Causal Effect of Relative Carbohydrate Intake on Hypertension through Psychological Well-Being and Adiposity: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
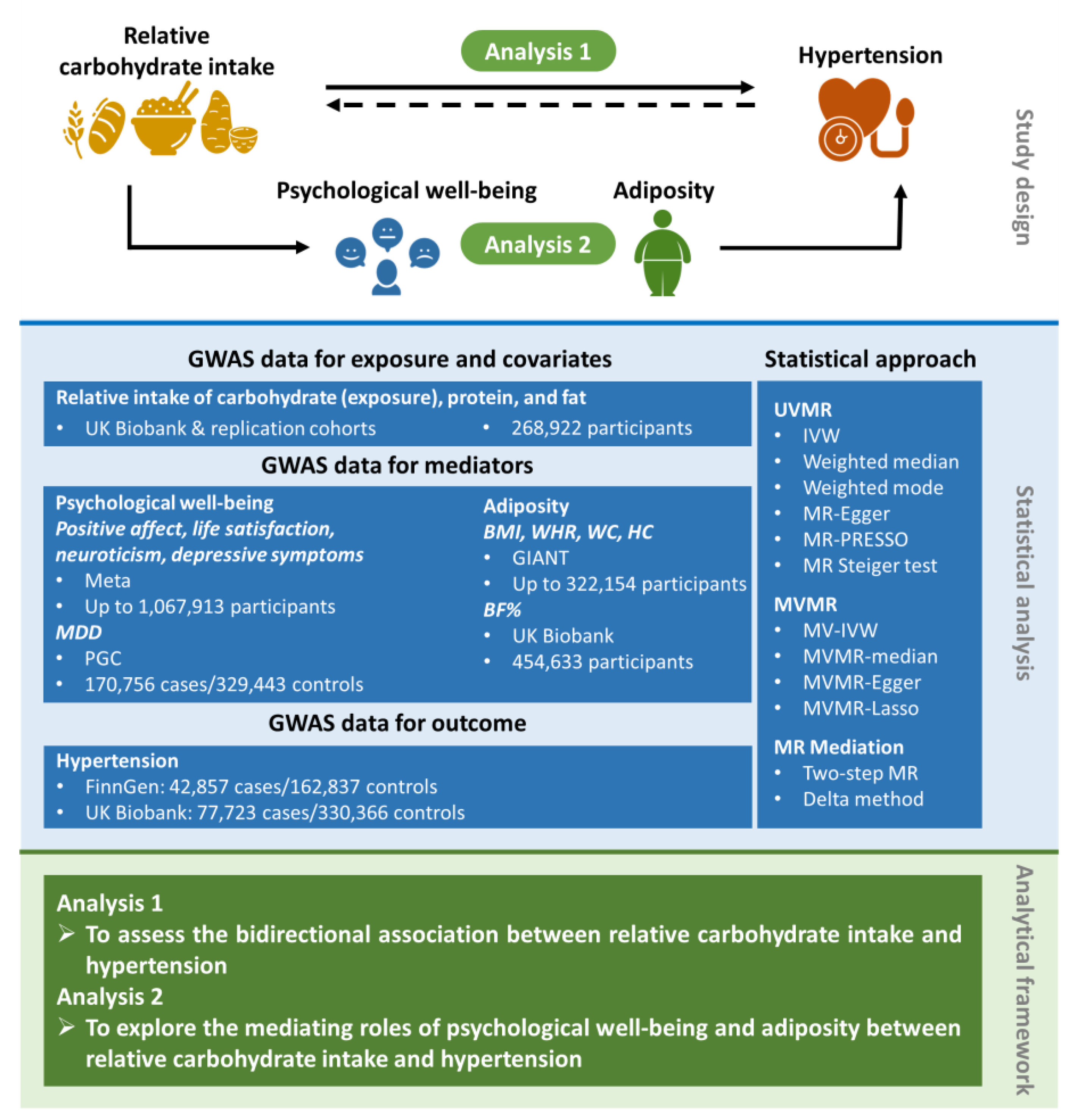
2.1. Study Design
2.2. Data Sources for the Exposure, Covariates, Mediators, and Outcome
2.2.1. Exposure and Covariates
2.2.2. Mediators
2.2.3. Outcome
2.3. Statistical Analysis
2.3.1. UVMR and MVMR Analyses
2.3.2. Mediation MR Analyses
2.3.3. MR Sensitivity Analyses
2.3.4. Meta-Analyses of Estimates from Two Outcome Databases
3. Results
3.1. Effect of Relative Carbohydrate Intake on Hypertension and the Reverse Effect
3.2. Effect of Relative Carbohydrate Intake on Psychological Well-Being and Adiposity
3.3. Effects of Psychological Well-Being and Adiposity on Hypertension
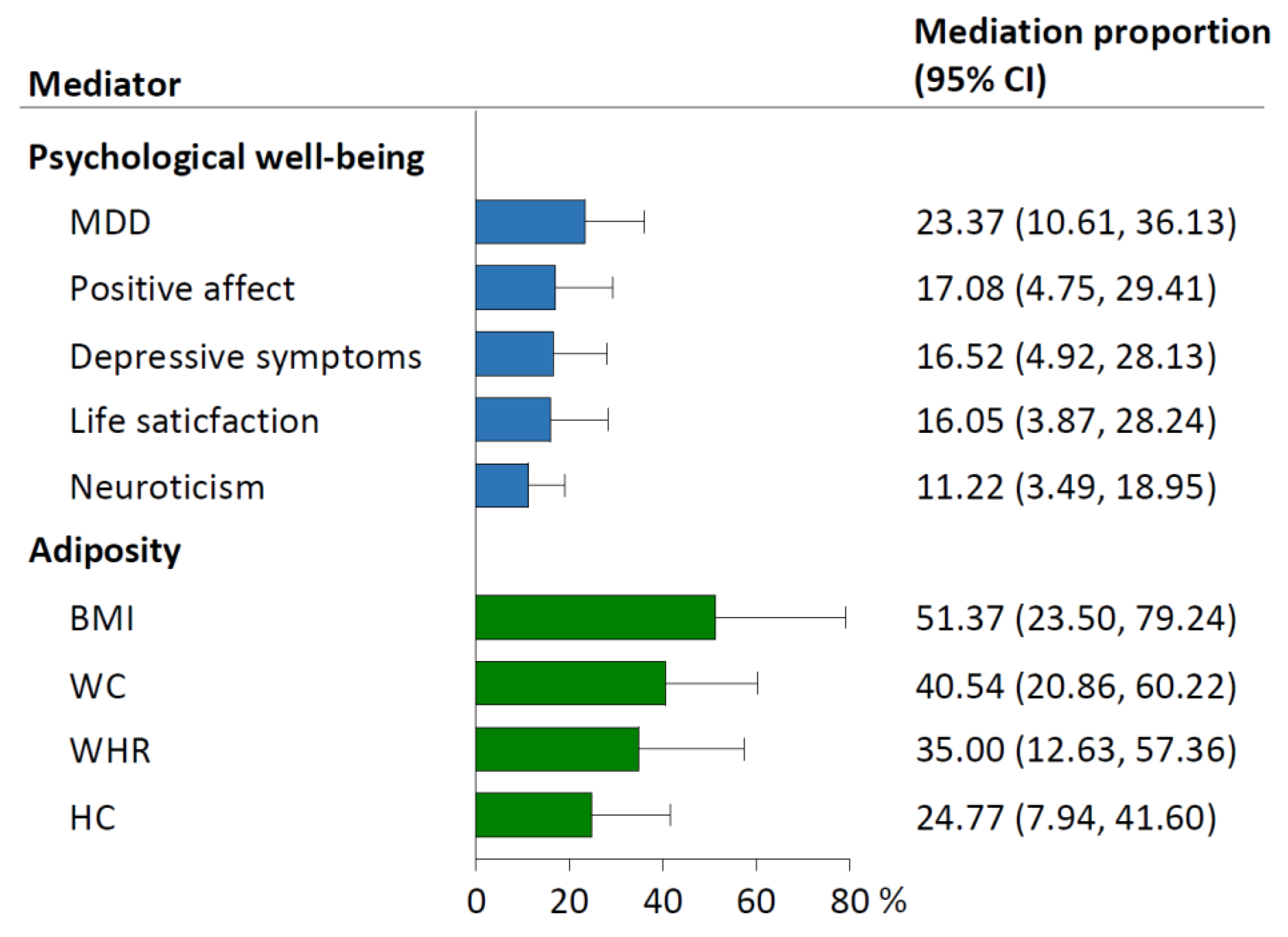
3.4. Mediating Effects of Psychological Well-Being and Adiposity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Hou, W.; Han, T.; Sun, X.; Chen, Y.; Xu, J.; Wang, Y.; Yang, X.; Jiang, W.; Sun, C. Relationship Between Carbohydrate Intake (Quantity, Quality, and Time Eaten) and Mortality (Total, Cardiovascular, and Diabetes): Assessment of 2003–2014 National Health and Nutrition Examination Survey Participants. Diabetes Care 2022, 45, 3024–3031. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Zhang, S.; Li, R.; Zhang, Y.; He, P.; Zhang, Z.; Liu, M.; Zhou, C.; Ye, Z.; et al. Dietary Carbohydrate Intake and New-Onset Hypertension: A Nationwide Cohort Study in China. Hypertension 2021, 78, 422–430. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef]
- Kelly, R.K.; Tong, T.Y.N.; Watling, C.Z.; Reynolds, A.; Piernas, C.; Schmidt, J.A.; Papier, K.; Carter, J.L.; Key, T.J.; Perez-Cornago, A. Associations between types and sources of dietary carbohydrates and cardiovascular disease risk: A prospective cohort study of UK Biobank participants. BMC Med. 2023, 21, 34. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, M.; Dong, S.S.; Wang, J.H.; Zhang, K.; Guo, J.; Guo, Y.; Yang, T.L. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat. Hum. Behav. 2022, 6, 1569–1576. [Google Scholar] [CrossRef]
