Pregnancy Protects against Abnormal Gut Permeability Promoted via the Consumption of a High-Fat Diet in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Mating
2.3. Microbiota Analysis
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Immunohistochemistry and Image Analysis
2.6. Quantitative Analysis of Gut Permeability
2.7. Statistical Analysis
3. Results
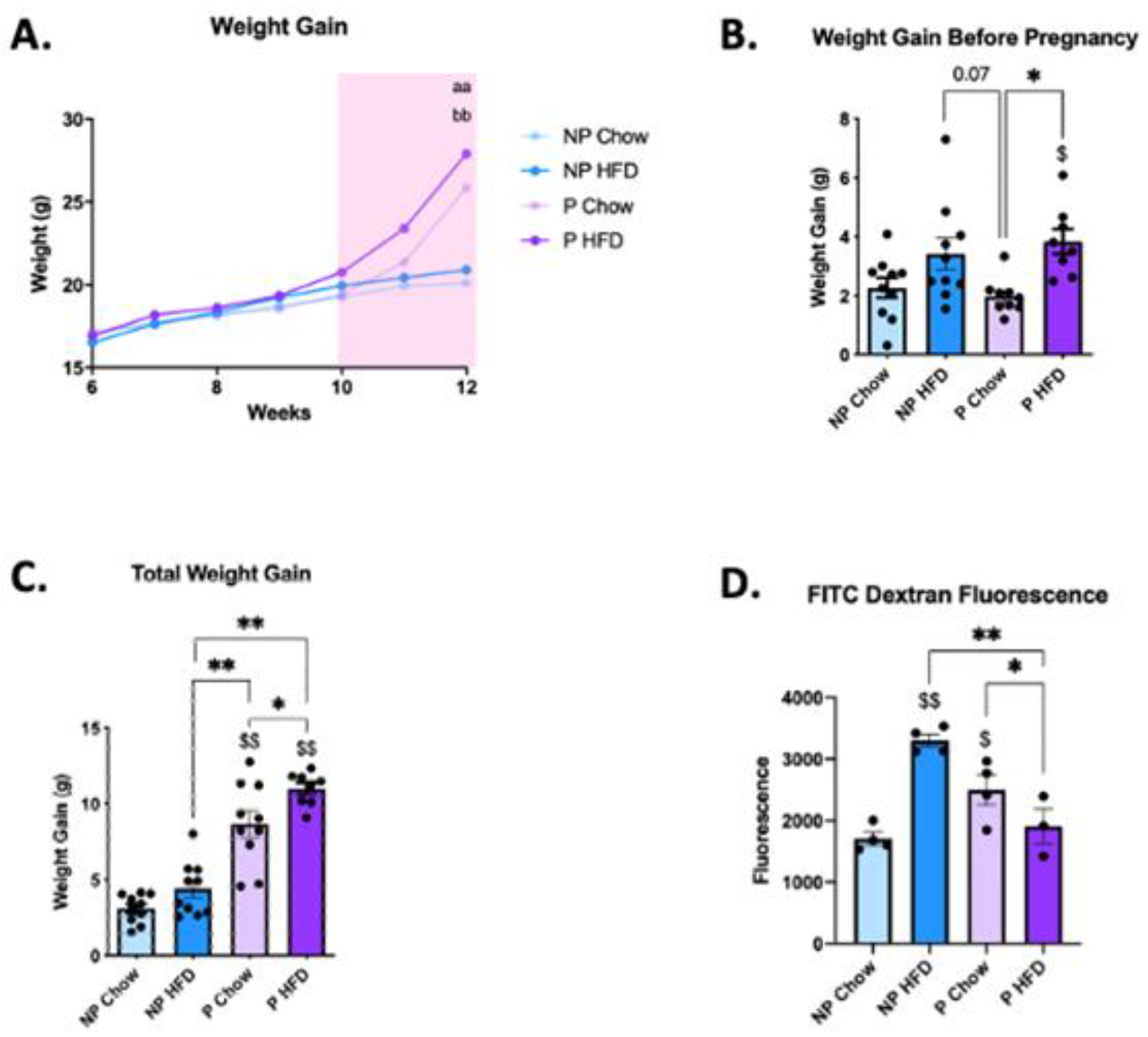
3.1. Pregnancy Protects against Diet-Induced Increase in Gut Permeability
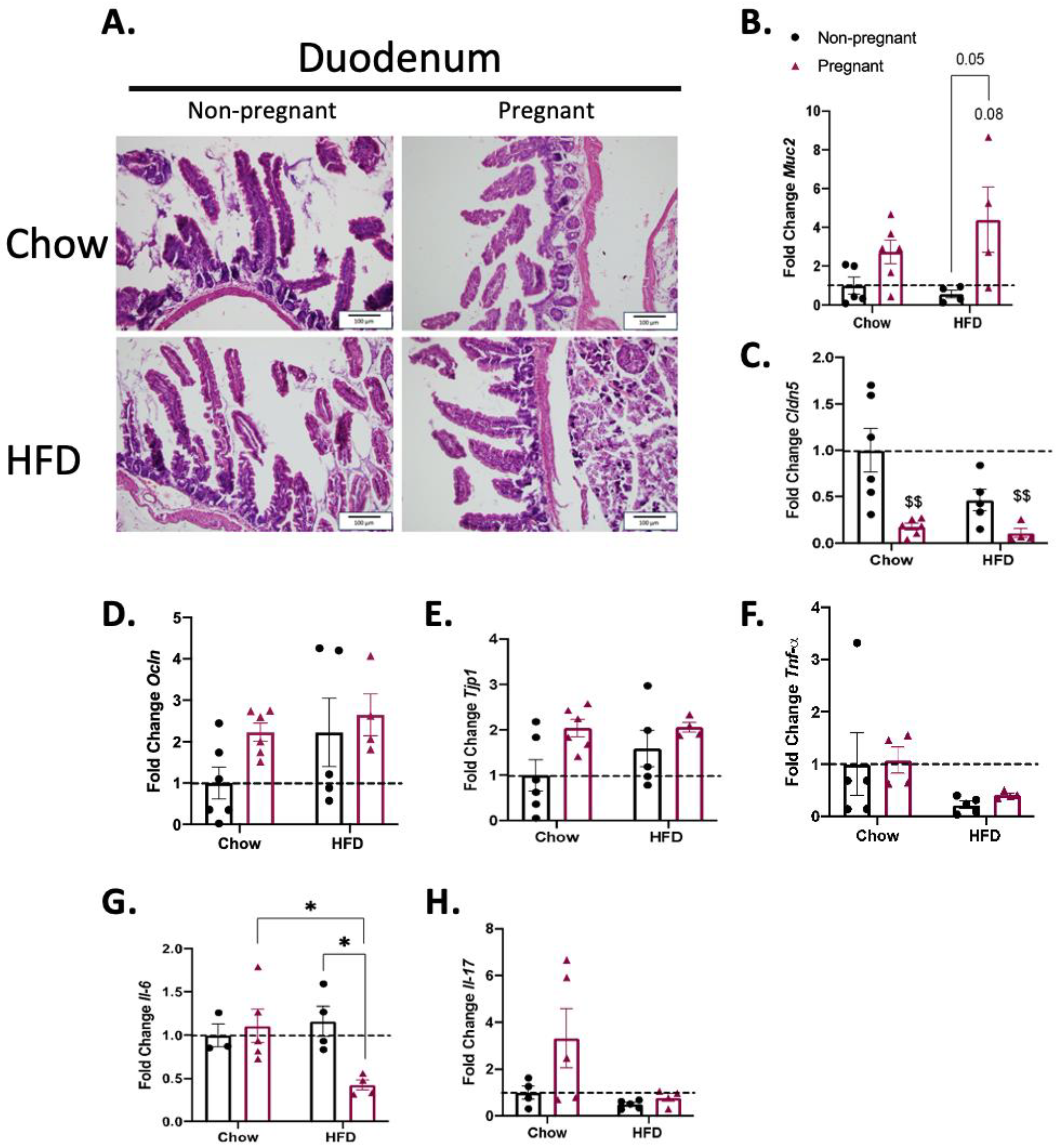
3.2. Consumption of a High-Fat Diet in Pregnant Mice Promotes Minimal Changes in the Duodenum
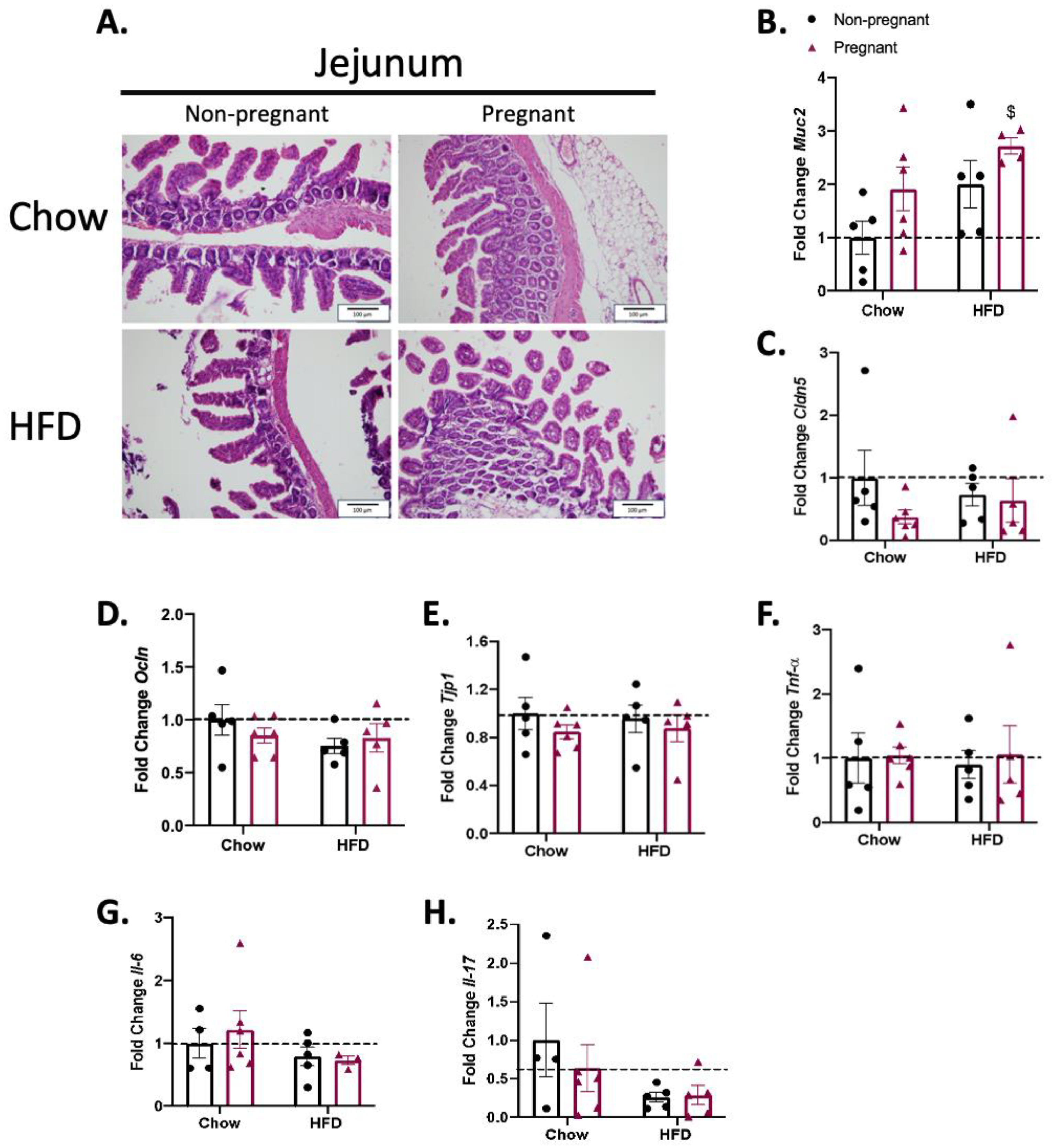
3.3. Consumption of a High-Fat Diet in Pregnant Mice Promotes Minimal Changes in the Jejunum
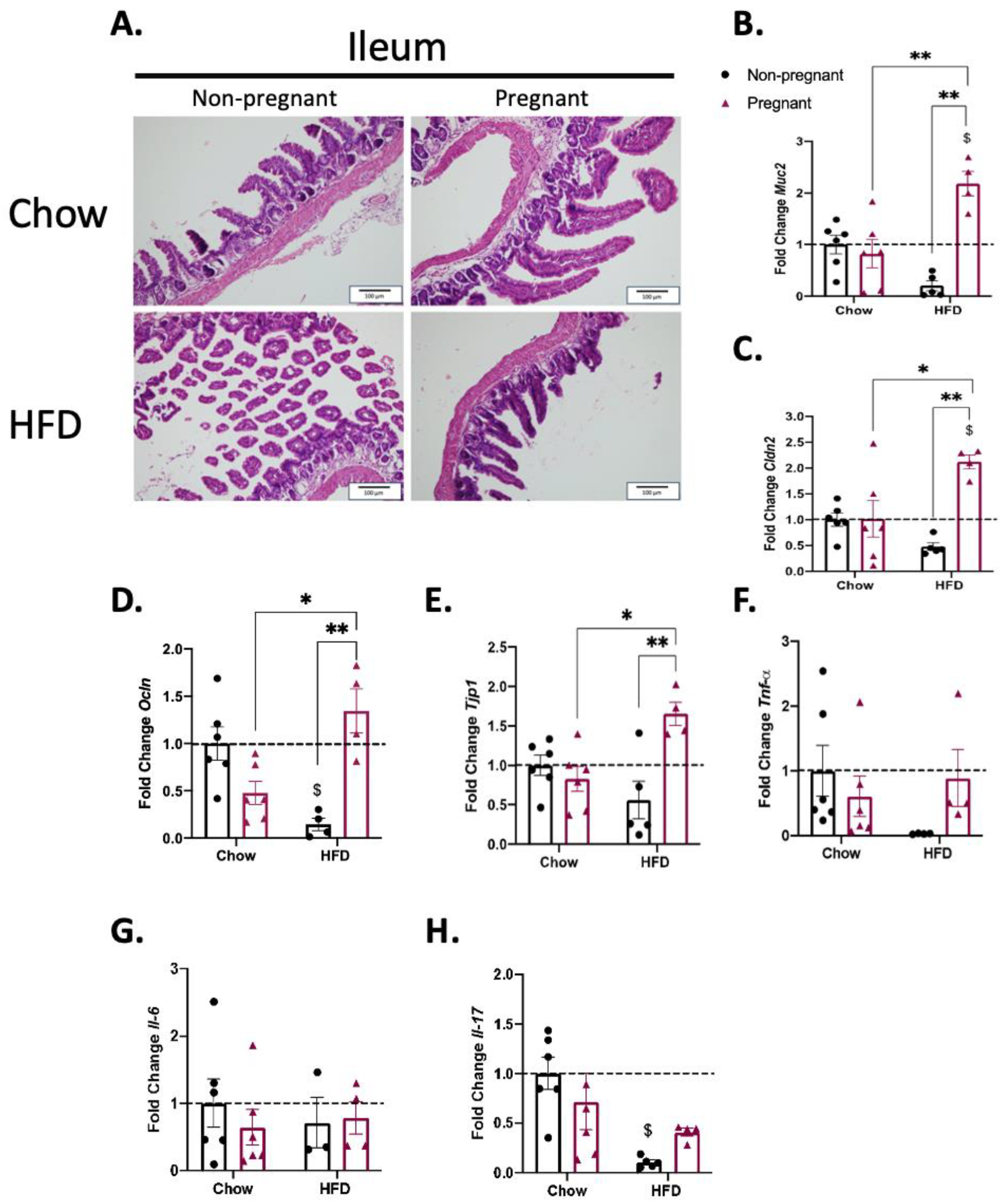
3.4. Pregnancy Promotes the Increased Expression of Gut Permeability-Related Transcripts in the Ileum of Mice on a High-Fat Diet
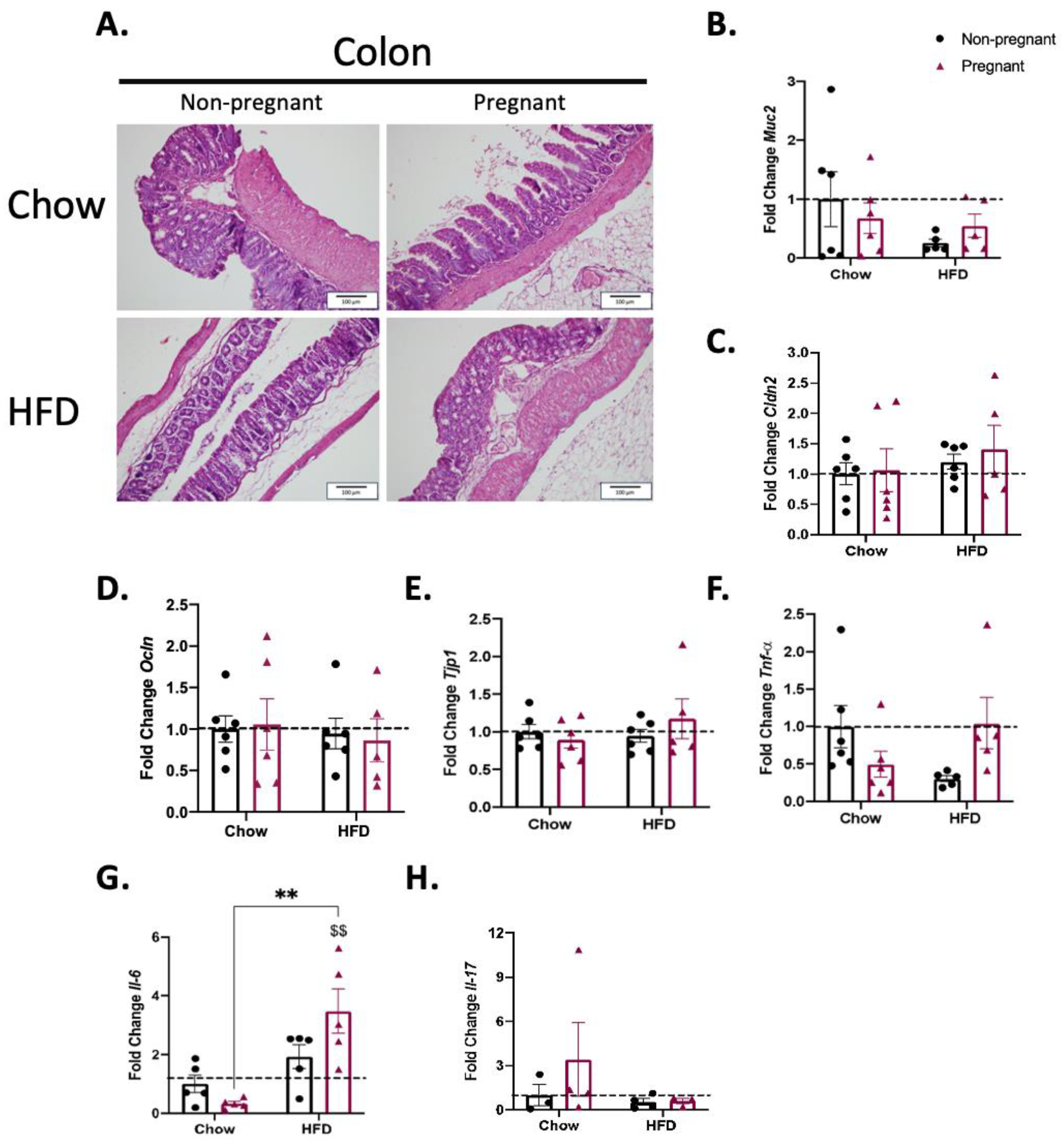
3.5. Consumption of a High-Fat Diet in Pregnant Mice Promotes Minimal Changes in the Colon
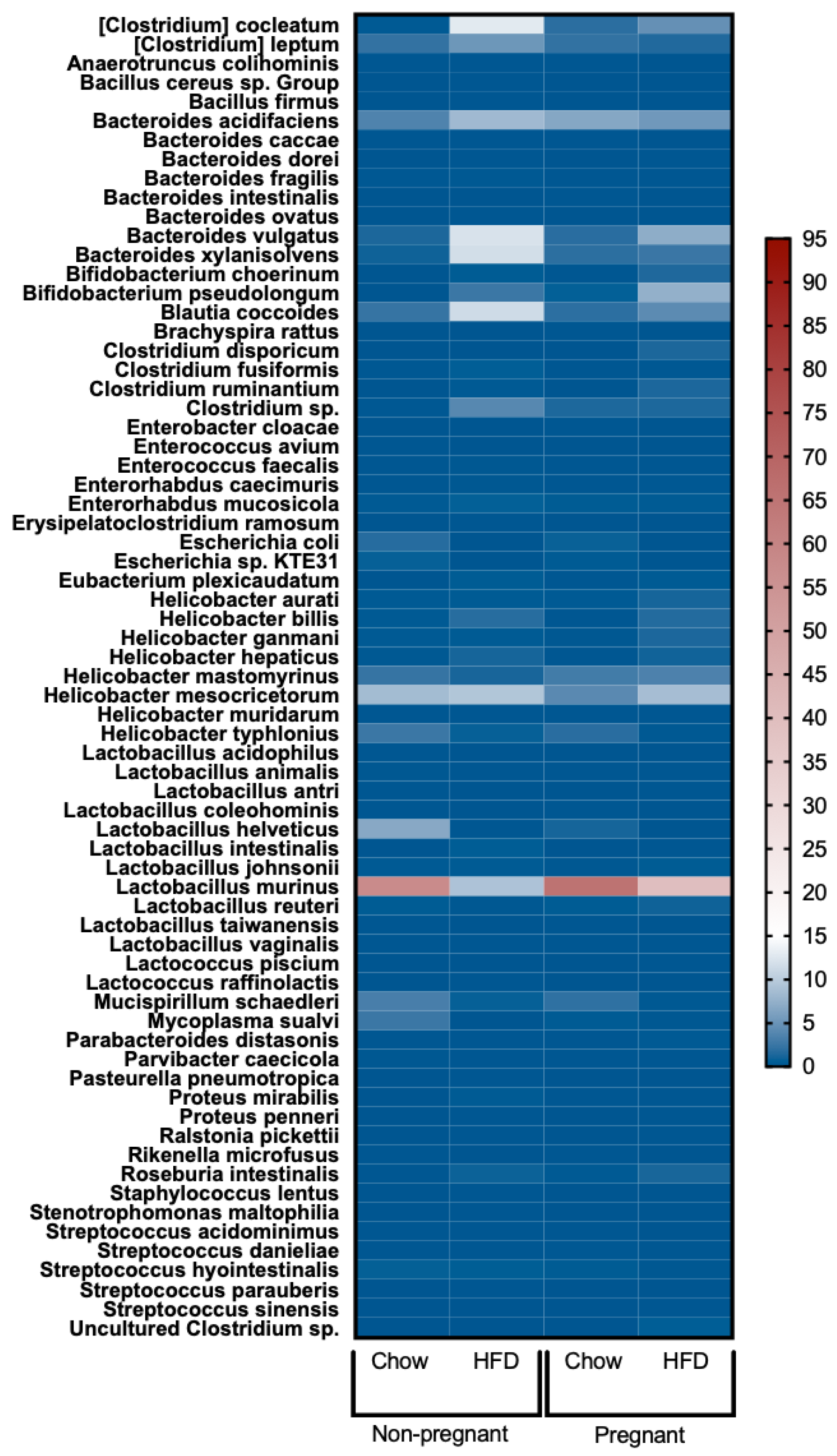
3.6. Increased Lactobacillus Murinus and Reduced Bacteria of the Genus Clostridia in the Gut of Pregnant Mice Fed a High-Fat Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of Maternal Obesity on the Long-Term Health of Offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.L. Maternal Obesity, Large Babies, and Diabetes. BMJ 1949, 1, 702–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The Association between Maternal Body Mass Index and Child