Diet-Wide Association, Genetic Susceptibility and Colorectal Cancer Risk: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Assessment of Food and Nutrients Intake
2.3. Outcome Ascertainment
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Results in the UK Biobank
3.3. Sensitivity Analysis
3.4. Interaction of Dietary Factors and Genetic Predisposition in CRC Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zygulska, A.L.; Pierzchalski, P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Markozannes, G.; Kanellopoulou, A.; Critselis, E.; Alhardan, S.; Karafousia, V.; Kasimis, J.C.; Katsaraki, C.; Papadopoulou, A.; Zografou, M.; et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 2021, 12, 4579. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.C.; Yilmaz, Ö.H.; Roper, J. Metabolism and Colorectal Cancer. Annu. Rev. Pathol. 2023, 18, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Kastrinos, F.; Kupfer, S.S.; Gupta, S. Colorectal Cancer Risk Assessment and Precision Approaches to Screening: Brave New World or Worlds Apart? Gastroenterology 2023, 164, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Castillo, M.; Muñoz de Mier, G.J.; de la Pinta, C.; Peña, C. The Diet as a Modulator of Tumor Microenvironment in Colorectal Cancer Patients. Int. J. Mol. Sci. 2023, 24, 7317. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Bouras, E.; van den Brandt, P.A.; Muller, D.C.; Papadopoulou, A.; Heath, A.K.; Critselis, E.; Gunter, M.J.; Vineis, P.; Ferrari, P.; et al. A Prospective Diet-Wide Association Study for Risk of Colorectal Cancer in EPIC. Clin. Gastroenterol. Hepatol. 2022, 20, 864–873. [Google Scholar] [CrossRef]
- McAllister, K.; Mechanic, L.E.; Amos, C.; Aschard, H.; Blair, I.A.; Chatterjee, N.; Conti, D.; Gauderman, W.J.; Hsu, L.; Hutter, C.M.; et al. Current Challenges and New Opportunities for Gene-Environment Interaction Studies of Complex Diseases. Am. J. Epidemiol. 2017, 186, 753–761. [Google Scholar] [CrossRef]
