Changes in Anxiety following Taste Education Intervention: Fussy Eating Children with and without Neurodevelopmental Disorders
Abstract
:1. Introduction
2. Materials and Methods
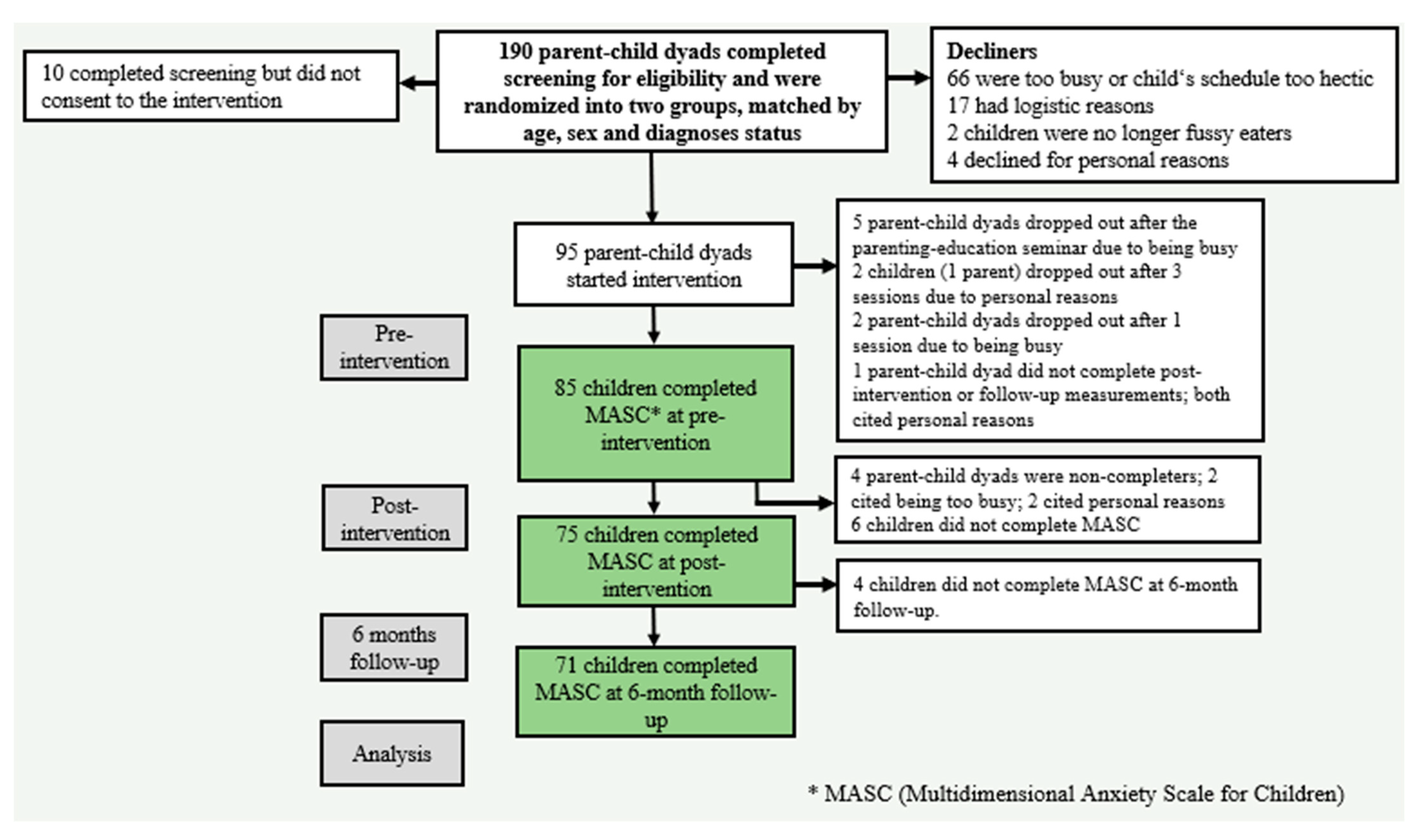
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Measures
2.4.1. Multidimensional Anxiety Scale for Children (MASC)
2.4.2. Background Information
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Changes in Anxiety Scores
3.3. Changes in Anxiety Scores Based on Neurodevelopmental Disorder Status
4. Discussion
4.1. Changes in Anxiety Scores
4.2. Changes in Anxiety Scores Based on Neurodevelopmental Disorder Status
4.3. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeCosta, P.; Moller, P.; Frost, M.B.; Olsen, A. Changing children’s eating behaviour—A review of experimental research. Appetite 2017, 113, 327–357. [Google Scholar] [CrossRef]
- Olsen, A. Reflections on current practice for taste learning in children. Int. J. Gastron. Food Sci. 2019, 15, 26–29. [Google Scholar] [CrossRef]
- Dovey, T.M.; Staples, P.A.; Gibson, E.L.; Halford, J.C.G. Food neophobia and ‘picky/fussy’ eating in children: A review. Appetite 2008, 50, 181–193. [Google Scholar] [CrossRef]
- Mascola, A.J.; Bryson, S.W.; Agras, W.S. Picky eating during childhood: A longitudinal study to age 11 years. Eat. Behav. 2010, 11, 253–257. [Google Scholar] [CrossRef]
- Taylor, C.M.; Wernimont, S.M.; Northstone, K.; Emmett, P.M. Picky/fussy eating in children: Review of definitions, assessment, prevalence and dietary intakes. Appetite 2015, 95, 349–359. [Google Scholar] [CrossRef]
- Gibson, E.L.; Cooke, L. Understanding Food Fussiness and Its Implications for Food Choice, Health, Weight and Interventions in Young Children: The Impact of Professor Jane Wardle. Curr. Obes. Rep. 2017, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.K.; Freeze, B.; Provost, E.; King, L.; Sanders, M. Maternal perceptions of nutrition, stress, time, and assistance during mealtimes: Similarities and differences between mothers of children with autism spectrum disorders and mothers of children with typical development. J. Occup. Ther. Sch. Early Interv. 2016, 9, 242–257. [Google Scholar] [CrossRef]
- Mitchell, G.L.; Farrow, C.; Haycraft, E.; Meyer, C. Parental influences on children’s eating behaviour and characteristics of successful parent-focussed interventions. Appetite 2013, 60, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Farrow, C.V.; Blissett, J. Controlling feeding practices: Cause or consequence of early child weight? Pediatrics 2008, 121, e164–e169. [Google Scholar] [CrossRef]
- Bandini, L.G.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 439–446. [Google Scholar] [CrossRef]
- Curtis, L.T.; Patel, K. Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): A review. J. Altern. Complement. Med. 2008, 14, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Farrow, C.V.; Coulthard, H. Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite 2012, 58, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Maratos, F.A.; Sharpe, E.E. The origins of disordered eating and childhood food neophobia: Applying an anxiety perspective. In Food Neophobia; Reilly, S., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 305–328. [Google Scholar] [CrossRef]
