Association between Asymptomatic Hyperuricemia with Adiposity Indices: A Cross-Sectional Study in a Spanish Population
Abstract
1. Introduction
2. Methods
2.1. Study Variable and Definitions
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keenan, R.T. The Biology of Urate. Semin. Arthritis Rheum. 2020, 50, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, L.A.; Kim, S.C. Gout: A Review of Nonmodifiable and Modifiable Risk Factors. Rheum. Dis. Clin. N. Am. 2014, 40, 581–604. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Saag, K.G.; Becker, M.A.; Borer, J.S.; Gorelick, P.B.; Whelton, A.; Hunt, B.; Castillo, M.; Gunawardhana, L. CARES Investigators Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. N. Engl. J. Med. 2018, 378, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Domienik-Karłowicz, J.; Tykarski, A.; Widecka, K.; Filipiak, K.J.; Jaguszewski, M.J.; Narkiewicz, K.; Mancia, G. Expert Consensus for the Diagnosis and Treatment of Patient with hyperuricemia and High Cardiovascular Risk: 2021 Update. Cardiol. J. 2021, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.W.; Glynn, R.J.; DeLabry, L.O. Asymptomatic Hyperuricemia. Risks and Consequences in the Normative Aging Study. Am. J. Med. 1987, 82, 421–426. [Google Scholar] [CrossRef]
- Wang, J.; Qin, T.; Chen, J.; Li, Y.; Wang, L.; Huang, H.; Li, J. Hyperuricemia and Risk of Incident Hypertension: A Systematic and Meta-Analysis of Observational Studies. PLoS ONE 2014, 9, e114259. [Google Scholar] [CrossRef]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Hara, S.; Roncal-Jimenez, C.A.; Bjornstad, P.; Nakagawa, T.; Andres-Hernando, A.; Sato, Y.; Jensen, T.; et al. Uric Acid Is a Strong Risk Marker for Developing Hypertension From Prehypertension: A 5-Year Japanese Cohort Study. Hypertension 2017, 71, 78–86. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Kang, S.; Wu, Y. Serum Uric Acid Levels and Incidence of Impaired Fasting glucose and Type 2 Diabetes Mellitus: A Meta-Analysis of Cohort Studies. Diabetes Res. Clin. Pract. 2013, 101, 88–96. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, C.; Li, X.; Sun, L.; Zhu, X.; Zhao, C.; Zhang, Z.; Yang, Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2015, 100, 4198–4207. [Google Scholar] [CrossRef]
