High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study
Abstract
:1. Introduction
2. Materials and Methods
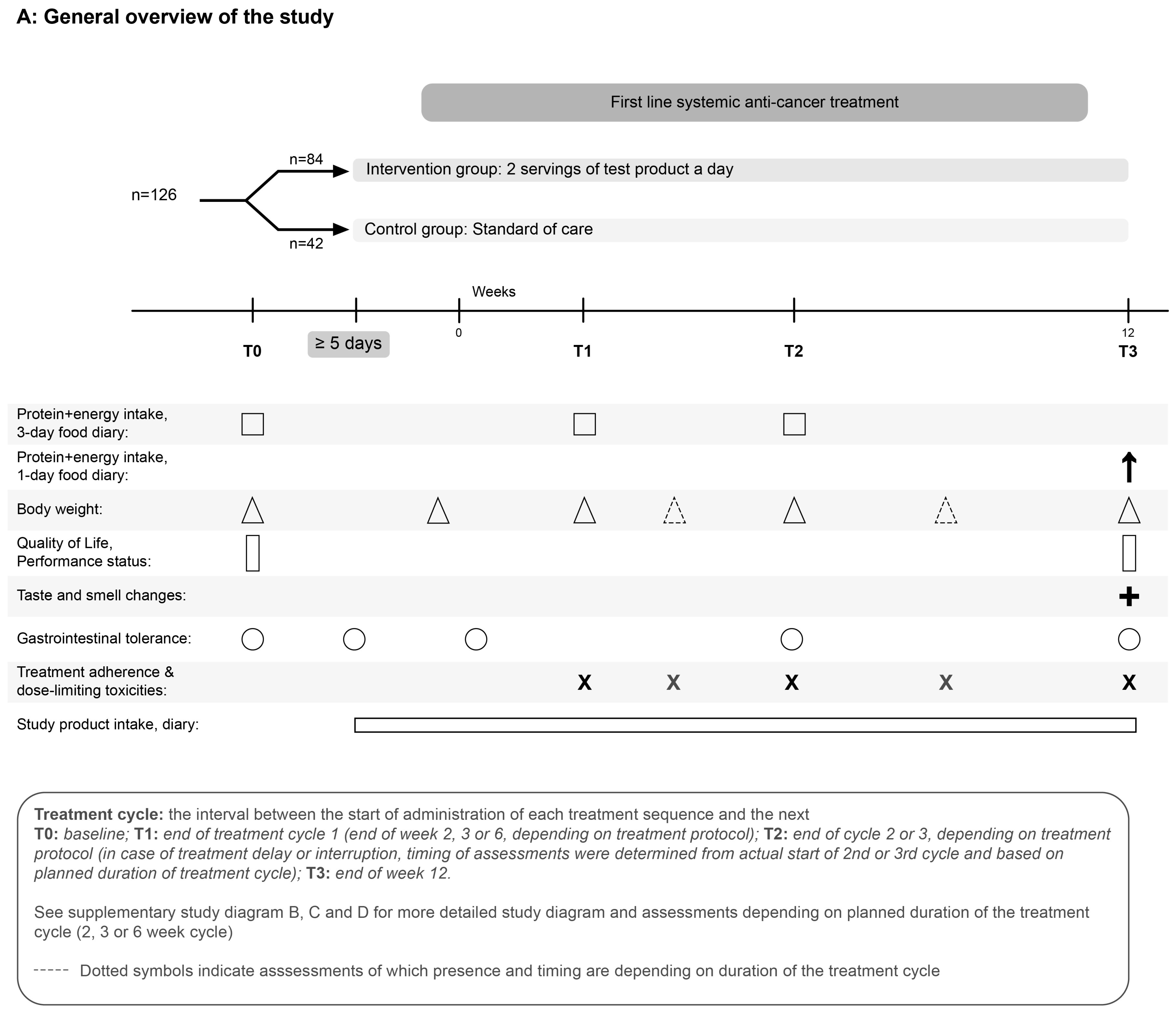
2.1. Study Design and Population
2.2. Nutritional Intervention
2.3. Randomisation Procedure
2.4. Outcome Measures
2.5. Statistics and Data Analysis
3. Results
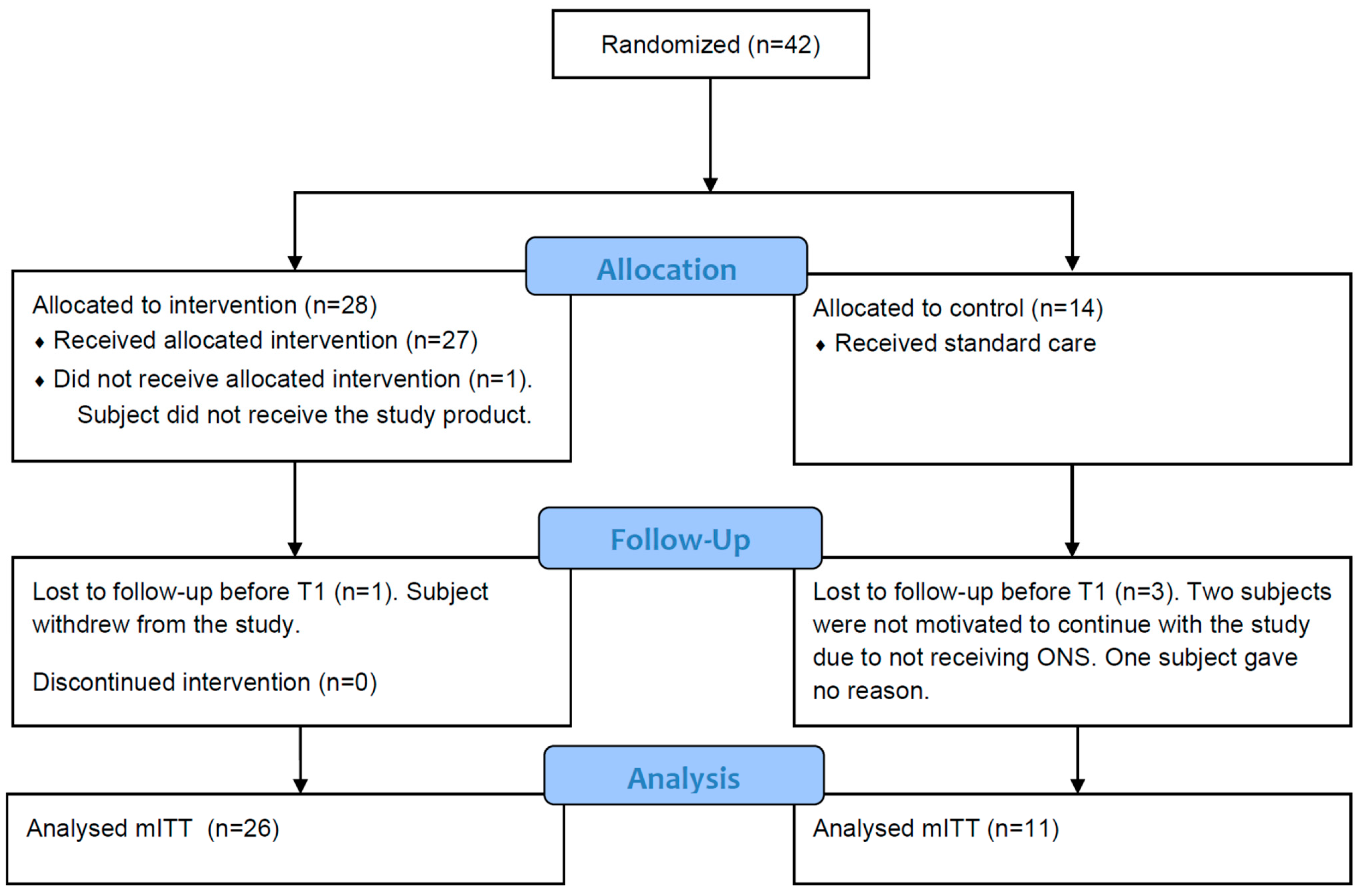
3.1. Patient Characteristics
3.2. Protein Intake
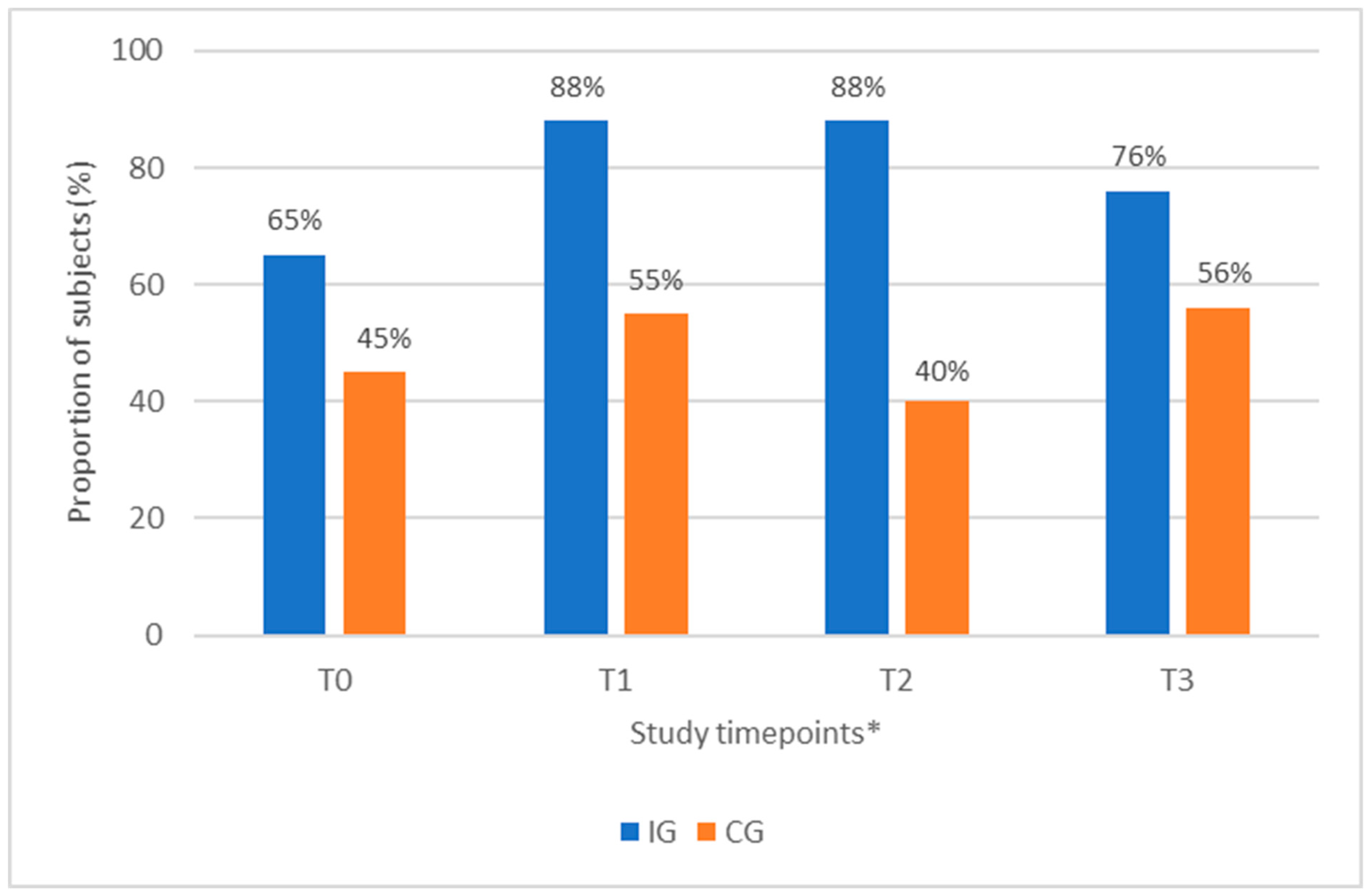
3.3. Proportion of Subjects with a Protein Intake ≥1.0 g/kg BW/day
3.4. Change in Body Weight during 12 Weeks of Anti-Cancer Treatment
3.5. ONS Compliance
3.6. Gastrointestinal (GI) Tolerance and Safety
3.7. Exploratory Outcome Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.P.; Guo, Z.Q.; Xu, H.X.; Yuan, K.T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021, 40, 1224–1232. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Planas, M.; Álvarez-Hernández, J.; León-Sanz, M.; Celaya-Pérez, S.; Araujo, K.; García de Lorenzo, A.; on Behalf of the PREDyCES® Researchers. Prevalence of hospital malnutrition in cancer patients: A sub-analysis of the PREDyCES® study. Support. Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Cancer Research UK. What is Prehabilitation? Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/prehabilitation/what-is-prehabilitation (accessed on 6 December 2022).