- Freuer, D.; Meisinger, C.; Linseisen, J. Causal relationship between dietary macronutrient composition and anthropometric measures: A bidirectional two-sample Mendelian randomization analysis. Clin. Nutr. 2021, 40, 4120–4131. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, C.; Kong, L.; Zheng, J.; Xu, M.; Xu, Y.; Li, M.; Zhao, Z.; Lu, J.; Chen, Y.; et al. Independent Associations of Education, Intelligence, and Cognition with Hypertension and the Mediating Effects of Cardiometabolic Risk Factors: A Mendelian Randomization Study. Hypertension 2023, 80, 192–203. [Google Scholar] [CrossRef]
- Kubzansky, L.D.; Huffman, J.C.; Boehm, J.K.; Hernandez, R.; Kim, E.S.; Koga, H.K.; Feig, E.H.; Lloyd-Jones, D.M.; Seligman, M.E.P.; Labarthe, D.R. Positive Psychological Well-Being and Cardiovascular Disease: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1382–1396. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Meddens, S.F.W.; de Vlaming, R.; Bowers, P.; Burik, C.A.P.; Linnér, R.K.; Lee, C.; Okbay, A.; Turley, P.; Rietveld, C.A.; Fontana, M.A.; et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol. Psychiatry 2021, 26, 2056–2069. [Google Scholar] [CrossRef]
- Baselmans, B.M.L.; Jansen, R.; Ip, H.F.; van Dongen, J.; Abdellaoui, A.; van de Weijer, M.P.; Bao, Y.; Smart, M.; Kumari, M.; Willemsen, G.; et al. Multivariate genome-wide analyses of the well-being spectrum. Nat. Genet. 2019, 51, 445–451. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef]
- Staley, J.R.; Blackshaw, J.; Kamat, M.A.; Ellis, S.; Surendran, P.; Sun, B.B.; Paul, D.S.; Freitag, D.; Burgess, S.; Danesh, J.; et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics 2016, 32, 3207–3209. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Mitchell, R.E.; Elsworth, B.L.; Mitchell, R.E.; Raistrick, C.A.; Paternoster, L.; Hemani, G.; Gaunt, T.R. MRC IEU UK Biobank GWAS Pipeline Version 2; University of Bristol: Bristol, UK, 2019. [Google Scholar]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.R.; Sanderson, E.; Hammerton, G.; Richmond, R.C.; Davey Smith, G.; Heron, J.; Taylor, A.E.; Davies, N.M.; Howe, L.D. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 2021, 36, 465–478. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Lockwood, C.M.; Hoffman, J.M.; West, S.G.; Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods 2002, 7, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Ortiz, R.; Kluwe, B.; Lazarus, S.; Teruel, M.N.; Joseph, J.J. Cortisol and cardiometabolic disease: A target for advancing health equity. Trends Endocrinol. Metab. 