- Rahimi-Sakak, F.; Maroofi, M.; Rahmani, J.; Bellissimo, N.; Hekmatdoost, A. Serum Uric Acid and Risk of Cardiovascular Mortality: A Review and Dose-Response Meta-Analysis of Cohort of over a Million Participants. BMC Cardiovasc. Disord. 2019, 19, 218. [Google Scholar] [CrossRef]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and Risk of Stroke: A Systematic Review and meta-Analysis. Arthritis Rheum. 2009, 61, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the Risk for Coronary Heart Disease morbidity and Mortality a Systematic Review and Dose-Response Meta-Analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.; Fang, C.; Pendse, J.; Toprover, M.; Pillinger, M.H. Narrative Review: Peripheral Arterial Disease in Patients with Hyperuricemia and Gout. Curr. Rheumatol. Rep. 2023, 25, 83–97. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, Uric Acid, and the Etiology of Diabetes and Obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice and The Obesity Society. Circulation 2013, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.M.; Tjønneland, A.; et al. General and Abdominal Adiposity and Risk of Death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef]
- Nevill, A.M.; Stewart, A.D.; Olds, T.; Holder, R. Relationship between Adiposity and Body Size Reveals limitations of BMI. Am. J. Phys. Anthropol. 2006, 129, 151–156. [Google Scholar] [CrossRef]
- Tobias, D.K.; Pan, A.; Jackson, C.L.; O’Reilly, E.J.; Ding, E.L.; Willett, W.C.; Manson, J.E.; Hu, F.B. Body-Mass Index and Mortality among Adults with Incident Type 2. N. Engl. J. Med. 2014, 370, 233–244. [Google Scholar] [CrossRef]
- Niedziela, J.; Hudzik, B.; Niedziela, N.; Gasior, M.; Gierlotka, M.; Wasilewski, J.; Myrda, K.; Lekston, A.; Poloński, L.; Rozentryt, P. The Obesity Paradox in Acute Coronary Syndrome: A Meta-Analysis. Eur. J. Epidemiol. 2014, 29, 801–812. [Google Scholar] [CrossRef]
- Oesch, L.; Tatlisumak, T.; Arnold, M.; Sarikaya, H. Obesity Paradox in Stroke—Myth or Reality? A Systematic Review. PLoS ONE 2017, 12, e0171334. [Google Scholar] [CrossRef]
- Li, C.; Ford, E.S.; McGuire, L.C.; Mokdad, A.H. Increasing Trends in Waist Circumference and Abdominal Obesity among US Adults. Obesity 2007, 15, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Mirmiran, P.; Azizi, F. Waist-to-Hip Ratio Is a Better Screening Measure for Cardiovascular Risk Factors than Other Anthropometric Indicators in Tehranian Adult Men. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1325–1332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-Height Ratio Is a Better Screening Tool than Waist Circumference and BMI for Adult Cardiometabolic Risk Factors: Systematic Review and Meta-Analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body Fat Distribution and Noncommunicable Diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur. J. Clin. Nutr. 2009, 64, 2–5. [Google Scholar] [CrossRef]