- Huyghe, J.R.; Bien, S.A.; Harrison, T.A.; Kang, H.M.; Chen, S.; Schmit, S.L.; Conti, D.V.; Qu, C.; Jeon, J.; Edlund, C.K.; et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat. Genet. 2019, 51, 76–87. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7, e6. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Smith, E.; Melander, O.; Ottosson, F. The association between plasma metabolites and future risk of all-cause mortality. J. Intern. Med. 2022, 292, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Bradbury, K.E.; Carter, M.; Elliott, P.; Evans, C.E.L.; Ford, H.E.; Hancock, N.; et al. Validation of the Oxford WebQ Online 24-Hour Dietary Questionnaire Using Biomarkers. Am. J. Epidemiol. 2019, 188, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Chai, J.C.; Xing, J.; Moon, J.-Y.; Shan, Z.; Yu, B.; Mossavar-Rahman, Y.; Sotres-Alvarez, D.; Li, J.; Mattei, J.; et al. Healthful eating patterns, serum metabolite profile and risk of diabetes in a population-based prospective study of US Hispanics/Latinos. Diabetologia 2022, 65, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Greenland, S. Dose-response and trend analysis in epidemiology: Alternatives to categorical analysis. Epidemiology 1995, 6, 356–365. [Google Scholar] [CrossRef]
- Jia, G.; Lu, Y.; Wen, W.; Long, J.; Liu, Y.; Tao, R.; Li, B.; Denny, J.C.; Shu, X.-O.; Zheng, W. Evaluating the Utility of Polygenic Risk Scores in Identifying High-Risk Individuals for Eight Common Cancers. JNCI Cancer Spectr. 2020, 4, pkaa021. [Google Scholar] [CrossRef]
- Li, X.; Timofeeva, M.; Spiliopoulou, A.; McKeigue, P.; He, Y.; Zhang, X.; Svinti, V.; Campbell, H.; Houlston, R.S.; Tomlinson, I.P.M.; et al. Prediction of colorectal cancer risk based on profiling with common genetic variants. Int. J. Cancer 2020, 147, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Myung, S.-K.; Lee, J.