The Role of Cyanidin-3-O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Condition
2.2. Establishment of OXA-Resistant Cell
2.3. Determination of OXA-Induced Morphological Changes in Resistant Cells
2.4. Cytotoxicity Assay
2.5. Hoechst 33342/PI Staining for Apoptosis Assay
2.6. Wound-Healing Assay
2.7. Immunofluorescence Staining
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. OXA Resistance Induces Spindle-Shaped Morphology in CRC Cells
3.2. C3G Reverses OXA Resistance in HCT-116-ROx Cells
3.3. The Combination of C3G and OXA Increases the Apoptotic Rate in HCT-116-ROx Cells
3.4. C3G Attenuates the Increased Migration Rate in HCT-116-ROx Cells
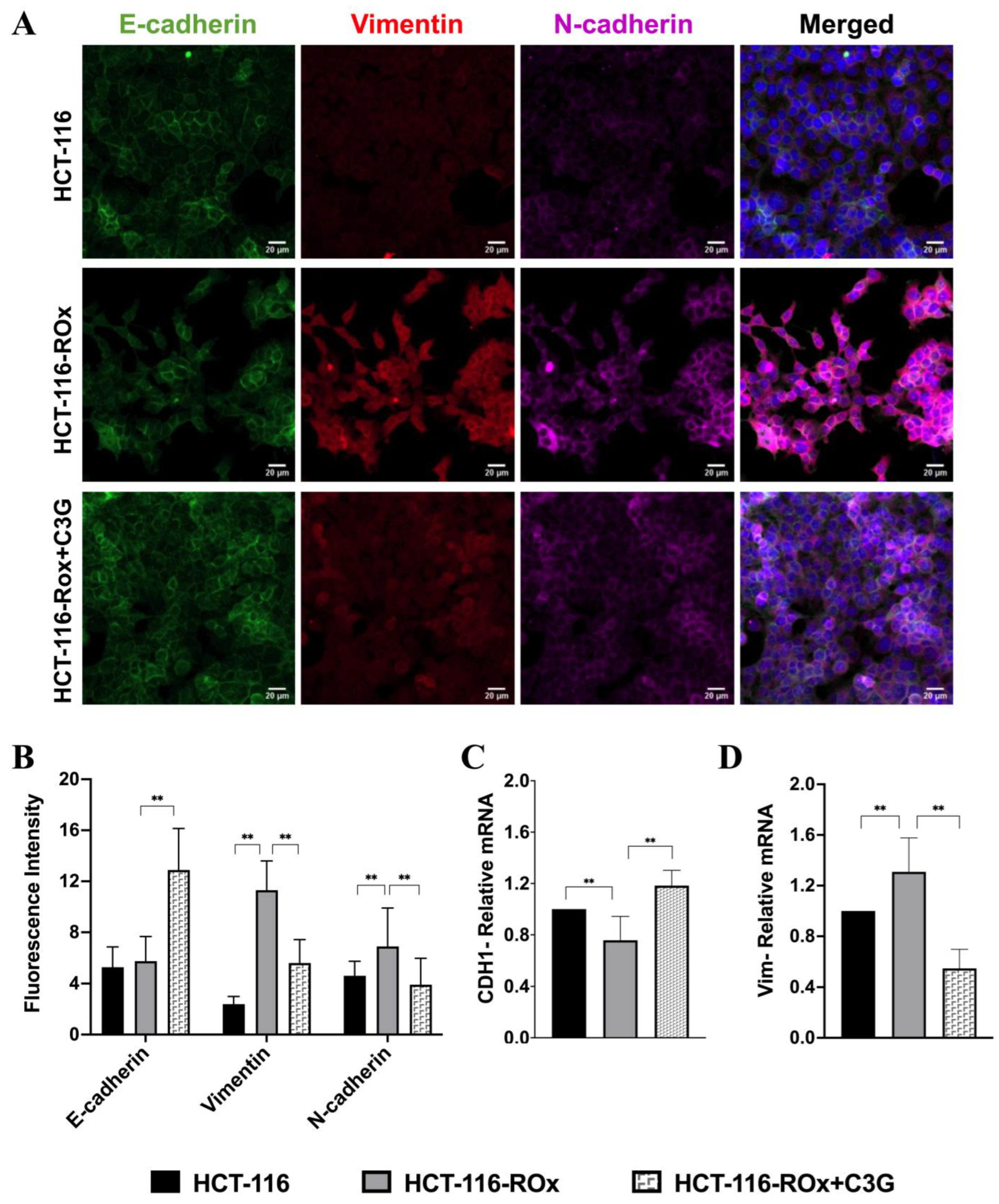
3.5. C3G Reverses OXA Resistance-Induced Mesenchymal Phenotype in HCT-116-ROx Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar]
- Greenlee, J.D.; Lopez-Cavestany, M.; Ortiz-Otero, N.; Liu, K.; Subramanian, T.; Cagir, B.; King, M.R. Oxaliplatin resistance in colorectal cancer enhances TRAIL sensitivity via death receptor 4 upregulation and lipid raft localization. Elife 2021, 10, e67750. [Google Scholar] [CrossRef]
- Werner, J.; Heinemann, V. Standards and Challenges of Care for Colorectal Cancer Today. Visc. Med. 2016, 32, 156–157. [Google Scholar] [CrossRef]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Giuliano, S.R.; Tralongo, P. Early colorectal cancer: Diagnosis, treatment and survivorship care. Crit. Rev. Oncol. Hematol. 2019, 136, 20–30. [Google Scholar]
- Ray, B.; Gupta, B.; Mehrotra, R. Binding of platinum derivative, oxaliplatin to deoxyribonucleic acid: Structural insight into antitumor action. J. Biomol. Struct. Dyn. 2019, 37, 3838–3847. [Google Scholar]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; de Porras, V.R.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar]
- Cao, G.; Li, P.; He, X.; Jin, M.; Li, M.; Chen, S.; Xu, X.; Sun, Q.; Xiong, M.; Chen, B. FHL3 Contributes to EMT and Chemotherapy Resistance Through Up-Regulation of Slug and Activation of TGFβ/Smad-Independent Pathways in Gastric Cancer. Front. Oncol. 2021, 11, 649029. [Google Scholar]
- Chen, X.; Zhou, Z.; Zhang, Z.; Zhao, C.; Li, J.; Jiang, J.; Huang, B.; Qin, Y. Puerarin inhibits EMT induced by oxaliplatin via targeting carbonic anhydrase XII. Front. Pharmacol. 2022, 13, 969422. [Google Scholar]
- Liang, R.; Zhang, J.; Liu, Z.; Liu, Z.; Li, Q.; Luo, X.; Li, Y.; Ye, J.; Lin, Y. Mechanism and Molecular Network of RBM8A-Mediated Regulation of Oxaliplatin Resistance in Hepatocellular Carcinoma. Front. Oncol. 2021, 10, 585452. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar]
- Babaei, G.; Aziz, S.G.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021, 133, 110909. [Google Scholar]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar]