- de Barse, L.M.; Cardona Cano, S.; Jansen, P.W.; Jaddoe, V.V.W.; Verhulst, F.C.; Franco, O.H.; Tiemeier, H.; Tharner, A. Are parents’ anxiety and depression related to child fussy eating? Arch. Dis. Child. 2016, 101, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Lafraire, J.; Rioux, C.; Giboreau, A.; Picard, D. Food rejections in children: Cognitive and social/environmental factors involved in food neophobia and picky/fussy eating behavior. Appetite 2016, 96, 347–357. [Google Scholar] [CrossRef]
- Zucker, N.; Copeland, W.; Franz, L.; Carpenter, K.; Keeling, L.; Angold, A.; Egger, H. Psychological and Psychosocial Impairment in Preschoolers With Selective Eating. Pediatrics 2015, 136, e582–e590. [Google Scholar] [CrossRef]
- Manning-Courtney, P.; Murray, D.; Currans, K.; Johnson, H.; Bing, N.; Kroeger-Geoppinger, K.; Sorensen, R.; Bass, J.; Reinhold, J.; Johnson, A.; et al. Autism Spectrum Disorders. Curr. Probl. Pediatr. Adolesc. Health Care 2013, 43, 2–11. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Smith, B.; Rogers, S.L.; Blissett, J.; Ludlow, A.K. The relationship between sensory sensitivity, food fussiness and food preferences in children with neurodevelopmental disorders. Appetite 2020, 150, 104643. [Google Scholar] [CrossRef]
- Evans, E.W.; Must, A.; Anderson, S.E.; Curtin, C.; Scampini, R.; Maslin, M.; Bandini, L. Dietary patterns and body mass index in children with autism and typically developing children. Res. Autism Spectr. Disord. 2012, 6, 399–405. [Google Scholar] [CrossRef]
- Hubbard, K.L.; Anderson, S.E.; Curtin, C.; Must, A.; Bandini, L.G. A comparison of food refusal related to characteristics of food in children with autism spectrum disorder and typically developing children. J. Acad. Nutr. Diet. 2014, 114, 1981–1987. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Mayes, R.D.; Molitoris, S. Autism and ADHD: Overlapping and discriminating symptoms. Res. Autism Spectr. Disord. 2012, 6, 277–285. [Google Scholar] [CrossRef]
- Råstam, M.; Täljemark, J.; Tajnia, A.; Lundström, S.; Gustafsson, P.; Lichtenstein, P.; Gillberg, C.; Anckarsäter, H.; Kerekes, N. Eating Problems and Overlap with ADHD and Autism Spectrum Disorders in a Nationwide Twin Study of 9- and 12-Year-Old Children. Sci. World J. 2013, 2013, 315429. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Zickgraf, H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res. Autism Spectr. Disord. 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Cortese, S.; Angriman, M.; Maffeis, C.; Isnard, P.; Konofal, E.; Lecendreux, M.; Purper-Ouakil, D.; Vincenzi, B.; Bernardina, B.D.; Mouren, M.-C. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: A Systematic Review of the Literature. Crit. Rev. Food Sci. Nutr. 2008, 48, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Tessari, L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr. Psychiatry Rep. 2017, 19, 4. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Dassen, F.C.; Franken, L.; Resch, C.; Houben, K. Impulsivity and overeating in children in the absence and presence of hunger. Appetite 2015, 93, 57–61. [Google Scholar] [CrossRef]