Obesity: A Systematic Review and Meta-Analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- EGG Consortium; Warrington, N.M.; Beaumont, R.N.; Horikoshi, M.; Day, F.R.; Helgeland, Ø.; Laurin, C.; Bacelis, J.; Peng, S.; Hao, K.; et al. Maternal and Fetal Genetic Effects on Birth Weight and Their Relevance to Cardio-Metabolic Risk Factors. Nat. Genet. 2019, 51, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Kankowski, L.; Ardissino, M.; McCracken, C.; Lewandowski, A.J.; Leeson, P.; Neubauer, S.; Harvey, N.C.; Petersen, S.E.; Raisi-Estabragh, Z. The Impact of Maternal Obesity on Offspring Cardiovascular Health: A Systematic Literature Review. Front. Endocrinol. 2022, 13, 868441. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Ozanne, S.E. Developmental Programming by Maternal Obesity: Lessons from Animal Models. Diabet. Med. 2021, 38, e14694. [Google Scholar] [CrossRef]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y.; Simmons, R.A. Diet-Induced Obesity Alters the Maternal Metabolome and Early Placenta Transcriptome and Decreases Placenta Vascularity in the Mouse. Biol. Reprod. 2018, 98, 795–809. [Google Scholar] [CrossRef]
- Rolls, B.A.; Gurr, M.I.; Van Duijvenvoorde, P.M.; Rolls, B.J.; Rowe, E.A. Lactation in Lean and Obese Rats: Effect of Cafeteria Feeding and of Dietary Obesity on Milk Composition. Physiol. Behav. 1986, 38, 185–190. [Google Scholar] [CrossRef]
- Dunn, G.A.; Bale, T.L. Maternal High-Fat Diet Promotes Body Length Increases and Insulin Insensitivity in Second-Generation Mice. Endocrinology 2009, 150, 4999–5009. [Google Scholar] [CrossRef]