2022, 33, 786–797. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Association of Symptoms of Depression with Cardiovascular Disease and Mortality in Low-, Middle-, and High-Income Countries. JAMA Psychiatry 2020, 77, 1052–1063. [Google Scholar]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e763–e783. [Google Scholar] [CrossRef]
- Plackett, B. The vicious cycle of depression and obesity. Nature 2022, 608, S42–S43. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G.; EPIC-InterAct Consortium. Instrumental variable analysis with a nonlinear exposure-outcome relationship. Epidemiology 2014, 25, 877–885. [Google Scholar] [CrossRef] [PubMed]


| Phenotype | Unit | Sample Size (Case/Control) | Ancestry | Consortium or Cohort Study | Data Source |
|---|---|---|---|---|---|
| Exposure | |||||
| Relative carbohydrate intake | 1-SD | 268,922 | European | UK Biobank, DietGen, 14 studies | Meddens SFW et al., 2021 (PMID: 32393786) [14] |
| Covariate | |||||
| Relative protein intake | 1-SD | 268,922 | European | UK Biobank, DietGen, 14 studies | Meddens SFW et al., 2021 (PMID: 32393786) [14] |
| Relative fat intake | 1-SD | 268,922 | European | ||
| Outcome | |||||
| Hypertension | Event | 42,857/162,837 | European | FinnGen | https://FinnGen.gitbook.io/documentation/ (accessed on 14 May 2023) |
| Event | 77,723/330,366 | European | UK Biobank | https://pan.ukbb.broadinstitute.org/ (accessed on 14 May 2023) | |
| Mediator | |||||
| Psychological well-being | |||||
| Positive affect | Z score | 410,603 | European | Meta | Baselmans BML et al., 2019 (PMID: 30643256) [15] |
| Life satisfaction | Z score | 80,852 | European | ||
| Neuroticism | Z score | 582,989 | European | ||
| Depressive symptoms | Z score | 1,295,946 | European | ||
| MDD | Event | 170,756/329,443 | European | PGC | Howard DM et al., 2019 (PMID: 30718901) [16] |
| Adiposity | |||||
| BMI | 1-SD (4.77 kg/m2) | 322,154 | European | GIANT | Locke AE et al., 2015 (PMID: 25673413) [17] |
| WHR | 1-SD (0.076) | 212,244 | European | Shungin D et al., 2015 (PMID: 25673412) [18] | |
| WC | 1-SD (12.52 cm) | 232,101 | European | ||
| HC | 1-SD (8.45 cm) | 213,038 | European | ||
| BF% | 1-SD | 454,633 | European | UK Biobank | https://gwas.mrcieu.ac.uk/datasets/ (accessed on 14 May 2023) |