- Abramovitch, A.; Dar, R.; Mittelman, A.; Wilhelm, S. Comorbidity Between Attention Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder Across the Lifespan: A Systematic and Critical Review. Harv. Rev. Psychiatry 2015, 23, 245–262. [Google Scholar] [CrossRef]
- Antshel, K.M.; Zhang-James, Y.; Wagner, K.E.; Ledesma, A.; Faraone, S.V. An update on the comorbidity of ADHD and ASD: A focus on clinical management. Expert Rev. Neurother. 2016, 16, 279–293. [Google Scholar] [CrossRef]
- Essawy, H.E.; Abdelgawad, A.A.; Khamis, M.E.; Zakaria, A. Study of disturbed eating behaviors in children with attention deficit hyperactivity disorder. Middle East Curr. Psychiatry 2020, 27, 8. [Google Scholar] [CrossRef]
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef]
- Kuusikko, S.; Pollock-Wurman, R.; Jussila, K.; Carter, A.S.; Mattila, M.L.; Ebeling, H.; Pauls, D.L.; Moilanen, I. Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. J. Autism Dev. Disord. 2008, 38, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Maric, M.; van Steensel, F.J.A.; Bögels, S.M. Parental Involvement in CBT for Anxiety-Disordered Youth Revisited: Family CBT Outperforms Child CBT in the Long Term for Children With Comorbid ADHD Symptoms. J. Atten. Disord. 2015, 22, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Brynjolfsdottir, B.; Bjarnason, R.; Helgason, T.; Olafsdottir, A.S. Offitumeðferð barna í Heilsuskóla Barnaspítalans: Breytingar á algengi ADHD, einhverfu, kvíða og þunglyndis. Sálfræðiritið 2017, 22, 85–102. [Google Scholar]
- Mayes, S.D.; Baweja, R.; Waschbusch, D.A.; Calhoun, S.L. Relationship between IQ and Internalizing and Externalizing Symptoms in Children with Autism and Children with ADHD. J. Ment. Health Res. Intellect. Disabil. 2022, 15, 95–110. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Olafsdottir, A.S.; Brynjolfsdottir, B.; Bjarnason, R.; Njardvik, U. Odds of fussy eating are greater among children with obesity and anxiety. Obes. Sci. Pract. 2022, 8, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, G.M.; Njardvik, U. Prevalence and Gender Differences of ODD, Anxiety, and Depression in a Sample of Children with ADHD. J. Atten. Disord. 2019, 23, 1339–1345. [Google Scholar] [CrossRef]
- Beighley, J.S.; Matson, J.L.; Rieske, R.D.; Adams, H.L. Food selectivity in children with and without an autism spectrum disorder: Investigation of diagnosis and age. Res. Dev. Disabil. 2013, 34, 3497–3503. [Google Scholar] [CrossRef]
- Efron, D.; Bryson, H.; Lycett, K.; Sciberras, E. Children referred for evaluation for ADHD: Comorbidity profiles and characteristics associated with a positive diagnosis. Child Care Health Dev. 2016, 42, 718–724. [Google Scholar] [CrossRef]
- Gordon-Lipkin, E.; Marvin, A.R.; Law, J.K.; Lipkin, P.H. Anxiety and Mood Disorder in Children With Autism Spectrum Disorder and ADHD. Pediatrics 2018, 141, e20171377. [Google Scholar] [CrossRef]
- Bellini, S. Social Skill Deficits and Anxiety in High-Functioning Adolescents With Autism Spectrum Disorders. Focus Autism Other Dev. Disabil. 2004, 19, 78–86. [Google Scholar] [CrossRef]