- Kodama, S.; Horikawa, C.; Fujihara, K.; Heianza, Y.; Hirasawa, R.; Yachi, Y.; Sugawara, A.; Tanaka, S.; Shimano, H.; Iida, K.T.; et al. Comparisons of the Strength of Associations with Future Type 2 Risk among Anthropometric Obesity Indicators, Including-to-Height Ratio: A Meta-Analysis. Am. J. Epidemiol. 2012, 176, 959–969. [Google Scholar] [CrossRef]
- Jayawardena, R.; Ranasinghe, P.; Ranathunga, T.; Mathangasinghe, Y.; Wasalathanththri, S.; Hills, A.P. Novel Anthropometric Parameters to Define Obesity And-Related Disease in Adults: A Systematic Review. Nutr. Rev. 2020, 78, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Rico-Martín, S.; Calderón-García, J.F.; Sánchez-Rey, P.; Franco-Antonio, C.; Martínez Álvarez, M.; Sánchez Muñoz-Torrero, J.F. Effectiveness of Body Roundness Index in Predicting Metabolic: A Systematic Review and Meta-Analysis. Obes. Rev. 2020, 21, e13023. [Google Scholar] [CrossRef]
- Calderón-García, J.F.; Roncero-Martín, R.; Rico-Martín, S.; De Nicolás-Jiménez, J.M.; López-Espuela, F.; Santano-Mogena, E.; Alfageme-García, P.; Sánchez Muñoz-Torrero, J.F. Effectiveness of Body Roundness Index (BRI) and a Body Shape (ABSI) in Predicting Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public. Health 2021, 18, 11607. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, S.; An, R. Effectiveness of A Body Shape Index (ABSI) in Predicting Diseases and Mortality: A Systematic Review and meta-Analysis. Obes. Rev. 2018, 19, 737–759. [Google Scholar] [CrossRef]
- Costo-Muriel, C.; Calderón-García, J.F.; Rico-Martín, S.; Sánchez-Bacaicoa, C.; Escudero-Sánchez, G.; Galán-González, J.; Rodríguez-Velasco, F.J.; Sánchez Muñoz-Torrero, J.F. Association of Subclinical Carotid Atherosclerosis Assessed By-Resolution Ultrasound With Traditional and Novel Indices. Curr. Probl. Cardiol. 2022, 48, 101574. [Google Scholar] [CrossRef]