| Mediator | Method | No. of SNP | β (95% CI) 1 | OR (95% CI) 1 | p Value |
|---|---|---|---|---|---|
| Psychological well-being | |||||
| Positive affect | IVW | 5 | 0.171 (0.063, 0.278) | / | 0.002 |
| Weighted median | 0.143 (0.037, 0.250) | / | 0.008 | ||
| Weighted mode | 0.140 (0.021, 0.259) | / | 0.082 | ||
| MR-Egger | 0.225 (−0.585, 1.035) | / | 0.62 | ||
| MR-PRESSO (no outliers) | 0.171 (0.063, 0.278) | / | 0.036 | ||
| Life satisfaction | IVW | 5 | 0.183 (0.069, 0.298) | / | 0.002 |
| Weighted median | 0.157 (0.039, 0.276) | / | 0.009 | ||
| Weighted mode | 0.155 (0.025, 0.285) | / | 0.079 | ||
| MR-Egger | 0.258 (−0.584, 1.110) | / | 0.59 | ||
| MR-PRESSO (no outliers) | 0.183 (0.069, 0.298) | / | 0.035 | ||
| Neuroticism | IVW | 5 | −0.171 (−0.270, −0.073) | / | 6.69 × 10−4 |
| Weighted median | −0.179 (−0.299, −0.059) | / | 0.004 | ||
| Weighted mode | −0.172 (−0.312, −0.032) | / | 0.073 | ||
| MR-Egger | −0.691 (−1.368, −0.014) | / | 0.14 | ||
| MR-PRESSO (no outliers) | −0.171 (−0.270, −0.073) | / | 0.027 | ||
| Depressive symptoms | IVW | 5 | −0.145 (−0.235, −0.056) | / | 0.001 |
| Weighted median | −0.110 (−0.184, −0.035) | / | 0.004 | ||
| Weighted mode | −0.111 (−0.189, −0.034) | / | 0.048 | ||
| MR-Egger | −0.126 (−0.855, 0.603) | / | 0.76 | ||
| MR-PRESSO (no outliers) | −0.145 (−0.235, −0.056) | / | 0.034 | ||
| MDD | IVW | 7 | −0.512 (−0.731, −0.294) | 0.60 (0.48, 0.75) | 4.15 × 10−6 |
| Weighted median | −0.541 (−0.802, −0.281) | 0.58 (0.45, 0.76) | 4.71 × 10−5 | ||
| Weighted mode | −0.528 (−0.948, −0.107) | 0.59 (0.39, 0.90) | 0.049 | ||
| MR-Egger | 0.255 (−0.845, 1.355) | 1.29 (0.43, 3.88) | 0.67 | ||
| MR-PRESSO (no outliers) | −0.512 (−0.731, −0.294) | 0.60 (0.48, 0.75) | 0.004 | ||
| Adiposity | |||||
| BMI | IVW | 5 | −0.669 (−1.006, −0.332) | / | 1.01 × 10−4 |
| Weighted median | −0.665 (−0.960, −0.369) | / | 1.08 × 10−5 | ||
| Weighted mode | −0.859 (−1.454, −0.264) | / | 0.047 | ||
| MR-Egger | −1.461 (−4.187, 1.266) | / | 0.37 | ||
| MR-PRESSO (no outliers) | −0.669 (−1.006, −0.332) | / | 0.018 | ||
| WHR | IVW | 5 | −0.357 (−0.562, −0.152) | / | 6.35 × 10−4 |
| Weighted median | −0.345 (−0.596, −0.094) | / | 0.007 | ||
| Weighted mode | −0.320 (−0.642, 0.001) | / | 0.12 | ||
| MR-Egger | −0.613 (−2.146, 0.920) | / | 0.49 | ||