- Elliott, J.A.; Doyle, S.L.; Murphy, C.F.; King, S.; Guinan, E.M.; Beddy, P.; Ravi, N.; Reynolds, J.V. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann. Surg. 2017, 266, 822–830. [Google Scholar] [CrossRef]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Lee, V.S.; Prado, C.M.; Meyerhardt, J.A.; Alexeeff, S.; Kroenke, C.H.; Xiao, J.; Castillo, A.L.; Caan, B.J. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer 2017, 123, 4868–4877. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non–small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef]
- Nakamura, R.; Inage, Y.; Tobita, R.; Yoneyama, S.; Numata, T.; Ota, K.; Yanai, H.; Endo, T.; Inadome, Y.; Sakashita, S.; et al. Sarcopenia in Resected NSCLC: Effect on Postoperative Outcomes. J. Thorac. Oncol. 2018, 13, 895–903. [Google Scholar] [CrossRef]
- Yang, M.; Shen, Y.; Tan, L.; Li, W. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis. Chest 2019, 156, 101–111. [Google Scholar] [CrossRef]
- Tobberup, R.; Rasmussen, H.H.; Holst, M.; Jensen, N.A.; Falkmer, U.G.; Bøgsted, M.; Delekta, A.M.; Carus, A. Exploring the dietary protein intake and skeletal muscle during first-line anti-neoplastic treatment in patients with non-small cell lung cancer. Clin. Nutr. ESPEN 2019, 34, 94–100. [Google Scholar] [CrossRef]
- Capitão, C.; Coutinho, D.; Neves, P.M.; Capelas, M.L.; Pimenta, N.M.; Santos, T.; Mäkitie, A.; Ravasco, P. Protein intake and muscle mass maintenance in patients with cancer types with high prevalence of sarcopenia: A systematic review. Support. Care Cancer 2022, 30, 3007–3015. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ijichi, H.; Saito, K.; Ishigaki, K.; Takami, M.; Sekine, R.; Usami, S.; Nakai, Y.; Koike, K.; Kubota, N. Protein intake after the initiation of chemotherapy is an independent prognostic factor for overall survival in patients with unresectable pancreatic cancer: A prospective cohort study. Clin. Nutr. 2021, 40, 4792–4798. [Google Scholar] [CrossRef]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- McCurdy, B.; Nejatinamini, S.; Debenham, B.J.; Álvarez-Camacho, M.; Kubrak, C.; Wismer, W.V.; Mazurak, V.C. Meeting Minimum ESPEN Energy Recommendations Is Not Enough to Maintain Muscle Mass in Head and Neck Cancer Patients. Nutrients 2019, 11, 2743. [Google Scholar] [CrossRef]
- Sonneborn-Papakostopoulos, M.; Dubois, C.; Mathies, V.; Heß, M.; Erickson, N.; Ernst, T.; Huebner, J. Quality of life, symptoms and dietary habits in oncology outpatients with malnutrition: A cross-sectional study. Med. Oncol. 2021, 38, 20. [Google Scholar] [CrossRef]
- Ford, K.L.; Orsso, C.E.; Kiss, N.; Johnson, S.B.; Purcell, S.A.; Gagnon, A.; Laviano, A.; Prado, C.M. Dietary choices after a cancer diagnosis: A narrative review. Nutrition 2022, 103–104, 111838. [Google Scholar] [CrossRef]
- Tan, S.; Meng, Q.; Jiang, Y.; Zhuang, Q.; Xi, Q.; Xu, J.; Zhao, J.; Sui, X.; Wu, G. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: A randomised clinical trial. Clin. Nutr. 2021, 40, 47–53. [Google Scholar] [CrossRef]
- Meng, Q.; Tan, S.; Jiang, Y.; Han, J.; Xi, Q.; Zhuang, Q.; Wu, G. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin. Nutr. 2021, 40, 40–46. [Google Scholar] [CrossRef]