- Haddad-Tóvolli, R.; Altirriba, J.; Obri, A.; Sánchez, E.E.; Chivite, I.; Milà-Guasch, M.; Ramírez, S.; Gómez-Valadés, A.G.; Pozo, M.; Burguet, J.; et al. Pro-Opiomelanocortin (POMC) Neuron Translatome Signatures Underlying Obesogenic Gestational Malprogramming in Mice. Mol. Metab. 2020, 36, 100963. [Google Scholar] [CrossRef]
- Haddad-Tóvolli, R.; Morari, J.; Barbizan, R.; Bóbbo, V.C.; Carraro, R.S.; Solon, C.; Dragano, N.R.; Torsoni, M.A.; Araujo, E.P.; Velloso, L.A. Maternal Obesity Damages the Median Eminence Blood-Brain Barrier Structure and Function in the Progeny: The Beneficial Impact of Cross-Fostering by Lean Mothers. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E154–E166. [Google Scholar] [CrossRef] [PubMed]
- Paul, H.A.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Diet-Induced Changes in Maternal Gut Microbiota and Metabolomic Profiles Influence Programming of Offspring Obesity Risk in Rats. Sci. Rep. 2016, 6, 20683. [Google Scholar] [CrossRef]
- Rubini, E.; Schenkelaars, N.; Rousian, M.; Sinclair, K.D.; Wekema, L.; Faas, M.M.; Steegers-Theunissen, R.P.M.; Schoenmakers, S. Maternal Obesity during Pregnancy Leads to Derangements in One-Carbon Metabolism and the Gut Microbiota: Implications for Fetal Development and Offspring Wellbeing. Am. J. Obstet. Gynecol. 2022, 227, 392–400. [Google Scholar] [CrossRef]
- Guo, F.; Jen, K.-L.C. High-Fat Feeding during Pregnancy and Lactation Affects Offspring Metabolism in Rats. Physiol. Behav. 1995, 57, 681–686. [Google Scholar] [CrossRef]