-H. Light Alcohol Drinking and Risk of Cancer: A Meta-Analysis of Cohort Studies. Cancer Res. Treat. 2018, 50, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Ward, H.A.; Jenab, M.; Rothwell, J.A.; Boutron-Ruault, M.-C.; Carbonnel, F.; Kvaskoff, M.; Kaaks, R.; Kühn, T.; Boeing, H.; et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin. Gastroenterol. Hepatol. 2019, 17, 1323–1331.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Xiao, J.; Sun, J.; Yu, L.; Zhang, H.; Meng, X.; Yuan, S.; Timofeeva, M.; Law, P.J.; et al. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer 2022, 151, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.D. Phosphorus and calcium: A review for the adult nutrition support clinician. Nutr. Clin. Pract. 2015, 30, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Papadimitriou, N.; Dimou, N.; Gill, D.; Lewis, S.J.; Martin, R.M.; Murphy, N.; Markozannes, G.; Zuber, V.; Cross, A.J.; et al. Genetically predicted circulating concentrations of micronutrients and risk of colorectal cancer among individuals of European descent: A Mendelian randomization study. Am. J. Clin. Nutr. 2021, 113, 1490–1502. [Google Scholar] [CrossRef]
- Gholamalizadeh, M.; Behrad Nasab, M.; Ahmadzadeh, M.; Doaei, S.; Jonoush, M.; Shekari, S.; Afsharfar, M.; Hosseinzadeh, P.; Abbastorki, S.; Akbari, M.E.; et al. The association among calorie, macronutrient, and micronutrient intake with colorectal cancer: A case-control study. Food Sci. Nutr. 2022, 10, 1527–1536. [Google Scholar] [CrossRef]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.M.; Slade, A.N.; Schwartz, B.; Arthur, A.E. A Case-Control Study Examining the Association of Fiber, Fruit, and Vegetable Intake and the Risk of Colorectal Cancer in a Palestinian Population. Int. J. Environ. Res. Public Health 2022, 19, 7181. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.d.F.; Fabi, J.P. Dietary fiber as a wide pillar of colorectal cancer prevention and adjuvant therapy. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Wang, M.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Fiber Intake and Survival After Colorectal Cancer Diagnosis. JAMA Oncol. 2018, 4, 71–79. [Google Scholar] [CrossRef]