| MR-PRESSO (no outliers) | −0.357 (−0.415, −0.299) | / | 2.67 × 10−4 | ||
| WC | IVW | 5 | −0.498 (−0.706, −0.290) | / | 2.80 × 10−6 |
| Weighted median | −0.447 (−0.734, −0.160) | / | 0.002 | ||
| Weighted mode | −0.331 (−0.768, 0.107) | / | 0.21 | ||
| MR-Egger | −0.905 (−2.539, 0.729) | / | 0.36 | ||
| MR-PRESSO (no outliers) | −0.498 (−0.694, −0.303) | / | 0.008 | ||
| HC | IVW | 5 | −0.468 (−0.739, −0.197) | / | 7.22 × 10−4 |
| Weighted median | −0.354 (−0.697, −0.010) | / | 0.044 | ||
| Weighted mode | −0.275 (−0.869, 0.318) | / | 0.41 | ||
| MR-Egger | −0.617 (−2.925, 1.692) | / | 0.64 | ||
| MR-PRESSO (no outliers) | −0.468 (−0.739, −0.197) | / | 0.028 | ||
| BF% | IVW | 7 | −0.427 (−0.771, −0.082) | / | 0.015 |
| Weighted median | −0.406 (−0.542, −0.270) | / | 4.56 × 10−9 | ||
| Weighted mode | −0.388 (−0.532, −0.244) | / | 0.002 | ||
| MR-Egger | −0.939 (−2.919, 1.043) | / | 0.40 | ||
| MR-PRESSO (3 outliers) | −0.408 (−0.500, −0.315) | / | 0.003 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Kong, L.; Wang, Y.; Dou, C.; Xu, M.; Zheng, J.; Zheng, R.; Xu, Y.; Li, M.; Zhao, Z.; et al. Causal Effect of Relative Carbohydrate Intake on Hypertension through Psychological Well-Being and Adiposity: A Mendelian Randomization Study. Nutrients 2023, 15, 4817. https://doi.org/10.3390/nu15224817
Ye C, Kong L, Wang Y, Dou C, Xu M, Zheng J, Zheng R, Xu Y, Li M, Zhao Z, et al. Causal Effect of Relative Carbohydrate Intake on Hypertension through Psychological Well-Being and Adiposity: A Mendelian Randomization Study. Nutrients. 2023; 15(22):4817. https://doi.org/10.3390/nu15224817
Chicago/Turabian StyleYe, Chaojie, Lijie Kong, Yiying Wang, Chun Dou, Min Xu, Jie Zheng, Ruizhi Zheng, Yu Xu, Mian Li, Zhiyun Zhao, and et al. 2023. "Causal Effect of Relative Carbohydrate Intake on Hypertension through Psychological Well-Being and Adiposity: A Mendelian Randomization Study" Nutrients 15, no. 22: 4817. https://doi.org/10.3390/nu15224817
APA StyleYe, C., Kong, L., Wang, Y., Dou, C., Xu, M., Zheng, J., Zheng, R., Xu, Y., Li, M., Zhao, Z., Lu, J., Chen, Y., Wang, W., Bi, Y., Wang, T., & Ning, G. (2023). Causal Effect of Relative Carbohydrate Intake on Hypertension through Psychological Well-Being and Adiposity: A Mendelian Randomization Study. Nutrients, 15(22), 4817. https://doi.org/10.3390/nu15224817








