Vitamin D Attenuates Ulcerative Colitis by Inhibiting ACSL4-Mediated Ferroptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Cell Culture and Drug Treatment
2.3. ACSL4 Transfection in HCT116
2.4. Assessment of Severity of Colitis
2.5. Evaluation of the Colon Macroscopic Damage Index (CMDI)
2.6. Histopathology and Immunohistochemistry (IHC)
2.7. Western Blot Analysis
2.8. Real-Time PCR
2.9. Measurement of Glutathione (GSH) and Malondialdehyde (MDA) in Colonic Tissues and Cells
2.10. Measurement of Myeloperoxidase (MPO)
2.11. Iron Measurements
2.12. Statistical Analysis
3. Results
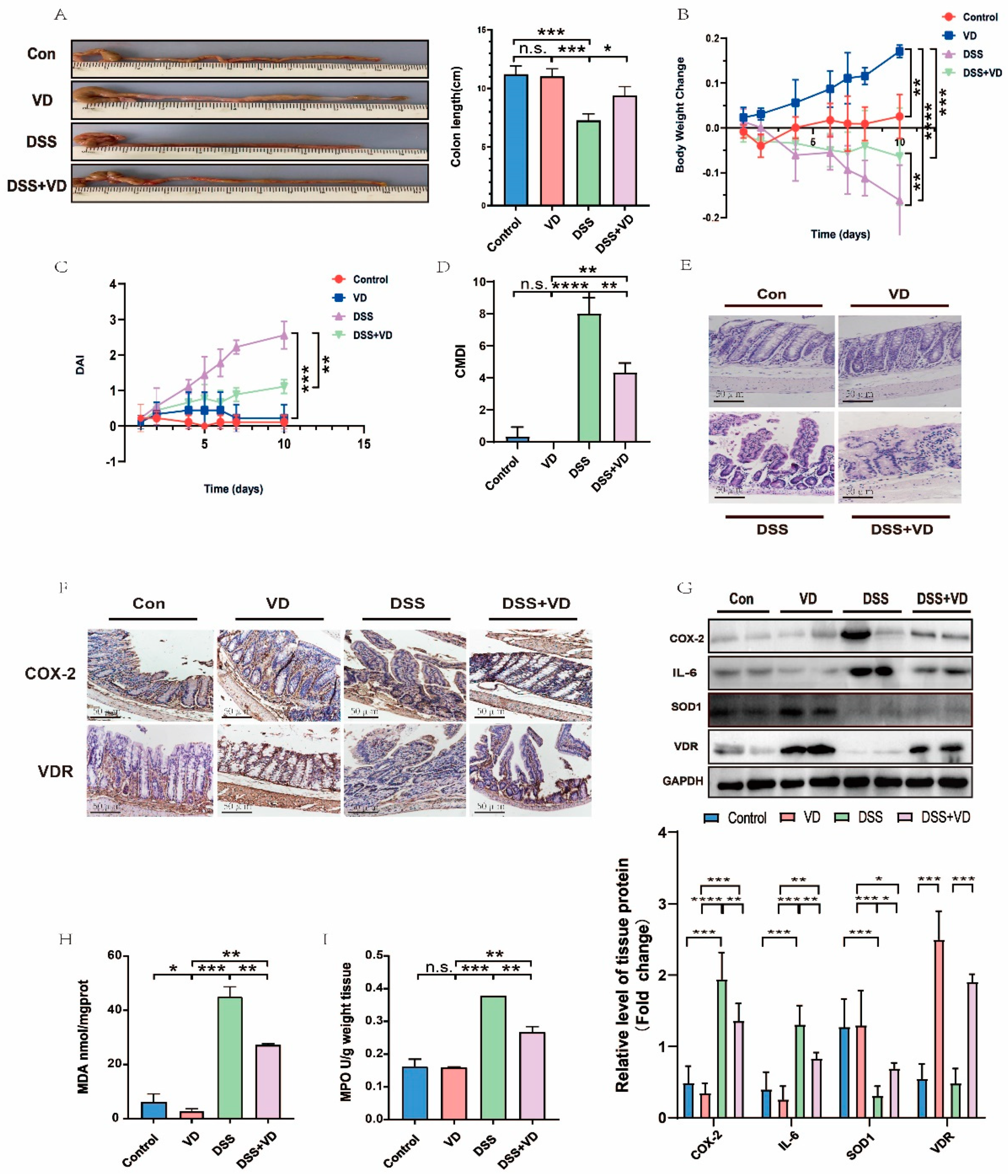
3.1. VD/VDR Attenuated DSS-Induced UC in Mice
3.2. VD/VDR Inhibits UC Inflammation In Vitro
3.3. Ferroptosis Was Involved in the Pathological Process of UC
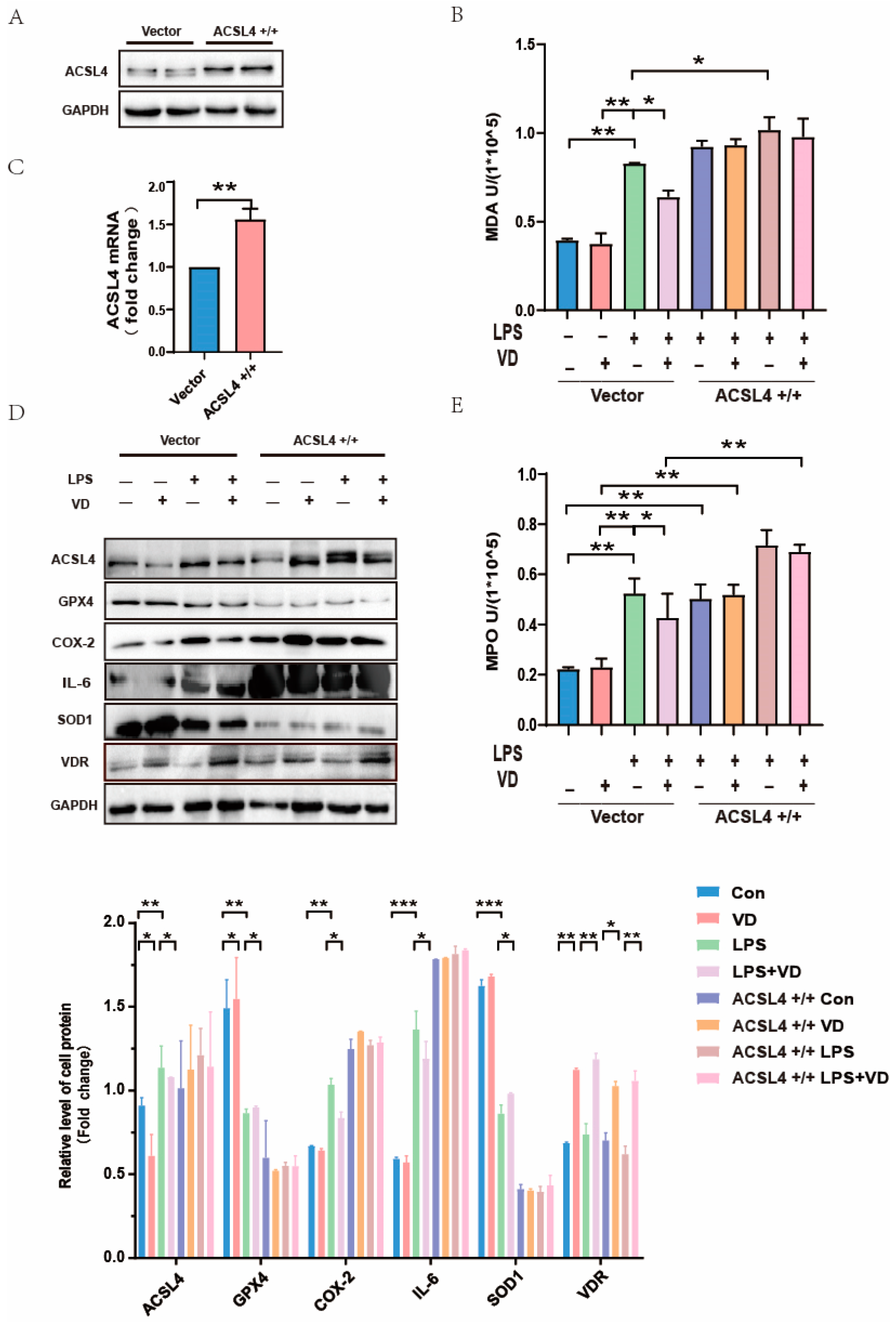
3.4. VD/VDR Regulated the Expression of ACSL4 and Other Ferroptosis Relative Proteins In Vivo and In Vitro
3.5. ACSL4 Plays an Indispensable Role in Vitamin D Mitigation of UC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | 1,25-dihydroxyvitamin D3 |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AA | Arachidonic acid |
| CCE | Cholecalciferol cholesterol emulsion |
| CMDI | Colon Macroscopic Damage Index |
| Con | Control |
| CD | Crohn’s disease |
| COX-2 | Cyclooxygenase-2 |
| DSS | Dextran Sulfate Sodium Salt |
| DAB | Diaminobenzidine |
| DAI | Disease Activity Index |
| Fer-1 | Ferrostatin-1 |
| GIT | Gastrointestinal tract |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| IHC | Immunohistochemistry |
| IBD | Inflammatory bowel disease |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| ACSLs | Long-chain acyl coenzyme A synthase family |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| PBS | Phate-buffered saline |
| PUFAs | Polyunsaturated fatty acid |
| GPX4 | Recombinant Glutathione Peroxidase 4 |
| SLC7A11 | Recombinant Solute Carrier Family 7, Member 11 |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SOD1 | Superoxide Dismutase 1 |
| UC | Ulcerative Colitis |