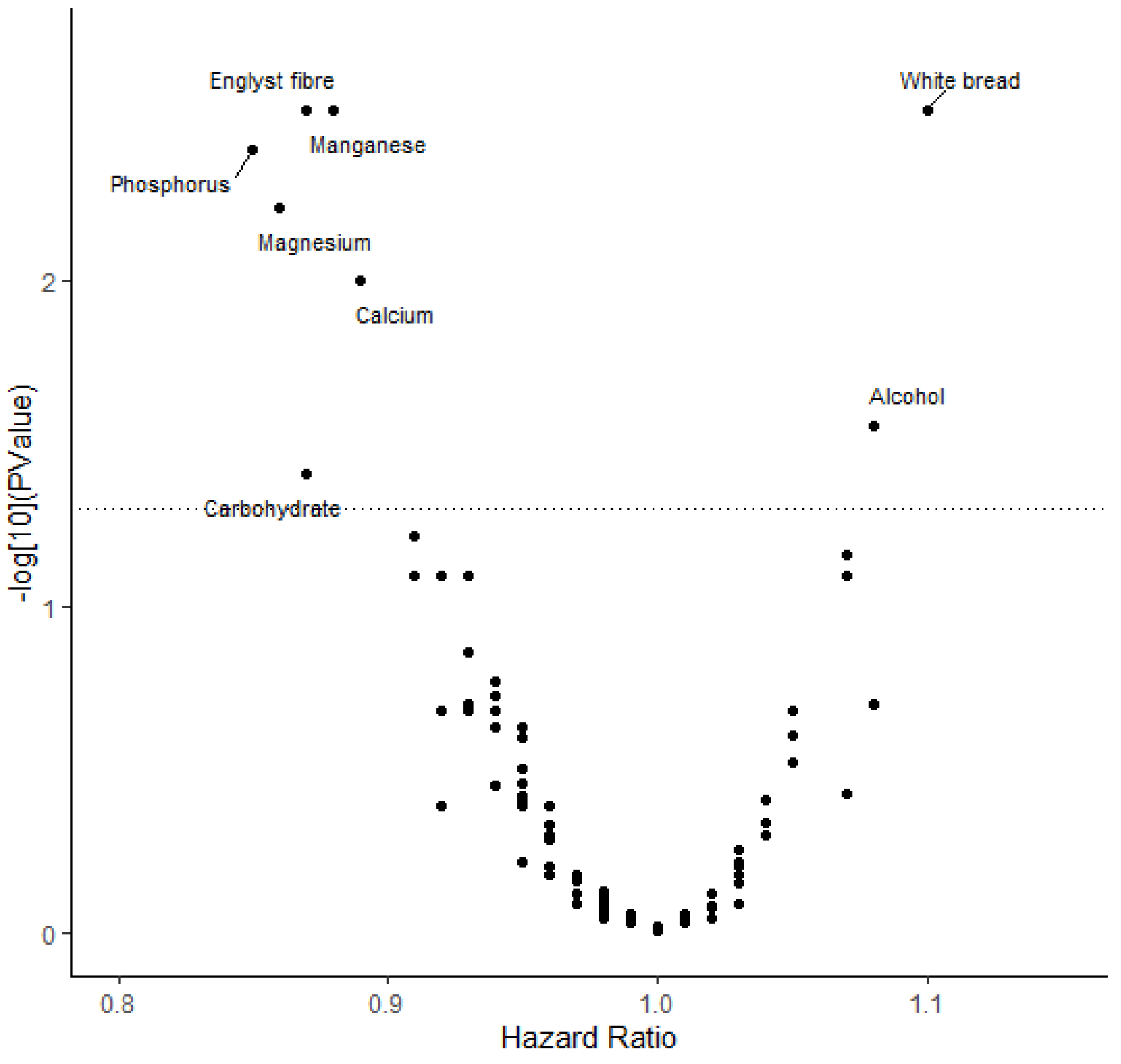
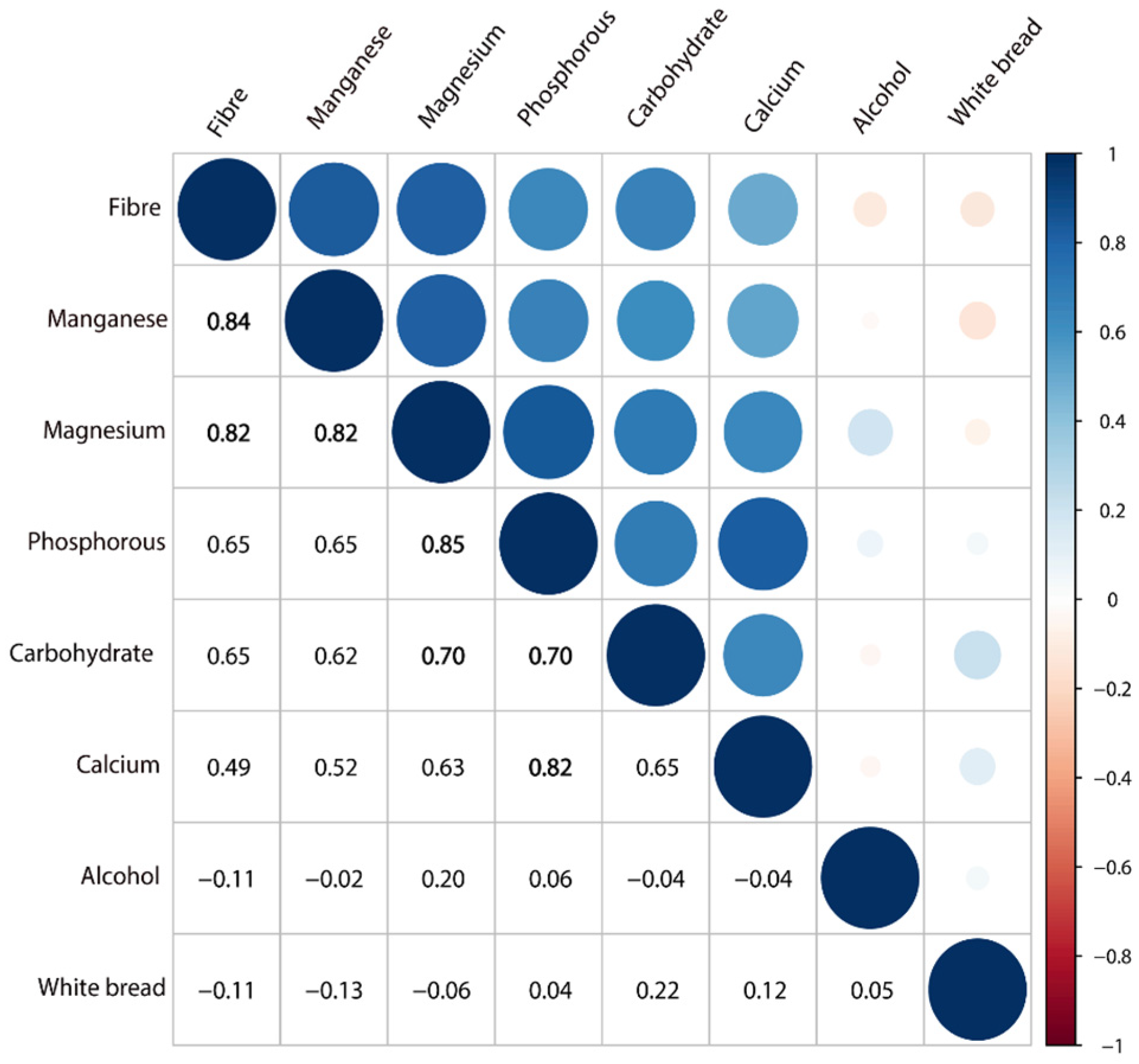
| Foods and Nutrients | Colorectal Cancer | Colon Cancer | Rectal Cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases/Controls | Multivariable HR (95% CI) | p Value | Cases/Controls | Multivariable HR (95% CI) | p Value | Cases/Controls | Multivariable HR (95% CI) | p Value | ||
| Carbohydrate | T1 | 452/38,953 | Ref. | 269/39,015 | Ref. | 104/39,019 | Ref. | |||
| T2 | 508/38,894 | 1.00 (0.87, 1.14) | 0.966 | 279/39,004 | 0.95 (0.79, 1.14) | 0.559 | 128/38,995 | 1.07 (0.81, 1.42) | 0.628 | |
| T3 | 506/38,897 | 0.84 (0.70, 1.01) | 0.064 | 294/38,990 | 0.89 (0.70, 1.12) | 0.317 | 127/38,995 | 0.87 (0.61, 1.26) | 0.472 | |
| Dietary fiber | T1 | 468/38,936 | Ref. | 252/39,032 | Ref. | 123/39,000 | Ref. | |||
| T2 | 521/38,882 | 0.98 (0.86, 1.12) | 0.769 | 306/38,977 | 1.10 (0.92, 1.30) | 0.295 | 129/38,993 | 0.91 (0.70, 1.17) | 0.463 | |
| T3 | 477/38,926 | 0.80 (0.69, 0.93) | 0.003 | 284/39,000 | 0.94 (0.78, 1.15) | 0.557 | 107/39,016 | 0.64 (0.48, 0.87) | 0.004 | |
| Calcium | T1 | 478/38,926 | Ref. | 280/39,004 | Ref. | 113/39,010 | Ref. | |||
| T2 | 504/38,900 | 0.93 (0.82, 1.06) | 0.305 | 296/38,987 | 0.94 (0.79, 1.11) | 0.465 | 125/38,997 | 0.98 (0.75, 1.28) | 0.874 | |
| T3 | 484/38,918 | 0.80 (0.68, 0.93) | 0.003 | 266/39,018 | 0.75 (0.62, 0.92) | 0.006 | 121/39,002 | 0.84 (0.62, 1.14) | 0.261 | |
| Magnesium | T1 | 448/38,956 | Ref. | 259/39,025 | Ref. | 117/39,006 | Ref. | |||
| T2 | 513/38,890 | 0.98 (0.85, 1.12) | 0.727 | 295/38,988 | 0.99 (0.83, 1.18) | 0.912 | 118/39,004 | 0.83 (0.63, 1.09) | 0.172 | |