- Levin, B.E.; Govek, E. Gestational Obesity Accentuates Obesity in Obesity-Prone Progeny. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 275, R1374–R1379. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Rainone, V.; Schneider, L.; Saulle, I.; Ricci, C.; Biasin, M.; Al-Daghri, N.M.; Giani, E.; Zuccotti, G.V.; Clerici, M.; Trabattoni, D. Upregulation of Inflammasome Activity and Increased Gut Permeability Are Associated with Obesity in Children and Adolescents. Int. J. Obes. 2016, 40, 1026–1033. [Google Scholar] [CrossRef]
- Desaldeleer, C.; Ferret-Bernard, S.; De Quelen, F.; Le Normand, L.; Perrier, C.; Savary, G.; Romé, V.; Michel, C.; Mourot, J.; Le Huërou-Luron, I.; et al. Maternal 18:3n-3 Favors Piglet Intestinal Passage of LPS and Promotes Intestinal Anti-Inflammatory Response to This Bacterial Ligand. J. Nutr. Biochem. 2014, 25, 1090–1098. [Google Scholar] [CrossRef]
- Mokkala, K.; Röytiö, H.; Munukka, E.; Pietilä, S.; Ekblad, U.; Rönnemaa, T.; Eerola, E.; Laiho, A.; Laitinen, K. Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Related to Serum Zonulin Concentration, a Marker for Intestinal Permeability. J. Nutr. 2016, 146, 1694–1700. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and Energy Harvesting Capacity of the Gut Microbiota: Relationship to Diet, Obesity and Time in Mouse Models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.A.; Silva, C.; Ferreira, A.C.; Martins, D.; Leite-Moreira, A.; Miranda, I.M.; Barros, A.S. The Association between Gestational Diabetes and the Microbiome: A Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Jimenez, N.; Fore, R.; Cilleros-Portet, A.; Lepeule, J.; Perron, P.; Kvist, T.; Tian, F.