- Jarrett, M.A.; Ollendick, T.H. A conceptual review of the comorbidity of attention-deficit/hyperactivity disorder and anxiety: Implications for future research and practice. Clin. Psychol. Rev. 2008, 28, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Pliner, P.; Eng, A.; Krishnan, K. The effects of fear and hunger on food neophobia in humans. Appetite 1995, 25, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Haraldsson, H.; Olafsdottir, A.S. Taste education—A food-based intervention in a school setting, focusing on children with and without neurodevelopmental disorders and their families. A randomized controlled trial. Appetite 2021, 167, 105623. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Olafsdottir, A.S. Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients 2022, 14, 4000. [Google Scholar] [CrossRef]
- Houldcroft, L.; Farrow, C.; Haycraft, E. Perceptions of parental pressure to eat and eating behaviours in preadolescents: The mediating role of anxiety. Appetite 2014, 80, 61–69. [Google Scholar] [CrossRef]
- Kuschner, E.S.; Eisenberg, I.W.; Orionzi, B.; Simmons, W.K.; Kenworthy, L.; Martin, A.; Wallace, G.L. A preliminary study of self-reported food selectivity in adolescents and young adults with autism spectrum disorder. Res. Autism Spectr. Disord. 2015, 15–16, 53–59. [Google Scholar] [CrossRef]
- Wardle, J.; Cooke, L.J.; Gibson, E.L.; Sapochnik, M.; Sheiham, A.; Lawson, M. Increasing children’s acceptance of vegetables; a randomized trial of parent-led exposure. Appetite 2003, 40, 155–162. [Google Scholar] [CrossRef]
- Lakkakula, A.; Geaghan, J.; Zanovec, M.; Pierce, S.; Tuuri, G. Repeated taste exposure increases liking for vegetables by low-income elementary school children. Appetite 2010, 55, 226–231. [Google Scholar] [CrossRef]
- Laureati, M.; Bergamaschi, V.; Pagliarini, E. School-based intervention with children. Peer-modeling, reward and repeated exposure reduce food neophobia and increase liking of fruits and vegetables. Appetite 2014, 83, 26–32. [Google Scholar] [CrossRef]
- Skouteris, H.; Hill, B.; McCabe, M.; Swinburn, B.; Busija, L. A parent-based intervention to promote healthy eating and active behaviours in pre-school children: Evaluation of the MEND 2–4 randomized controlled trial. Pediatr. Obes. 2016, 11, 4–10. [Google Scholar] [CrossRef]
- Skouteris, H.; McCabe, M.; Swinburn, B.; Newgreen, V.; Sacher, P.; Chadwick, P. Parental influence and obesity prevention in pre-schoolers: A systematic review of interventions. Obes. Rev. 2011, 12, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Battjes-Fries, M.C.E.; Haveman-Nies, A.; Zeinstra, G.G.; van Dongen, E.J.I.; Meester, H.J.; van den Top-Pullen, R.; van’t Veer, P.; de Graaf, K. Effectiveness of Taste Lessons with and without additional experiential learning activities on children’s willingness to taste vegetables. Appetite 2017, 109, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ensaff, H.; Crawford, R.; Russell, J.M.; Barker, M.E. Preparing and sharing food: A quantitative analysis of a primary school-based food intervention. J. Public Health 2016, 39, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Upton, P.; Upton, D. Can a school-based intervention increase fruit and vegetable consumption for children with Autism? Educ. Health 2013, 31, 95–97. [Google Scholar]
- Curtin, C.; Hubbard, K.; Anderson, S.E.; Mick, E.; Must, A.; Bandini, L.G. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Olsen, A.; Olafsdottir, A.S. Fussy Eating among Children and Their Parents: Associations in Parent-Child Dyads, in a Sample of Children with and without Neurodevelopmental Disorders. Nutrients 2021, 13, 2196. [Google Scholar] [CrossRef]