| VD | Vitamin D |
| VDR | Vitamin D receptor |
| XcT | Xc-activity |
References
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Cheng, Z.; Wu, F.; Hu, M.; Liu, Z.; Dong, R.; Chen, G. Berberine in the treatment of ulcerative colitis: A possible pathway through Tuft cells. Biomed. Pharmacother. 2021, 134, 111129. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Feagan, B.G.; Panaccione, R.; Colombel, J.F.; Sandborn, W.J.; Sands, B.E.; Danese, S.; D’Haens, G.; Rubin, D.T.; Shafran, I.; et al. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, P.; Chen, W.; Chen, G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol. Lett. 2020, 225, 9–15. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Verway, M.; Behr, M.A.; White, J.H. Vitamin D, NOD2, autophagy and Crohn’s disease. Expert. Rev. Clin. Immunol. 2010, 6, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, L.; Properzi, G.; Marampon, F.; Gravina, G.L.; Festuccia, C.; Di Cesare, E.; Scarsella, L.; Ciccarelli, C.; Zani, B.M.; Ferri, C. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. J. Cardiovasc. Transl. Res. 2013, 6, 221–231. [Google Scholar] [CrossRef]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. Effects of vitamin D supplementation on blood markers in ulcerative colitis patients: A systematic review and meta-analysis. Eur. J. Nutr. 2022, 61, 23–35. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Du, J.; Ge, X.; Sun, Y.; Li, X.; Xun, Z.; Liu, W.; Wang, Z.Y.; Li, Y.C. Vitamin D Deficiency Exacerbates Colonic Inflammation Due to Activation of the Local Renin-Angiotensin System in the Colon. Dig. Dis. Sci. 2021, 66, 3813–3821. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S.R.; Dewitz, C.; De Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P.S.; et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of Ferroptosis and Relations with Regulated Cell Death: A Review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Chaudhary, G.; Mahajan, U.B.; Goyal, S.N.; Ojha, S.; Patil, C.R.; Subramanya, S.B. Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. Am. J. Transl. Res. 2017, 9, 1792–1800. [Google Scholar] [PubMed]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef]