| T3 | 505/38,898 | 0.82 (0.69, 0.97) | 0.023 | 288/38,996 | 0.85 (0.68, 1.07) | 0.167 | 124/38,999 | 0.70 (0.50, 0.98) | 0.038 | |
| Phosphorus | T1 | 445/38,959 | Ref. | 257/39,027 | Ref. | 108/39,015 | Ref. | |||
| T2 | 521/38,882 | 1.00 (0.88, 1.15) | 0.946 | 297/38,986 | 1.01 (0.85, 1.21) | 0.892 | 131/38,991 | 1.02 (0.78, 1.34) | 0.875 | |
| T3 | 500/38,903 | 0.81 (0.68, 0.97) | 0.020 | 288/38,996 | 0.86 (0.68, 1.08) | 0.195 | 120/39,003 | 0.76 (0.54, 1.09) | 0.134 | |
| Manganese | T1 | 492/38,912 | Ref. | 289/38,995 | Ref. | 119/39,004 | Ref. | |||
| T2 | 510/38,893 | 0.92 (0.81, 1.05) | 0.233 | 288/38,995 | 0.90 (0.76, 1.06) | 0.210 | 121/39,001 | 0.91 (0.70, 1.19) | 0.494 | |
| T3 | 464/38,939 | 0.76 (0.65, 0.88) | 2.19E-04 | 265/39,019 | 0.76 (0.63, 0.92) | 0.005 | 119/39,004 | 0.80 (0.59, 1.07) | 0.128 | |
| Alcohol | T1 | 465/38,946 | Ref. | 274/39,011 | Ref. | 102/39,024 | Ref. | |||
| T2 | 454/38,942 | 0.94 (0.83, 1.08) | 0.385 | 265/39,017 | 0.94 (0.79, 1.11) | 0.457 | 114/39,005 | 1.06 (0.81, 1.39) | 0.654 | |
| T3 | 547/38,856 | 1.02 (0.90, 1.17) | 0.715 | 303/38,981 | 0.97 (0.82, 1.15) | 0.731 | 143/38,980 | 1.17 (0.90, 1.52) | 0.254 | |
| White bread | T1 | 625/53789 | Ref. | 356/53,912 | Ref. | 146/53,912 | Ref. | |||
| T2 | 278/24,461 | 1.03 (0.89, 1.18) | 0.716 | 167/24,508 | 1.08 (0.90, 1.30) | 0.402 | 64/24,508 | 1.03 (0.77, 1.38) | 0.845 | |
| T3 | 563/38,494 | 1.22 (1.08, 1.37) | 0.001 | 319/38,589 | 1.22 (1.05, 1.43) | 0.012 | 149/38,589 | 1.35 (1.06, 1.70) | 0.013 | |
| Dietary Factors | HR (95% CI) | p Value | p for Interaction |
|---|---|---|---|
| Carbohydrate | 0.063 | ||
| Low | 0.93 (0.75, 1.15) | 0.488 | |
| Intermediate | 0.85 (0.72, 1.00) | 0.054 | |
| High | 0.87 (0.76, 0.99) | 0.035 | |
| Dietary fiber | 0.627 | ||
| Low | 0.81 (0.70, 0.95) | 0.008 | |
| Intermediate | 0.83 (0.74, 0.93) | 0.002 | |
| High | 0.93 (0.85, 1.02) | 0.139 | |
| Calcium | 0.295 | ||