-Y.; Lesseur, C.; Binder, A.M.; Lozano, M.; et al. A Meta-Analysis of Pre-Pregnancy Maternal Body Mass Index and Placental DNA Methylation Identifies 27 CpG Sites with Implications for Mother-Child Health. Commun. Biol. 2022, 5, 1313. [Google Scholar] [CrossRef]
- Yang, S.-T.; Liu, C.-H.; Ma, S.-H.; Chang, W.-H.; Chen, Y.-J.; Lee, W.-L.; Wang, P.-H. Association between Pre-Pregnancy Overweightness/Obesity and Pregnancy Outcomes in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9094. [Google Scholar] [CrossRef] [PubMed]
- Fries, W.; Mazzon, E.; Squarzoni, S.; Martin, A.; Martines, D.; Micali, A.; Sturniolo, G.C.; Citi, S.; Longo, G. Experimental Colitis Increases Small Intestine Permeability in the Rat. Lab. Investig. 1999, 79, 49–57. [Google Scholar]
- Duckworth, C.A.; Watson, A.J. Analysis of Epithelial Cell Shedding and Gaps in the Intestinal Epithelium. Methods Mol. Biol. 2011, 763, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Pärsson, H.; Andersson, R.; Soltesz, V.; Johansson, K.; Bengmark, S. Bacterial Translocation, Intestinal Ultrastructure and Cell Membrane Permeability Early after Major Liver Resection in the Rat. Br. J. Surg. 1994, 81, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.A.; Breznik, J.A.; Kennedy, K.M.; Yeo, E.; Kennelly, B.K.E.; Jazwiec, P.A.; Patterson, V.S.; Bellissimo, C.J.; Anhê, F.F.; Schertzer, J.D.; et al. Intestinal Permeability and Peripheral Immune Cell Composition Are Altered by Pregnancy and Adiposity at Mid- and Late-Gestation in the Mouse. PLoS ONE 2023, 18, e0284972. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, F.; Di, W.; Lv, Y.; Xia, F.; Sheng, Y.; Yu, J.; Ding, G. Impact of a High-fat Diet on Intestinal Stem Cells and Epithelial Barrier Function in Middle-aged Female Mice. Mol. Med. Rep. 2020, 21, 1133–1144. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Hornef, M. Innate Immune Signalling at the Intestinal Epithelium in Homeostasis and Disease. EMBO Rep. 2012, 13, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Randow, F.; MacMicking, J.D.; James, L.C. Cellular Self-Defense: How Cell-Autonomous Immunity Protects against Pathogens. Science 2013, 340, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.A.; de Oliveira, M.C.; Cordeiro, L.M.d.S.; Val, C.H.; Machado, F.S.; Fernandes, S.O.A.; Cardoso, V.N.; Teixeira, M.M.; Silveira, A.L.M.; Ferreira, A.V.M. Effect of High-Refined Carbohydrate Diet on Intestinal Integrity. Nutrition 2023, 113, 112084. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, M.; Sun, L.; Wang, T.; Zhu, Z.; Shu, R.; Wu, F.; Li, Z. Crocin-I Protects Against High-Fat Diet-Induced Obesity via Modulation of Gut Microbiota and Intestinal Inflammation in Mice. Front. Pharmacol. 2022, 13, 894089. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, Y.; Wang, Y.; Yang, Y.; Han, W.; Li, J.; Wang, Y.; Liu, X. Secoisolariciresinol Diglucoside Ameliorates High Fat Diet-Induced Colon Inflammation and Regulates Gut Microbiota in Mice. Food Funct. 2022, 13, 3009–3022. [Google Scholar] [CrossRef]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef]