- Xiong, Y.; Lou, Y.; Su, H.; Fu, Y.; Kong, J. Cholecalciterol cholesterol emulsion ameliorates experimental colitis via down-regulating the pyroptosis signaling pathway. Exp. Mol. Pathol. 2016, 100, 386–392. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Liu, N.; Zhang, Y.; Li, Y.; Pan, Y.; Yang, J.; Liu, Z.; Kong, J. Vitamin D alleviates liver fibrosis by inhibiting histidine-rich calcium binding protein (HRC). Chem. Biol. Interact. 2021, 334, 109355. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Y.; Su, H.; Wang, J.; Liu, Z.; Kong, J. Effects of cholecalciferol cholesterol emulsion on renal fibrosis and aquaporin 2 and 4 in mice with unilateral ureteral obstruction. Biomed. Pharmacother. 2018, 102, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Mehigan, G.A.; Villegas, F.; Mitsuhashi, S.; Longhi, M.S.; Malvar, G.; Csizmadia, E.; Robson, S.; Moss, A.C. Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells. Inflamm. Bowel Dis. 2020, 26, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Zhu, H.; Zhang, X.; Han, L.; Zhao, Z.; Wang, J.; Ning, L.; Zhou, W.; Lu, C.; et al. Inhibition of Histone Deacetylation by MS-275 Alleviates Colitis by Activating the Vitamin D Receptor. J. Crohns Colitis 2020, 14, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, M.; Duan, W.; Chen, L.; Wang, L.; Liu, P. Anemoside B4 prevents acute ulcerative colitis through inhibiting of TLR4/NF-kappaB/MAPK signaling pathway. Int. Immunopharmacol. 2020, 87, 106794. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; MacNaughton, W.K.; Morris, G.P.; Beck, P.L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology 1989, 96, 29–36. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Karmeli, F.; Takabayashi, K.; Hayashi, T.; Leider-Trejo, L.; Lee, J.; Leoni, L.M.; Raz, E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 2002, 122, 1428–1441. [Google Scholar] [CrossRef]