- Bargetzi, L.; Brack, C.; Herrmann, J.; Bargetzi, A.; Hersberger, L.; Bargetzi, M.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: Secondary analysis of a prospective randomized trial. Ann. Oncol. 2021, 32, 1025–1033. [Google Scholar] [CrossRef]
- Kabata, P.; Jastrzębski, T.; Kąkol, M.; Król, K.; Bobowicz, M.; Kosowska, A.; Jaśkiewicz, J. Preoperative nutritional support in cancer patients with no clinical signs of malnutrition—Prospective randomized controlled trial. Support. Care Cancer 2015, 23, 365–370. [Google Scholar] [CrossRef]
- Maňásek, V.; Bezděk, K.; Foltys, A.; Klos, K.; Smitka, J.; Smehlik, D. The Impact of High Protein Nutritional Support on Clinical Outcomes and Treatment Costs of Patients with Colorectal Cancer. Klin. Onkol. 2016, 29, 351–357. [Google Scholar] [CrossRef]
- Ziętarska, M.; Krawczyk-Lipiec, J.; Kraj, L.; Zaucha, R.; Małgorzewicz, S. Chemotherapy-Related Toxicity, Nutritional Status and Quality of Life in Precachectic Oncologic Patients with, or without, High Protein Nutritional Support. A Prospective, Randomized Study. Nutrients 2017, 9, 1108. [Google Scholar] [CrossRef]
- Grupińska, J.; Budzyń, M.; Maćkowiak, K.; Brzeziński, J.J.; Kycler, W.; Leporowska, E.; Gryszczyńska, B.; Kasprzak, M.P.; Iskra, M.; Formanowicz, D. Beneficial Effects of Oral Nutritional Supplements on Body Composition and Biochemical Parameters in Women with Breast Cancer Undergoing Postoperative Chemotherapy: A Propensity Score Matching Analysis. Nutrients 2021, 13, 3549. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Apolone, G.; Filiberti, A.; Cifani, S.; Ruggiata, R.; Mosconi, P. Evaluation of the EORTC QLQ-C30 questionnaire: A comparison with SF-36 Health Survey in a cohort of Italian long-survival cancer patients. Ann. Oncol. 1998, 9, 549–557. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0; U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Washington, DC, USA, 2009.
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- Van der Meij, B.S.; Langius, J.A.; Smit, E.F.; Spreeuwenberg, M.D.; von Blomberg, B.M.; Heijboer, A.C.; Paul, M.A.; van Leeuwen, P.A. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J. Nutr. 2010, 140, 1774–1780. [Google Scholar] [CrossRef]
- Bavry, J.L. STATPOWER: User’s Guide, 2nd ed.; Scientific Software Inc.: Chicago, IL, USA, 1991. [Google Scholar]
- Twisk, J.W.R. Applied Multilevel Analysis; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Regueme, S.C.; Echeverria, I.; Monéger, N.; Durrieu, J.; Becerro-Hallard, M.; Duc, S.; Lafargue, A.; Mertens, C.; Laksir, H.; Ceccaldi, J.; et al. Protein intake, weight loss, dietary intervention, and worsening of quality of life in older patients during chemotherapy for cancer. Support. Care Cancer 2021, 29, 687–696. [Google Scholar] [CrossRef]
- Molfino, A.; Emerenziani, S.; Tonini, G.; Santini, D.; Gigante, A.; Guarino, M.P.L.; Nuglio, C.; Imbimbo, G.; La Cesa, A.; Cicala, M.; et al. Early impairment of food intake in patients newly diagnosed with cancer. Front. Nutr. 2022, 9, 997813. [Google Scholar] [CrossRef]
- Le-Rademacher, J.; Lopez, C.; Wolfe, E.; Foster, N.R.; Mandrekar, S.J.; Wang, X.; Kumar, R.; Adjei, A.; Jatoi, A. Weight loss over time and survival: A landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 1501–1508. [Google Scholar] [CrossRef]