- Costo-Muriel, C.; Calderón-García, J.F.; Rico-Martín, S.; Galán-González, J.; Escudero-Sánchez, G.; Sánchez-Bacaicoa, C.; Rodríguez-Velasco, F.J.; Santano-Mogena, E.; Fonseca, C.; Sánchez Muñoz-Torrero, J.F. Relationship between the Novel and Traditional Anthropometric and Subclinical Atherosclerosis Evaluated by Carotid-Media Thickness (c-IMT). Front. Nutr. 2023, 10, 1170450. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. A New Body Shape Index Predicts Mortality Hazard Independently of body Mass Index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Abdominal Volume Index. An Anthropometry-Based Index for estimation of Obesity Is Strongly Related to Impaired Glucose and Type 2 Diabetes Mellitus. Arch. Med. Res. 2003, 34, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Stefanovski, D.; Buchanan Thomas A and Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A Better Index of Body Adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between Body Roundness with Body Fat and Visceral Tissue Emerging from a New Geometrical Model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef]
- Valdez, R. A Simple Model-Based Index of Abdominal Adiposity. J. Clin. Epidemiol. 1991, 44, 955–956. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical Usefulness of a New Equation for Estimating Body Fat. Diabetes Care 2011, 35, 383–388. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Kwon, T.Y.; Kim, S.G. A Novel Adiposity Index as an Integrated Predictor of cardiometabolic Disease Morbidity and Mortality. Sci. Rep. 2018, 8, 16753. [Google Scholar] [CrossRef]
- Zhang, N.; Chang, Y.; Guo, X.; Chen, Y.; Ye, N.; Sun, Y. A Body Shape Index and Body Roundness Index: Two New Body indices for Detecting Association between Obesity and Hyperuricemia in rural Area of China. Eur. J. Intern. Med. 2016, 29, 32–36. [Google Scholar] [CrossRef]
- Liu, X.Z.; Li, H.H.; Huang, S.; Zhao, D.B. Association between Hyperuricemia and Nontraditional Adiposity. Clin. Rheumatol. 2018, 38, 1055–1062. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Wang, S.; Qian, H.; Jia, P.; Chen, Y.; Li, Z.; Zhang, L. Body Adiposity Index, Lipid Accumulation Product, and cardiometabolic Index Reveal the Contribution of Adiposity in the Risk of Hyperuricemia among Chinese Rural. Clin. Rheumatol. 2018, 37, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, X.; Xu, Y.; Dong, H. Feasibility of Body Roundness Index for Identifying a clustering of Cardiometabolic Abnormalities Compared to BMI, Waist and Other Anthropometric Indices: The China Health and Nutrition Survey, 2008 to 2009. Medicine 2016, 95, e4642. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the Management of Arterial of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.J. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in clinical Practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Rubio, M.A.; Barbany, M.; Moreno, B.; Grupo Colaborativo de la SEEDO. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. 2007, 128, 184–196, quiz 200. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of Gout and Hyperuricemia in the US General: The National Health and Nutrition Examination Survey-2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Lyngdoh, T.; Vuistiner, P.; Marques-Vidal, P.; Rousson, V.; Waeber, G.; Vollenweider, P.; Bochud, M. Serum Uric Acid and Adiposity: Deciphering Causality Using a bidirectional Mendelian Randomization Approach. PLoS ONE 2012, 7, e39321. [Google Scholar] [CrossRef]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Curhan, G. Obesity, Weight Change, Hypertension, Diuretic Use, and Risk of gout in Men: The Health Professionals Follow-up Study. Arch. Intern. Med. 2005, 165, 742–748. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Krishnaswami, S.; Resnick, H.; Kelley, D.E.; Haggerty, C.; Harris, T.B.; Schwartz, A.V.; Kritchevsky, S.; Newman, A.B. Association between Regional Adipose Tissue Distribution and Both 2 Diabetes and Impaired Glucose Tolerance in Elderly Men and Women. Diabetes Care 2003, 26, 372–379. [Google Scholar] [CrossRef]
- Rathmann, W.; Funkhouser, E.; Dyer, A.R.; Roseman, J.M. Relations of Hyperuricemia with the Various Components of the insulin Resistance Syndrome in Young Black and White Adults: The Study. Coronary Artery Risk Development in Young Adults. Ann. Epidemiol. 1998, 8, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse Effects of the Classic Antioxidant Uric Acid in adipocytes: NADPH Oxidase-Mediated Oxidative/Nitrosative Stress. Am. J. Physiol. Cell Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Daimon, T. The “cardiometabolic Index” as a New Marker Determined by adiposity and Blood Lipids for Discrimination of Diabetes. Clin. Chim. Acta 2014, 438, 274–278. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid Accumulation Product” Performs Better than the Body Index for Recognizing Cardiovascular Risk: A-Based Comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed]
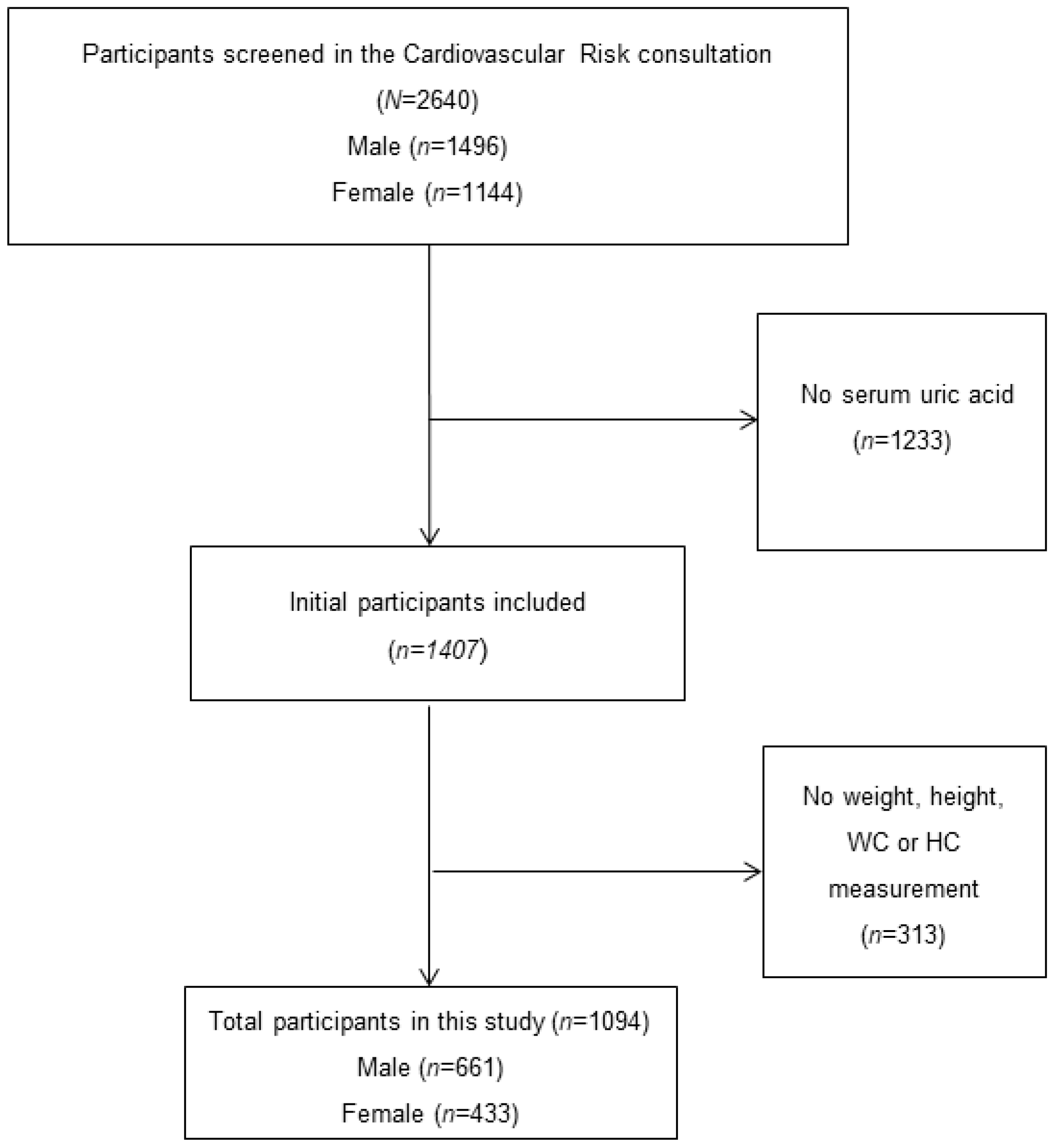
| Normouricemia (n = 862) | Asymptomatic Hyperuricemia (n = 232) | p-Value | |
|---|---|---|---|