- March, J.S.; Parker, J.D.A.; Sullivan, K.; Stallings, P.; Conners, C.K. The Multidimensional Anxiety Scale for Children (MASC): Factor Structure, Reliability, and Validity. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 554–565. [Google Scholar] [CrossRef]
- Ólason, D.T.; Blöndahl, M.S.; Smári, J. Psychometric properties of the Multidimensional Anxiety Scale for Children (MASC) among Icelandic schoolchildren. Scand. J. Psychol. 2004, 45, 429–436. [Google Scholar] [CrossRef]
- Jonsdottir, I.H.; Hansdottir, I.; Olafsson, R.P.; Johannsdottir, S.; Sigursteinsson, H.; Thorvaldsdottir, G.H. Mælitæki og sálfræðileg próf í notkun hjá sálfræðingum á Íslandi: Rafræn könnun prófanefndar Sálfræðingafélags Íslands. Salfraediritid (Icel. J. Psychol.) 2013, 18, 93–102. [Google Scholar]
- R: CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Little, R.J.A. A Test of Missing Completely at Random for Multivariate Data with Missing Values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Noor, N.M.; Al Bakri Abdullah, M.M.; Yahaya, A.S.; Ramli, N.A. Comparison of Linear Interpolation Method and Mean Method to Replace the Missing Values in Environmental Data Set. Mater. Sci. Forum 2015, 803, 278–281. [Google Scholar] [CrossRef]
- Svavarsdottir, E.K.; Gisladottir, M.; Tryggvadottir, G.B. Perception on family support and predictors’ of satisfaction with the healthcare service among families of children and adolescents with serious mental illnesses who are in active psychiatric treatment. J. Child Adolesc. Psychiatr. Nurs. 2019, 32, 6–15. [Google Scholar] [CrossRef]
- Zoëga, H.; Baldursson, G.; Hrafnkelsson, B.; Almarsdóttir, A.B.; Valdimarsdóttir, U.; Halldórsson, M. Psychotropic Drug Use among Icelandic Children: A Nationwide Population-Based Study. J. Child Adolesc. Psychopharmacol. 2009, 19, 757–764. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Parents reported oral sensory sensitivity processing and food preference in ADHD. J. Psychiatr. Ment. Health Nurs. 2013, 20, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Grondhuis, S.N.; Aman, M.G. Assessment of anxiety in children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2012, 6, 1345–1365. [Google Scholar] [CrossRef]
- van Steijn, D.J.; Oerlemans, A.M.; van Aken, M.A.; Buitelaar, J.K.; Rommelse, N.N. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J. Autism Dev. Disord. 2014, 44, 1064–1076. [Google Scholar] [CrossRef]
- Brown, S. The Rejection of Known and Previously Accepted Foods in Early Childhood. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2010. [Google Scholar]
- Werthmann, J.; Jansen, A.; Havermans, R.; Nederkoorn, C.; Kremers, S.; Roefs, A. Bits and pieces. Food texture influences food acceptance in young children. Appetite 2015, 84, 181–187. [Google Scholar] [CrossRef]
- Maratos, F.A.; Staples, P. Attentional biases towards familiar and unfamiliar foods in children. The role of food neophobia. Appetite 2015, 91, 220–225. [Google Scholar] [CrossRef]