| Low | 0.89 (0.76, 1.04) | 0.155 | |
| Intermediate | 0.86 (0.76, 0.97) | 0.017 | |
| High | 0.89 (0.81, 0.98) | 0.022 | |
| Magnesium | 0.310 | ||
| Low | 0.80 (0.66, 0.97) | 0.022 | |
| Intermediate | 0.84 (0.72, 0.97) | 0.018 | |
| High | 0.89 (0.79, 1.00) | 0.060 | |
| Phosphorus | 0.291 | ||
| Low | 0.79 (0.65, 0.96) | 0.019 | |
| Intermediate | 0.82 (0.70, 0.96) | 0.014 | |
| High | 0.88 (0.78, 1.00) | 0.045 | |
| Manganese | 0.246 | ||
| Low | 0.83 (0.71, 0.97) | 0.016 | |
| Intermediate | 0.86 (0.76, 0.97) | 0.011 | |
| High | 0.91 (0.83, 1.00) | 0.052 | |
| Alcohol | 0.779 | ||
| Low | 1.07 (0.96, 1.20) | 0.225 | |
| Intermediate | 1.06 (0.97, 1.16) | 0.170 | |
| High | 1.10 (1.02, 1.18) | 0.011 | |
| White bread | 0.762 | ||
| Low | 1.18 (1.07, 1.31) | 0.001 | |
| Intermediate | 1.07 (0.99, 1.17) | 0.103 | |
| High | 1.10 (1.03, 1.18) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.; Lu, Y.; Wu, W.; Jiang, F.; Li, Z.; Xu, L.; Zhang, R.; Li, X.; Chen, D. Diet-Wide Association, Genetic Susceptibility and Colorectal Cancer Risk: A Prospective Cohort Study. Nutrients 2023, 15, 4801. https://doi.org/10.3390/nu15224801
Jin D, Lu Y, Wu W, Jiang F, Li Z, Xu L, Zhang R, Li X, Chen D. Diet-Wide Association, Genetic Susceptibility and Colorectal Cancer Risk: A Prospective Cohort Study. Nutrients. 2023; 15(22):4801. https://doi.org/10.3390/nu15224801
Chicago/Turabian StyleJin, Dongqing, Ying Lu, Wei Wu, Fangyuan Jiang, Zihan Li, Liying Xu, Rongqi Zhang, Xue Li, and Dong Chen. 2023. "Diet-Wide Association, Genetic Susceptibility and Colorectal Cancer Risk: A Prospective Cohort Study" Nutrients 15, no. 22: 4801. https://doi.org/10.3390/nu15224801
APA StyleJin, D., Lu, Y., Wu, W., Jiang, F., Li, Z., Xu, L., Zhang, R., Li, X., & Chen, D. (2023). Diet-Wide Association, Genetic Susceptibility and Colorectal Cancer Risk: A Prospective Cohort Study. Nutrients, 15(22), 4801. https://doi.org/10.3390/nu15224801