| Age (years) | 53.62 ± 12.61 | 59.04 ± 12.59 | <0.001 |
| Gender-males (%) | 518 (60.1%) | 143 (61.6%) | 0.669 |
| CV Risk factors | |||
| Current smokers (%) | 164 (19.0%) | 31 (13.4%) | 0.047 |
| Hypertension (%) | 491 (57.0%) | 183 (78.9%) | <0.001 |
| Dyslipidemia (%) | 749 (86.9%) | 194 (83.6%) | 0.201 |
| Diabetes (%) | 236 (27.4%) | 84 (36.2%) | 0.009 |
| Obesity (%) | 312 (36.2%) | 115 (49.6%) | <0.001 |
| Metabolic Syndrome (%) | 274 (31.8%) | 117 (50.4%) | <0.001 |
| Sedentary (%) | 278 (32.3%) | 90 (38.8%) | 0.062 |
| CV event (%) | 191 (22.2%) | 71 (30.6%) | 0.008 |
| Clinical and laboratory evaluation | |||
| SBP (mmHg) | 138.22 ± 17.74 | 141.99 ± 18.84 | 0.005 |
| DBP (mmHg) | 81.41 ± 10.04 | 80.00 ± 11.13 | 0.063 |
| PP (mmHg) | 56.80 ± 16.39 | 61.99 ± 18.53 | <0.001 |
| Total cholesterol (mg/dL) | 177.96 ± 41.86 | 170.22 ± 39.24 | 0.011 |
| LDL (mg/dL) | 99.40 ± 36.56 | 91.73 ± 34.05 | 0.003 |
| Triglyceride (mg/dL) | 137.86 ± 95.47 | 164.89 ± 100.66 | <0.001 |
| FPG (mg/dL) | 107.45 ± 28.15 | 116.53 ± 40.38 | 0.001 |
| HbA1C (%) | 6.01 ± 0.99 | 6.16 ± 0.94 | 0.035 |
| Drugs | |||
| Antihypertensive drugs (%) | 453 (53.6%) | 174 (75.0%) | <0.001 |
| Lipid-lowering drugs (%) | 667 (77.3%) | 169 (72.8%) | 0.149 |
| Antidiabetic drugs (%) | 218 (25.3%) | 79 (34.1%) | 0.008 |
| Traditional anthropometric indices | |||
| BMI (kg/m2) | 29.03 ± 4.96 | 30.68 ± 5.01 | <0.001 |
| WHR | 0.93 ± 0.09 | 0.96 ± 0.07 | <0.001 |
| WHtR | 0.60 ± 0.08 | 0.64 ± 0.08 | <0.001 |
| Novel anthropometric indices | |||
| ABSI | 0.082 ± 0.008 | 0.084 ± 0.007 | 0.001 |
| AVI | 20.17 ± 5.83 | 22.41 ± 5.48 | <0.001 |
| BAI | 32.45 ± 6.41 | 34.35 ± 7.97 | 0.001 |
| BRI | 5.62 ± 2.03 | 6.54 ± 2.22 | <0.001 |
| CI | 1.31 ± 0.13 | 1.36 ± 0.11 | <0.001 |
| CUN-BAE | 34.75 ± 7.91 | 36.97 ± 8.37 | <0.001 |
| WWI | 12.04 ± 1.00 | 12.10 ± 1.17 | 0.496 |
| Correlation Analysis | Multiple Linear Regression Analysis | ||||||
|---|---|---|---|---|---|---|---|
| R | p-Value | Model R2 | Model Adjusted R2 | Standardized β | t | p-Value | |
| Traditional anthropometric indices | |||||||
| BMI (kg/m2) | 0.209 | <0.001 | 0.189 | 0.181 | 0.136 | 4.12 | <0.001 |
| WHR | 0.282 | <0.001 | 0.171 | 0.163 | 0.092 | 2.60 | 0.009 |
| WHtR | 0.195 | <0.001 | 0.179 | 0.171 | 0.136 | 4.18 | <0.001 |
| Novel anthropometric indices | |||||||
| ABSI | 0.112 | <0.001 | 0.166 | 0.157 | −0.003 | −0.055 | 0.649 |