- Arendt, K.; Thastum, M.; Hougaard, E. Efficacy of a Danish version of the Cool Kids program: A randomized wait-list controlled trial. Acta Psychiatr. Scand. 2016, 133, 109–121. [Google Scholar] [CrossRef]
- Sofronoff, K.; Attwood, T.; Hinton, S. A randomised controlled trial of a CBT intervention for anxiety in children with Asperger syndrome. J. Child Psychol. Psychiatry 2005, 46, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Esbjørn, B.H.; Breinholst, S.; Reinholdt-Dunne, M.L. Parental Involvement in Cognitive Behavior Therapy for Children with Anxiety Disorders: 3-Year Follow-Up. Child Psychiatry Hum. Dev. 2017, 48, 444–454. [Google Scholar] [CrossRef] [PubMed]


| Total Sample (n = 71) | Without ND (n = 40) | ND (ADHD, ASD, or Both) (n = 31) | |
|---|---|---|---|
| Child | |||
| Female, n (%) | 28 (39.4) | 20 (50.0) | 8 (25.8) |
| Male, n (%) | 43 (60.6) | 20 (50.0) | 23 (74.2) |
| Mean age in years (SD) | 9.2 (1.58) | 8.9 (1.63) | 9.4 (1.48) |
| Diagnosis, n (%) | |||
| ADHD, primarily | 11 (15.5) | - | 11 (35.5) |
| ASD, primarily | 5 (7.0) | - | 5 (16.1) |
| Anxiety | 8 (11.3) | 2 (5.0) | 6 (19.3) |
| Other | 7 (9.8) | 4 (10.0) | 3 (9.7) |
| Parent | |||
| Mother | 65 (91.5) | 37 (92.5) | 28 (90.3) |
| Father | 6 (8.5) | 3 (7.5) | 3 (9.7) |
| Education level, n (%) | |||
| No higher education | 4 (5.6) | 3 (7.5) | 1 (3.2) |
| Vocational education | 11 (15.5) | 5 (12.5) | 6 (19.4) |
| University level | 56 (78.9) | 29 (72.5) | 27 (87.1) |
| Occupational status, n (%) | |||
| Full-time occupation | 54 (76.1) | 33 (82.5) | 21 (67.7) |
| Part-time occupation or student | 17 (23.9) | 10 (25.0) | 7 (22.6) |
| Single-parent household, n (%) | 10 (14.1) | 3 (7.5) | 7 (22.6) |
| Children in the household, n (%) | |||
| 1 | 8 (11.3) | 4 (10.0) | 4 (12.9) |
| 2 | 30 (42.3) | 17 (42.5) | 13 (41.9) |
| 3 or more children | 33 (46.5) | 19 (47.5) | 14 (45.2) |
| MASC Total n = 71 | Pre-Intervention M (SD) | Post-Intervention M (SD) | 6 Month Follow-Up M (SD) | F | p | Cohen’s d |
|---|---|---|---|---|---|---|
| MASC Total | 56.6 (11.38) | 54.1 (10.89) | 53.1 (10.93) | 3.987 | 0.021 | 0.32 |
| Physical symptoms | 51.7 (12.25) | 48.7 (11.17) | 47.9 (10.42) | 4.323 | 0.015 | 0.33 |
| Harm avoidance | 53.8 (8.84) | 52.6 (9.99) | 53.2 (11.44) | 0.434 | 0.649 | 0.06 |
| Social anxiety | 55.0 (11.10) | 52.8 (11.12) | 52.1 (11.60) | 3.683 | 0.028 | 0.26 |
| Separation anxiety | 62.3 (12.87) | 60.8 (13.97) | 58.2 (12.11) | 4.488 | 0.013 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsteinsdottir, S.; Olafsdottir, A.S.; Traustadottir, O.U.; Njardvik, U. Changes in Anxiety following Taste Education Intervention: Fussy Eating Children with and without Neurodevelopmental Disorders. Nutrients 2023, 15, 4783. https://doi.org/10.3390/nu15224783
Thorsteinsdottir S, Olafsdottir AS, Traustadottir OU, Njardvik U. Changes in Anxiety following Taste Education Intervention: Fussy Eating Children with and without Neurodevelopmental Disorders. Nutrients. 2023; 15(22):4783. https://doi.org/10.3390/nu15224783
Chicago/Turabian StyleThorsteinsdottir, Sigrun, Anna S. Olafsdottir, Olof U. Traustadottir, and Urdur Njardvik. 2023. "Changes in Anxiety following Taste Education Intervention: Fussy Eating Children with and without Neurodevelopmental Disorders" Nutrients 15, no. 22: 4783. https://doi.org/10.3390/nu15224783