- Ryan, A.M.; Prado, C.M.; Sullivan, E.S.; Power, D.G.; Daly, L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019, 67–68, 110539. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Jouinot, A.; Ulmann, G.; Vazeille, C.; Durand, J.P.; Boudou-Rouquette, P.; Arrondeau, J.; Tlemsani, C.; Fournel, L.; Alifano, M.; Wislez, M.; et al. Hypermetabolism is an independent prognostic factor of survival in metastatic non-small cell lung cancer patients. Clin. Nutr. 2020, 39, 1893–1899. [Google Scholar] [CrossRef]
- Sjøblom, B.; Grønberg, B.H.; Benth, J.; Baracos, V.E.; Fløtten, Ø.; Hjermstad, M.J.; Aass, N.; Jordhøy, M. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer 2015, 90, 85–91. [Google Scholar] [CrossRef]
- Barret, M.; Antoun, S.; Dalban, C.; Malka, D.; Mansourbakht, T.; Zaanan, A.; Latko, E.; Taieb, J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr. Cancer 2014, 66, 583–589. [Google Scholar] [CrossRef]
- Chemama, S.; Bayar, M.A.; Lanoy, E.; Ammari, S.; Stoclin, A.; Goéré, D.; Elias, D.; Raynard, B.; Antoun, S. Sarcopenia is Associated with Chemotherapy Toxicity in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 3891–3898. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Yoshida, M.; et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Van Vledder, M.G.; Levolger, S.; Ayez, N.; Verhoef, C.; Tran, T.C.; Ijzermans, J.N. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br. J. Surg. 2012, 99, 550–557. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Cereda, E.; Cappello, S.; Colombo, S.; Klersy, C.; Imarisio, I.; Turri, A.; Caraccia, M.; Borioli, V.; Monaco, T.; Benazzo, M.; et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. 2018, 126, 81–88. [Google Scholar] [CrossRef]
- Upadhaya, S.; Yu, J.X.; Oliva, C.; Hooton, M.; Hodge, J.; Hubbard-Lucey, V.M. Impact of COVID-19 on oncology clinical trials. Nat. Rev. Drug Discov. 2020, 19, 376–377. [Google Scholar] [CrossRef]
- Avins, A.L. Can unequal be more fair? Ethics, subject allocation, and randomised clinical trials. J. Med. Ethics 1998, 24, 401–408. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- De van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Sullivan, E.S.; Rice, N.; Kingston, E.; Kelly, A.; Reynolds, J.V.; Feighan, J.; Power, D.G.; Ryan, A.M. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clin. Nutr. ESPEN 2021, 41, 331–339. [Google Scholar] [CrossRef]


| Parameter | IG (n = 26) | CG (n = 11) | Total (n = 37) |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 15 (57.7%) | 3 (27.3%) | 18 (48.6%) |
| Male | 11 (42.3%) | 8 (72.7%) | 19 (51.4%) |
| Age (years), mean (SD) | 66.1 (7.8) | 70.1(8.2) | 67.3 (8.0) |
| Smoking, n (%) | |||
| No | 23 (88.5%) | 10 (90.9%) | 33 (89.2%) |
| Yes | 3 (11.5%) | 1 (9.1%) | 4 (10.8%) |
| Previous cancer treatment in last 12 months, n (%) | |||
| No | 18 (69.2%) | 8 (72.7%) | 26 (70.3%) |
| Yes | 8 (30.8%) | 3 (27.3%) | 11 (29.7%) |
| Localisation primary tumour, n (%) | |||
| Colorectal | 12 (46.2%) | 3 (27.3%) | 15 (40.5%) |
| Lung | 14 (53.8%) | 8 (72.7%) | 22 (59.5%) |
| Tumour stage at diagnosis, n (%) | |||
| IIB | 1 (3.8%) | 0 (0.0%) | 1 (2.8%) |
| III | 6 (23.1%) | 2 (20.0%) | 8 (22.2%) |
| IV | 19 (73.1%) | 8 (80.0%) | 27 (75.0%) |
| Missing | 0 | 1 | 1 |