- Yan, D.; Qiang, Y.; Tian, T.; Lu, D.; Wu, C. The Effect of Endotoxin on the Intestinal Mucus Layer in Non- and Post-Pregnancy Mice. Front. Vet. Sci. 2021, 8, 824170. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic Cells Express Tight Junction Proteins and Penetrate Gut Epithelial Monolayers to Sample Bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Rahner, C.; Mitic, L.L.; Anderson, J.M. Heterogeneity in Expression and Subcellular Localization of Claudins 2, 3, 4, and 5 in the Rat Liver, Pancreas, and Gut. Gastroenterology 2001, 120, 411–422. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Kang, S. Fecal Microbiota Composition, Their Interactions, and Metagenome Function in US Adults with Type 2 Diabetes According to Enterotypes. Int. J. Mol. Sci. 2023, 24, 9533. [Google Scholar] [CrossRef]
- Eslinger, A.J.; Eller, L.K.; Reimer, R.A. Yellow Pea Fiber Improves Glycemia and Reduces Clostridium Leptum in Diet-Induced Obese Rats. Nutr. Res. 2014, 34, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Wiklund, P.; Pekkala, S.; Völgyi, E.; Xu, L.; Cheng, S.; Lyytikäinen, A.; Marjomäki, V.; Alen, M.; Vaahtovuo, J.; et al. Women with and without Metabolic Disorder Differ in Their Gut Microbiota Composition. Obesity 2012, 20, 1082–1087. [Google Scholar] [CrossRef]