| AVI | 0.274 | <0.001 | 0.182 | 0.174 | 0.147 | 4.59 | <0.001 |
| BAI | −0.055 | 0.040 | 0.141 | 0.163 | 0.098 | 2.68 | 0.007 |
| BRI | 0.188 | <0.001 | 0.181 | 0.173 | 0.141 | 4.40 | <0.001 |
| CI | 0.174 | <0.001 | 0.167 | 0.159 | 0.040 | 1.22 | 0.221 |
| CUN-BAE | −0.062 | 0.042 | 0.188 | 0.180 | 0.237 | 5.35 | <0.001 |
| WWI | −0.222 | <0.001 | 0.169 | 0.161 | −0.083 | −2.19 | 0.029 |
| Subjects with Hyperuricemia (n = 232) OR (CI%95) | p-Value | |
|---|---|---|
| Age (years) ≥ 65 | 1.77 (1.28–2.44) | <0.001 |
| Males (%) | 1.06 (0.79–1.43) | 0.669 |
| Non-smokers | 1.13 (0.83–1.54) | 0.434 |
| Current Smokers (%) | 0.65 (0.43–0.99) | 0.047 |
| Ex-smokers (%) | 1.12 (0.84–1.50) | 0.420 |
| Hypertension (%) | 2.82 (2.00–3.97) | <0.001 |
| Dyslipidemia (%) | 0.77 (0.51–1.14) | 0.201 |
| Diabetes (%) | 1.50 (1.10–2.04) | 0.009 |
| Sedentary (%) | 1.33 (0.98–1.79) | 0.062 |
| Metabolic Syndrome (%) | 2.18 (1.62–2.93) | <0.001 |
| CV event (%) | 1.54 (1.12–2.13) | 0.008 |
| SBP (mmHg) ≥ 140 | 1.43 (1.07–1.92) | 0.015 |
| DBP (mmHg) ≥ 90 | 0.90 (0.63–1.27) | 0.566 |
| PP (mmHg) ≥ 60 | 1.84 (1.39–2.49) | <0.001 |
| TC (mg/dL) ≥ 190 | 0.70 (0.50–0.96) | 0.031 |
| LDL (mg/dL) ≥ 100 | 0.70 (0.52–0.95) | 0.024 |
| Triglyceride (mg/dL) ≥ 200 | 1.57 (1.09–2.25) | 0.014 |
| FPG (mg/dL) ≥ 126 | 0.67 (0.47–0.95) | 0.028 |
| HbA1C (%) ≥ 6.5 | 1.31 (0.92–1.87) | 0.123 |
| Antihypertensive drugs (%) | 2.70 (1.95–3.75) | <0.001 |
| Lipid-lowering drugs (%) | 0.78 (0.56–1.09) | 0.149 |
| Antidiabetic drugs (%) | 1.52 (1.11–2.08) | 0.008 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| OR (CI%95) | p-Value | aOR (CI%95) | p-Value | |
| Traditional anthropometric indices | ||||
| BMI ≥ 30 kg/m2 | 1.73 (1.29–2.32) | <0.001 | 1.32 (0.96–1.82) | 0.084 |
| WHR > 0.85 in women or 0.94 in men | 1.97 (1.37–2.81) | <0.001 | 1.36 (0.92–2.01) | 0.113 |
| WHtR > 0.5 | 5.19 (1.87–14.37) | 0.002 | 2.93 (1.03–8.37) | 0.044 |
| Novel anthropometric indices | ||||
| ABSI ≥ 0.086 | 1.58 (1.15–2.17) | 0.005 | 1.15 (0.81–1.64) | 0.412 |
| AVI ≥ 23.85 | 1.95 (1.43–2.67) | <0.001 | 1.46 (1.04–2.04) | 0.026 |
| BAI ≥ 36.23 | 1.44 (1.04–1.98) | 0.026 | 1.13 (0.79–1.60) | 0.491 |
| BRI ≥ 6.92 | 2.23 (1.63–3.04) | <0.001 | 1.66 (1.19–2.32) | 0.003 |
| CI ≥ 1.39 | 1.88 (1.39–2.55) | <0.001 | 1.28 (0.91–1.81) | 0.150 |
| CUN-BAE ≥ 41.07 | 1.43 1.03–1.97 | 0.028 | 1.24 (0.88–1.76) | 0.208 |
| WWI ≥ 12.72 | 1.39 (1.01–1.92) | 0043 | 1.29 (0.90–1.83) | 0.155 |