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch. Pharm. Res. 2019, 42, 14–24. [Google Scholar]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updat. 2020, 53, 100715. [Google Scholar]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Park, S.; Choi, M.; Lee, M. Effects of Anthocyanin Supplementation on Reduction of Obesity Criteria: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Possible Effects of Dietary Anthocyanins on Diabetes and Insulin Resistance. Curr. Drug Targets 2017, 18, 629–640. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Ma, X.; Ning, S. Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phytother. Res. 2019, 33, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, W.; Huo, Y.; Song, G.; Sun, H.; Guo, X.; Wang, C. Cyanidin-3-glucoside attenuates 4-hydroxynonenal- and visible light-induced retinal damage in vitro and in vivo. Food Funct. 2019, 10, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Kim, M.S.; de Mejia, E.G. Anthocyanins, delphinidin-3-O-glucoside; cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, Y.; Li, H.; Wang, L.; Ponikwicka-Tyszko, D.; Lebiedzinska, W.; Pilaszewicz-Puza, A.; Liu, H.; Zhou, L.; Fan, H.; et al. Cyanidin-3-O-Glucoside Pharmacologically Inhibits Tumorigenesis via Estrogen Receptor β in Melanoma Mice. Front. Oncol. 2019, 9, 1110. [Google Scholar] [CrossRef]
- Matboli, M.; Hasanin, A.H.; Hussein, R.; El-Nakeep, S.; Habib, E.K.; Ellackany, R.; Saleh, L.A. Cyanidin 3-glucoside modulated cell cycle progression in liver precancerous lesion, in vivo study. World J. Gastroenterol. 2021, 27, 1435–1450. [Google Scholar] [CrossRef]
- Baster, Z.; Li, L.; Kukkurainen, S.; Chen, J.; Pentikäinen, O.; Győrffy, B.; Hytönen, V.P.; Zhu, H.; Rajfur, Z.; Huang, C. Cyanidin-3-glucoside binds to talin and modulates colon cancer cell adhesions and 3D growth. FASEB J. 2020, 34, 2227–2237. [Google Scholar] [CrossRef]
- Jing, N.; Song, J.; Liu, Z.; Wang, L.; Jiang, G. Glycosylation of anthocyanins enhances the apoptosis of colon cancer cells by handicapping energy metabolism. BMC Complement. Med. Ther. 2020, 20, 312. [Google Scholar] [CrossRef]
- Pinner, S.; Sahai, E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat. Cell Biol. 2008, 10, 127–137. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Wang, H.; Li, G.; Yan, H.; Fei, Z.; Xu, Y.; Li, W. Curcumin reverses irinotecan resistance in colon cancer cell by regulation of epithelial-mesenchymal transition. Anticancer. Drugs 2018, 29, 334–340. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J.; Transition, E.-M.; Cancer, P. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Hua, Y.; Zhu, Y.; Zhang, J.; Zhu, Z.; Ning, Z.; Chen, H.; Liu, L.; Chen, Z.; Meng, Z. miR-122 Targets X-Linked Inhibitor of Apoptosis Protein to Sensitize Oxaliplatin-Resistant Colorectal Cancer Cells to Oxaliplatin-Mediated Cytotoxicity. Cell Physiol. Biochem. 2018, 51, 2148–2159. [Google Scholar] [CrossRef]
- Qi, F.F.; Yang, Y.; Zhang, H.; Chen, H. Long non-coding RNAs: Key regulators in oxaliplatin resistance of colorectal cancer. Biomed. Pharmacother. 2020, 128, 110329. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chen, M.C.; Baskaran, R.; Lin, Y.M.; Day, C.H.; Lin, Y.J.; Tu, C.C.; Vijaya Padma, V.; Kuo, W.W. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell Physiol. 2018, 233, 5458–5467. [Google Scholar] [CrossRef]