- Ibrahim, S.; Zhu, X.; Luo, X.; Feng, Y.; Wang, J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int. Immunopharmacol. 2020, 85, 106610. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.; Wang, Z.; Zhao, Y.; Sun, R.; Tian, D.; Liu, D.; Zhang, F.; Ning, S.; Yao, J.; et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019, 26, 2284–2299. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Scoglio, S.; Serio, R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients 2020, 12, 3635. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.Y.; Kim, J.H.; Kang, J.E.; Park, M.H.; Kim, E.J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ostrowski, R.; Jiang, D.; Zhao, Q.; Liang, Y.; Che, X.; Zhao, J.; Xiang, X.; Qin, W.; He, Z. Hepcidin Promoted Ferroptosis through Iron Metabolism which Is Associated with DMT1 Signaling Activation in Early Brain Injury following Subarachnoid Hemorrhage. Oxidative Med. Cell. Longev. 2021, 2021, 9800794. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shih, D.Q.; Zhang, X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012, 12, 57. [Google Scholar] [CrossRef]
- Dai, Z.H.; Tan, B.; Yang, H.; Wang, O.; Qian, J.J.; Lv, H. 1,25-hydroxyvitamin D relieves colitis in rats via down-regulation of toll-like receptor 9 expression. Croat. Med. J. 2015, 56, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Cui, Z.; Wang, X.; Ning, T.; Wang, T.; Zhang, N.; Xie, S.; Min, L.; Zhang, S.; et al. Ferrostatin-1 alleviated TNBS induced colitis via the inhibition of ferroptosis. Biochem. Biophys. Res. Commun. 2021, 573, 48–54. [Google Scholar] [CrossRef]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73. [Google Scholar] [CrossRef]
- Fan, Y.G.; Pang, Z.Q.; Wu, T.Y.; Zhang, Y.H.; Xuan, W.Q.; Wang, Z.; Yu, X.; Li, Y.C.; Guo, C.; Wang, Z.Y. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic. Biol. Med. 2020, 161, 139–149. [Google Scholar] [CrossRef] [PubMed]



| Grade | Weight Loss (%) | Stool Consistency | Gross Bleeding |
|---|---|---|---|