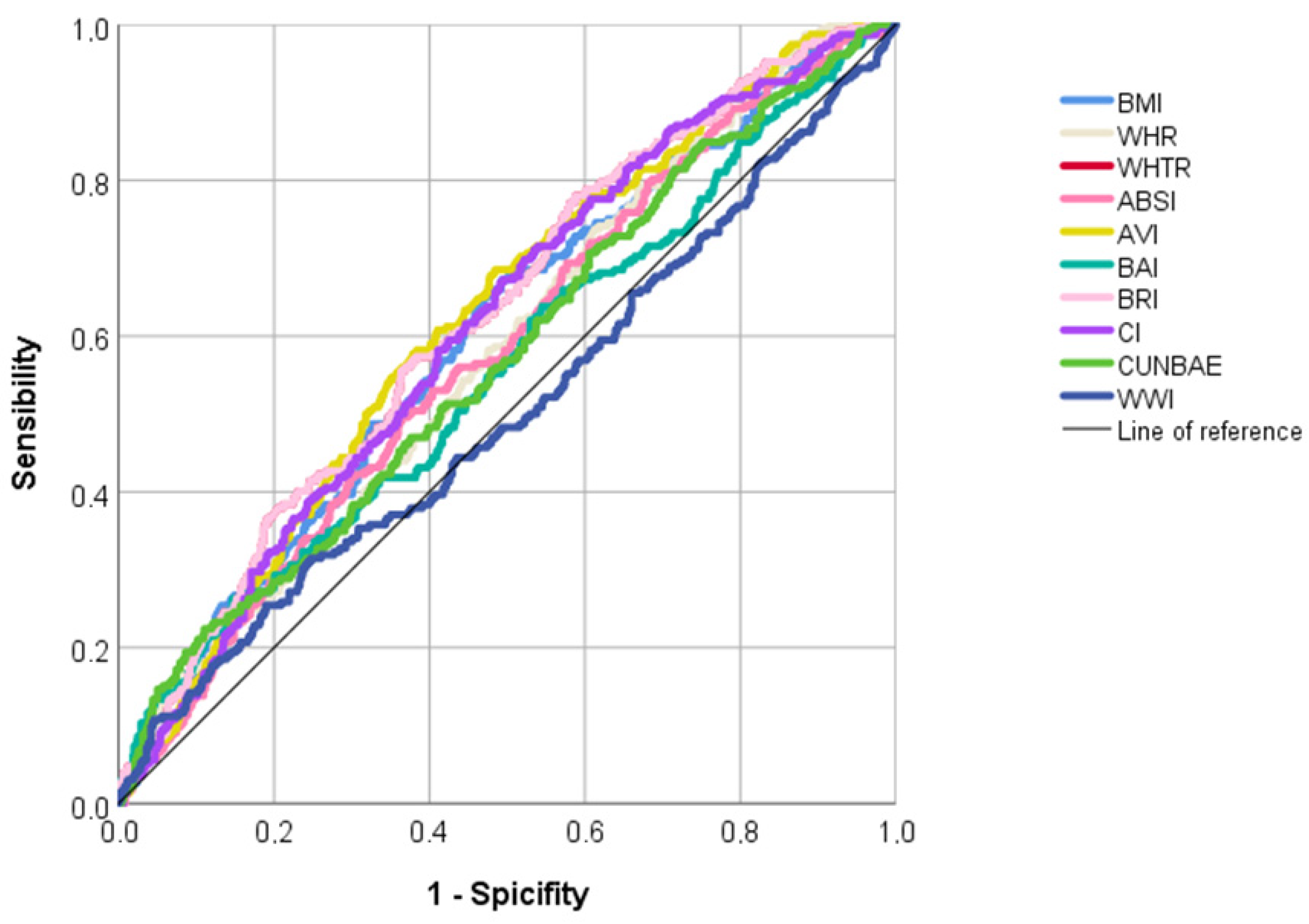
| AUC (95%IC) | p-Value | Sensitivity | Specificity | Youden’s Index | Cut-Off | |
|---|---|---|---|---|---|---|
| Traditional anthropometric indices | ||||||
| BMI | 0.600 (0.560–0.640) | <0.001 | 0.659 | 0.523 | 1.182 | 28.63 |
| WHR | 0.582 (0.542–0.622) | <0.001 | 0.737 | 0.387 | 1.124 | 0.91 |
| WHTR | 0.624 (0.582–0.664) | <0.001 | 0.780 | 0.411 | 1.192 | 0.57 |
| Novel anthropometric indices | ||||||
| ABSI | 0.579 (0.539–0.619) | <0.001 | 0.496 | 0.635 | 1.131 | 0.083 |
| AVI | 0.621 (0.582–0.660) | <0.001 | 0.685 | 0.515 | 1.200 | 19.55 |
| BAI | 0.555 (0.513–0.598) | 0.009 | 0.263 | 0.852 | 1.115 | 38.90 |
| BRI | 0.624 (0.585–0.664) | <0.001 | 0.556 | 0.635 | 1.191 | 5.97 |
| CI | 0.611 (0.571–0.650) | <0.001 | 0.672 | 0.505 | 1.178 | 1.31 |
| CUNBAE | 0.570 (0.529–0.612) | 0.001 | 0.224 | 0.889 | 1.113 | 44.87 |
| WWI | 0.503 (0.459–0.547) | 0.904 | 0.315 | 0.752 | 1.067 | 12.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bacaicoa, C.; Santano-Mogena, E.; Rico-Martín, S.; Rey-Sánchez, P.; Juárez-Vela, R.; Sánchez Muñoz-Torrero, J.F.; López-Espuela, F.; Calderón-García, J.F. Association between Asymptomatic Hyperuricemia with Adiposity Indices: A Cross-Sectional Study in a Spanish Population. Nutrients 2023, 15, 4798. https://doi.org/10.3390/nu15224798
Sánchez-Bacaicoa C, Santano-Mogena E, Rico-Martín S, Rey-Sánchez P, Juárez-Vela R, Sánchez Muñoz-Torrero JF, López-Espuela F, Calderón-García JF. Association between Asymptomatic Hyperuricemia with Adiposity Indices: A Cross-Sectional Study in a Spanish Population. Nutrients. 2023; 15(22):4798. https://doi.org/10.3390/nu15224798
Chicago/Turabian StyleSánchez-Bacaicoa, Carmen, Esperanza Santano-Mogena, Sergio Rico-Martín, Purificación Rey-Sánchez, Raúl Juárez-Vela, Juan F. Sánchez Muñoz-Torrero, Fidel López-Espuela, and Julián F. Calderón-García. 2023. "Association between Asymptomatic Hyperuricemia with Adiposity Indices: A Cross-Sectional Study in a Spanish Population" Nutrients 15, no. 22: 4798. https://doi.org/10.3390/nu15224798
APA StyleSánchez-Bacaicoa, C., Santano-Mogena, E., Rico-Martín, S., Rey-Sánchez, P., Juárez-Vela, R., Sánchez Muñoz-Torrero, J. F., López-Espuela, F., & Calderón-García, J. F. (2023). Association between Asymptomatic Hyperuricemia with Adiposity Indices: A Cross-Sectional Study in a Spanish Population. Nutrients, 15(22), 4798. https://doi.org/10.3390/nu15224798