- Liang, H.; Xu, Y.; Zhang, Q.; Yang, Y.; Mou, Y.; Gao, Y.; Chen, R.; Chen, C.; Dai, P. MiR-483-3p regulates oxaliplatin resistance by targeting FAM171B in human colorectal cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 725–736. [Google Scholar] [CrossRef]
- Noordhuis, P.; Laan, A.C.; van de Born, K.; Honeywell, R.J.; Peters, G.J. Coexisting Molecular Determinants of Acquired Oxaliplatin Resistance in Human Colorectal and Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2019, 20, 3619. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Reactive oxygen species induce epithelial-mesenchymal transition, glycolytic switch, and mitochondrial repression through the Dlx-2/Snail signaling pathways in MCF-7 cells. Mol. Med. Rep. 2019, 20, 2339–2346. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, G.; Fu, Y.; Han, Y.; Guo, C.; Yin, L.; Cai, C.; Shen, H.; Wu, S.; Zeng, S. FOXC2 Promotes Oxaliplatin Resistance by Inducing Epithelial-Mesenchymal Transition via MAPK/ERK Signaling in Colorectal Cancer. OncoTargets Ther. 2020, 13, 1625–1635. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Mao, L.; Li, Y.; Zhao, J.; Li, Q.; Yang, B.; Wang, Y.; Zhu, Z.; Sun, H.; Zhai, Z. Transforming growth factor-β1 contributes to oxaliplatin resistance in colorectal cancer via epithelial to mesenchymal transition. Oncol. Lett. 2017, 14, 647–654. [Google Scholar] [CrossRef]
- Wei, W.; Ma, X.D.; Jiang, G.M.; Shi, B.; Zhong, W.; Sun, C.L.; Zhao, L.; Hou, Y.J.; Wang, H. The AKT/GSK3-Mediated Slug Expression Contributes to Oxaliplatin Resistance in Colorectal Cancer via Upregulation of ERCC1. Oncol. Res. 2020, 28, 423–438. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.S.; Zhang, X.H.; Yang, S.N.; Liu, D.; Diao, C.R.; Wang, H.; Zheng, F.P. Cyanidin inhibits EMT induced by oxaliplatin via targeting the PDK1-PI3K/Akt signaling pathway. Food Funct. 2019, 10, 592–601. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Wang, Y.; Shen, H.; Wang, X.; Wu, L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Onco Targets Ther. 2019, 12, 3893–3903. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, M.; Ye, Q.; Wang, X.; Xu, J.; Shi, G.; Hu, Z. Cyanidin-3-O-glucoside inhibits epithelial-to-mesenchymal transition, and migration and invasion of breast cancer cells by upregulating KLF4. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurter, H.; Basbinar, Y.; Ellidokuz, H.; Calibasi-Kocal, G. The Role of Cyanidin-3-O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer. Nutrients 2023, 15, 4705. https://doi.org/10.3390/nu15224705
Kurter H, Basbinar Y, Ellidokuz H, Calibasi-Kocal G. The Role of Cyanidin-3-O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer. Nutrients. 2023; 15(22):4705. https://doi.org/10.3390/nu15224705
Chicago/Turabian StyleKurter, Hasan, Yasemin Basbinar, Hulya Ellidokuz, and Gizem Calibasi-Kocal. 2023. "The Role of Cyanidin-3-O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer" Nutrients 15, no. 22: 4705. https://doi.org/10.3390/nu15224705
APA StyleKurter, H., Basbinar, Y., Ellidokuz, H., & Calibasi-Kocal, G. (2023). The Role of Cyanidin-3-O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer. Nutrients, 15(22), 4705. https://doi.org/10.3390/nu15224705