| 0 | 0 | Normal | N/A |
| 1 | 1–5 | Mild soft | — |
| 2 | 5–10 | Soft and wet | Hemoccult positive |
| 3 | 10–20 | Half-loose stool | — |
| 4 | >20 | Loose stool | Gross bleeding |
| Score | Criteria |
|---|---|
| 0 | No damage |
| 1 | Hyperemia |
| 2 | Hyperemia and thickening without ulceration |
| 3 | Ulceration at a single site |
| 4 | Two or more sites of ulceration or inflammation |
| 5 | Ulceration or inflammation extending > 1 cm along the length |
| 6–10 | Damage covering > 2 cm along the length of colon, with the score being increased by 1 for each additional cm of involvement |
| Gene | Mouse | Human |
|---|---|---|
| ACSL4 | F: TCCTTCGTGACTACTGCCGAG R: GTTATAGGTGGTTTCGTGGAT | CATCCCTGGAGCAGATACTCT TCACTTAGGATTTCCCTGGTC |
| VDR | F: CAAGGACAACCGACGCCACTG R: CCTCCTCCTCCTTCCGCTTCAG | CTGGTGACTTTGACCGGAAT CTGCACCTCCTCATCTGTGA |
| COX-2 | F: GGTTGCTGGTGGTAGGAATC R: TAAAGCGTTTGGGGTACTCA | CTAGAGCCCTTCCTCCTGCG GCTGGGCAAAGAATGCAAT |
| SOD1 | F: CTCAGGAGACCATTGCATCA R: ACAAGCCAAACGACTTCCAG | TGTCATGCAGTCCCTTGGAT CATTCTCGCCCTGGATCTCT |
| GPX4 | F: GCCCCTCCATCTACGACTTC R: TTGGTGATGATGCAGACGAAC | GGAGCCAGGGAGTAACGAAG GACGGTGTCCAAACTTGGTG |
| GAPDH | F: AAATCAAGTGGGGCGATGCT R: TGGTTCACACCCATGACGAA | ACCCAGAAGACTGTGGATGG TCAGCTCAGGGATGACCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Sun, C.; Kong, J. Vitamin D Attenuates Ulcerative Colitis by Inhibiting ACSL4-Mediated Ferroptosis. Nutrients 2023, 15, 4845. https://doi.org/10.3390/nu15224845
Gao S, Sun C, Kong J. Vitamin D Attenuates Ulcerative Colitis by Inhibiting ACSL4-Mediated Ferroptosis. Nutrients. 2023; 15(22):4845. https://doi.org/10.3390/nu15224845
Chicago/Turabian StyleGao, Shuo, Can Sun, and Juan Kong. 2023. "Vitamin D Attenuates Ulcerative Colitis by Inhibiting ACSL4-Mediated Ferroptosis" Nutrients 15, no. 22: 4845. https://doi.org/10.3390/nu15224845
APA StyleGao, S., Sun, C., & Kong, J. (2023). Vitamin D Attenuates Ulcerative Colitis by Inhibiting ACSL4-Mediated Ferroptosis. Nutrients, 15(22), 4845. https://doi.org/10.3390/nu15224845