| Anti-cancer treatment during intervention, n (%) | |||
| Chemotherapy | 19 (73.1%) | 7 (63.6%) | 26 (70.3%) |
| Concurrent chemoradiotherapy | 2 (7.7%) | 1 (9.1%) | 3 (8.1%) |
| Immunotherapy | 5 (19.2%) | 3 (27.3%) | 8 (21.6%) |
| Planned duration treatment cycle, n (%) | |||
| 2 weeks | 8 (30.8%) | 2 (18.2%) | 10 (27.0%) |
| 3 weeks | 17 (65.4%) | 8 (72.7%) | 25 (67.6%) |
| 6 weeks | 1 (3.8%) | 1 (9.1%) | 2 (5.4%) |
| Parameter | IG (n = 26) | CG (n = 11) | Total (n = 37) |
|---|---|---|---|
| Body weight (kg) mean (SD) | 75.2 (10.6) | 82.3 (15.9) | 77.4 (12.6) |
| Body mass index (kg/m2) mean (SD) | 26.2 (3.7) | 27.2 (3.0) | 26.5 (3.4) |
| Proportion of patients who experienced unplanned WL n (%) | 10 (38%) | 8 (73%) | 18 (49%) |
| Unplanned WL in last 6 months (kg) mean (SD) | 3.0 (2.3) a* | 3.9 (2.5) b | 3.4 (2.4) c |
| Unplanned WL in last 6 months (% BW) mean (SD) | 3.9 (2.8) a | 4.7 (2.5) b | 4.3 (2.6) c |
| Energy intake (kcal/kg BW/day) mean (SD) | 28.0 (9.1) | 24.5 (7.3) | 27.0 (8.6) |
| Protein intake (g/kg BW/day) mean (SD) | 1.12 (0.33) | 1.04 (0.30) | 1.10 (0.32) |
| Protein intake ≥ 1.0 g/kg BW/day (lower limit of ESPEN recommendation for protein intake in adult cancer patients) n (%) | |||
| Yes | 17 (65%) | 5 (45%) | n/a |
| No | 9 (35%) | 6 (55%) | n/a |
| Parameter | Number of Subjects (IG vs. CG) | IG (n = 26) | CG (n = 11) |
|---|---|---|---|
| Protein intake (g/kg BW/day) mean (SD) | |||
| T0 a | n = 26 vs. n = 11 | 1.12 (0.33) | 1.04 (0.30) |
| T1 | n = 25 vs. n = 11 | 1.40 (0.33) | 1.07 (0.30) |
| T2 | n = 16 vs. n = 10 | 1.32 (0.27) | 0.94 (0.27) |
| T3 | n = 17 vs. n = 9 | 1.25 (0.58) | 1.08 (0.30) |
| Parameter | LS Means ± SE | 95% CI | Treatment Difference ± SE | 95% CI | p-Value 1 |
|---|---|---|---|---|---|
| Protein intake (g/day) | |||||
| IG | 106.1 ± 5.3 | 95.2–117.0 | 17.2 ± 7.0 | 3.0–31.5 | 0.019 * |
| CG | 88.8 ± 7.2 | 74.2–103.4 | |||
| Protein intake (g/kg BW/day) | |||||
| IG | 1.40 ± 0.08 | 1.24–1.56 | 0.25 ± 0.10 | 0.05–0.46 | 0.018 * |
| CG | 1.15 ± 0.10 | 0.94–1.36 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dingemans, A.-M.; van Walree, N.; Schramel, F.; Soud, M.Y.-E.; Baltruškevičienė, E.; Lybaert, W.; Veldhorst, M.; van den Berg, C.A.; Kaasa, S. High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study. Nutrients 2023, 15, 5030. https://doi.org/10.3390/nu15245030
Dingemans A-M, van Walree N, Schramel F, Soud MY-E, Baltruškevičienė E, Lybaert W, Veldhorst M, van den Berg CA, Kaasa S. High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study. Nutrients. 2023; 15(24):5030. https://doi.org/10.3390/nu15245030
Chicago/Turabian StyleDingemans, Anne-Marie, Nico van Walree, Franz Schramel, Magdolen Youssef-El Soud, Edita Baltruškevičienė, Willem Lybaert, Margriet Veldhorst, Claudia. A. van den Berg, and Stein Kaasa. 2023. "High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study" Nutrients 15, no. 24: 5030. https://doi.org/10.3390/nu15245030
APA StyleDingemans, A.-M., van Walree, N., Schramel, F., Soud, M. Y.-E., Baltruškevičienė, E., Lybaert, W., Veldhorst, M., van den Berg, C. A., & Kaasa, S. (2023). High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study. Nutrients, 15(24), 5030. https://doi.org/10.3390/nu15245030






