2 | L. bulgaricus, S. thermophiles |
| [50] | 2 | 4.00 × 109 | 3 | 1.20 × 1010 | Bread | 120 g/day (Probiotic) (Sahar Bread Company, Tehran, Iran) | Lacking the probiotics | Lactobacillus | 1 | L. sporogenes (heat-resistant) (Tak Gen Zist Company, Tehran, Iran) |
| [51,52] | 1.5 | 7.74 × 106 | 1 | 7.74 × 106 | Yogurt | Conventional yogurt (L. bulgaricus, S. thermophilus) (Iran Dairy Industries Co., Tehran, Iran) | N/A | Lactobacillus, Bifidobacterium | 2 | B. lactis Bb12, L. acidophilus La5 (Chr. Hansen, Hoersholm, Denmark) |
| [53] | 1 | 8.00 × 108 | 2 | 1.60 × 109 | Shake | Probiotics, 9% skim milk powder, 23% whey powder, 21% maltodextrin, 15% oatmeal, 7% texturized soy-bean protein TSP, 5% soybean fiber, 3.5% guar gum, 3.5% collagen, 5% soybean extract, 4.5% fructooligosaccharide, and other | Lacking the probiotics and fructooligosaccharide. | Lactobacillus, Bifidobacterium | 2 | L. acidophillus, B. bifidum |
| Study | Probiotic Composition (Prebiotics, Genus) | Probiotic Duration | Glycemic Parameters | Lipid Parameters | Blood Pressure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c | FPG | PPG | Insulin | HOMA-IR | TC | HDL | LDL | VLDL | TG | SBP | DBP | |||
| [20] (T2DM patients on metformin) | Lactobacillus, Bifidobacterium | 3 months | D | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [27] (T2DM patients on metformin) | Not specified | 3 months | D | N.S. | N.S. | N.T. | N.T. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [25] (T2DM patients on metformin) | Lactobacillus, Streptococcus, Clostridium | 3 months | D | D | D | N.T. | N.T. | D | N.S. | D | N.T. | D | N.T. | N.T. |
| [36] (T2DM patients on metformin) | Lactobacillus, Bifidobacterium, Streptococcus, Bacillus | 3 months | D | N.S. | N.T. | N.S. | N.S. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [19] (T2DM patients on metformin) | Lactobacillus, Bifidobacterium, Streptococcus, Saccharomyces | 3 months | D | D | N.T. | D | N.S. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [42] | Lactobacillus, Bifidobacterium | 3 months | D | N.S. | N.T. | D | N.S. | N.S. | N.S. | N.S. | N.T. | N.S. | N.S. | N.S. |
| [23] | Lactobacillus, Bifidobacterium | 3 months | D | N.S. | N.S. | N.S. | N.S. | D | N.S. | D | N.T. | N.S. | N.T. | N.T. |
| [31] | Lactobacillus, Bifidobacterium, Streptococcus | 3 months | D | D | N.S. | N.S. | N.S. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. |
| [21] | Lactobacillus, Bifidobacterium, Bacillus, Leuconostoc, Pediococcus | 3 months | N.S. | N.S. | N.S. | D | N.S. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [28] | Bacillus | 3 months | N.T. | D | N.T. | N.T. | N.T. | N.S. | I | N.S. | N.S. | N.S. | N.S. | N.S. |
| [33] | Lactobacillus | 3 months | N.S. | D | N.T. | N.T. | N.T. | N.S. | I | D | N.T. | N.S. | N.T. | N.T. |
| [29] | Bacillus | 3 months | N.S. | N.S. | N.T. | N.T. | N.T. | N.S. | N.S. | N.S. | N.T. | N.S. | N.S. | N.S. |
| [40] | Vitamin D, Lactobacillus, Bifidobacterium | 3 months | N.T. | N.S. | N.T. | D | D | N.S. | I | N.S. | N.S. | N.S. | N.S. | N.S. |
| [30] | Bifidobacterium | 4 months | D | D | N.S. | N.T. | N.T. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [44] | Lactobacillus | 4 months | N.S. | N.S. | N.T. | N.T. | N.T. | N.S. | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [37,38] | Lactobacillus, Bifidobacterium | 6 months | N.T. | D | N.T. | D | D | D | N.S. | N.S. | N.T. | D | N.T. | N.T. |
| [32] | Lacticaseibacillus, Bifidobacterium | 6 months | N.S. | N.S. | N.T. | N.T. | N.T. | N.S. | N.S. | N.T. | N.T. | N.S. | N.T. | N.T. |
| [39] | Lactobacillus | 6 months | D | N.S. | N.T. | N.S. | N.T. | D | N.S. | N.S. | N.T. | N.S. | D | D |
| Study | Probiotic Composition (Prebiotics, Genus) | Probiotic Duration | Glycemic Parameters | Lipid Parameters | Blood Pressure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c | FPG | PPG | Insulin | HOMA-IR | TC | HDL | LDL | VLDL | TG | SBP | DBP | |||
| [26] | Bifidobacterium | 2.5 months | N.S. | N.S. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. |
| [34] | Lactobacillus | 2 months | N.S. | D | N.T. | D | D | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. |
| [24] | Fructooligosaccharides, Lactobacillus, Bifidobacterium, Streptococcus | 1.5 months | N.T. | N.T. | N.T. | N.T. | N.T. | N.S. | N.S. | N.S. | N.T. | N.S. | D | D |
| [47] | Fructooligosaccharides, Lactobacillus | 2 months | N.T. | N.T. | N.T. | N.T. | N.T. | D | N.S. | N.S. | N.T. | N.S. | N.T. | N.T. |
| [22] | Fructooligosaccharides, Lactobacillus, Bifidobacterium, Streptococcus | 1.5 months | N.T. | N.S. | N.T. | D | N.S. | N.S. | I | I | N.T. | N.S. | D | D |
| [53] | Fructooligosaccharides, Lactobacillus, Bifidobacterium | 1 month | N.T. | D | N.T. | N.T. | N.T. | N.S. | I | N.S. | N.S. | N.S. | N.T. | N.T. |
| [51,52] | Lactobacillus, Bifidobacterium | 1.5 months | D | D | N.T. | N.S. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. |
| [35] | Lactobacillus, Bifidobacterium, Streptococcus | 1 month | N.T. | N.S. | N.T. | N.T. | N.T. | N.S. | I | N.S. | N.T. | N.S. | N.T. | N.T. |
| [50] | Lactobacillus, Bifidobacterium | 2 months | N.T. | N.T. | N.T. | N.T. | N.T. | N.S. | I | D | N.T. | N.S. | N.T. | N.T. |
| [48] | Lactobacillus | 1 month | N.S. | N.S. | N.T. | N.S. | N.T. | N.S. | N.S. | N.S. | N.T. | D | N.T. | N.T. |
| [41] | Lactobacillus | 2 months | N.T. | N.S. | N.T. | N.T. | N.T. | N.T. | I | D | N.T. | N.S. | N.T. | N.T. |
| [43] | Lacticaseibacillus, Bifidobacterium | 1.5 months | D | N.S. | N.T. | N.S. | N.S. | D | N.S. | D | N.T. | N.S. | N.T. | N.T. |
| [46] | Lactobacillus | 2 months | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.S. | N.S. |
| [49] | Lactobacillus | 2 months | N.T. | N.T. | N.T. | N.T. | N.T. | N.S. | N.S. | N.S. | N.S. | N.S. | N.T. | N.T. |
| [45] | Inulin, β-carotene, Lactobacillus | 1.5 months | N.T. | N.S. | N.T. | D | D | D | N.S. | N.S. | D | D | N.S. | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paquette, S.; Thomas, S.C.; Venkataraman, K.; Appanna, V.D.; Tharmalingam, S. The Effects of Oral Probiotics on Type 2 Diabetes Mellitus (T2DM): A Clinical Trial Systematic Literature Review. Nutrients 2023, 15, 4690. https://doi.org/10.3390/nu15214690
Paquette S, Thomas SC, Venkataraman K, Appanna VD, Tharmalingam S. The Effects of Oral Probiotics on Type 2 Diabetes Mellitus (T2DM): A Clinical Trial Systematic Literature Review. Nutrients. 2023; 15(21):4690. https://doi.org/10.3390/nu15214690
Chicago/Turabian StylePaquette, Simon, Sean C. Thomas, Krishnan Venkataraman, Vasu D. Appanna, and Sujeenthar Tharmalingam. 2023. "The Effects of Oral Probiotics on Type 2 Diabetes Mellitus (T2DM): A Clinical Trial Systematic Literature Review" Nutrients 15, no. 21: 4690. https://doi.org/10.3390/nu15214690
APA StylePaquette, S., Thomas, S. C., Venkataraman, K., Appanna, V. D., & Tharmalingam, S. (2023). The Effects of Oral Probiotics on Type 2 Diabetes Mellitus (T2DM): A Clinical Trial Systematic Literature Review. Nutrients, 15(21), 4690. https://doi.org/10.3390/nu15214690