- Nadal, I.; Santacruz, A.; Marcos, A.; Warnberg, J.; Garagorri, J.M.; Moreno, L.A.; Martin-Matillas, M.; Campoy, C.; Martí, A.; Moleres, A.; et al. Shifts in Clostridia, Bacteroides and Immunoglobulin-Coating Fecal Bacteria Associated with Weight Loss in Obese Adolescents. Int. J. Obes. 2009, 33, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.R.; Blosser, E.G.; Zindl, C.L.; Silberger, D.J.; Conlan, S.; Laufer, V.A.; DiToro, D.; Deming, C.; Kumar, R.; Morrow, C.D.; et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med. 2019, 25, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]

| Component | Chow (g) | HFD (g) |
|---|---|---|
| Starch | 437 | 118 |
| Protein (casein 85%) | 192 | 240 |
| Dextrinized corn starch | 136 | 70 |
| Sucrose | 100 | 70 |
| Soybean oil | 43 | 43 |
| Lard | 0 | 367 |
| Fiber (cellulose) | 49 | 49 |
| Mineral mix (AIN-93) | 30 | 30 |
| Vitamin mix (AIN-93) | 8 | 8 |
| L-Cystine | 3 | 3 |
| Choline bitartrate | 2 | 2 |
| Total | 1000 | 1000 |
| Applied Biosystems | Assay | Ref Seq | Exon Location |
|---|---|---|---|
| Actb | Mm04394036_g1 | AK075973.1 | 1–2 |
| Gapdh | Mm99999915_g1 | Nm_008084.2 | 2–3 |
| Integrated DNA Tech | Assay | Ref Seq | Exon Location |
| Cldn2 | Mm.PT.58.32319262 | NM_016675(1) | 1–2 |
| Cldn5 | Mm.PT.58.33394738.g | NM_013805(1) | 1–1 |
| Il17a | Mm.PT.58.6531092 | NM_010552(1) | 2–3 |
| Il6 | Mm.PT.58.13354106 | NM_031168(1) | 2–3 |
| Muc2 | Mm.PT.58.30808474.gs | XM_003945707(1) | 24–25b |
| Ocln | Mm.PT.58.30118962 | NM_008756(1) | 6–7 |
| Tjp1 | Mm.PT.58.12952721 | NM_001163574(2) | 25–26 |
| Tnf | Mm.PT.58.12575861 | NM_013693(1) | 2–4 |
| Rplp0 | Mm.PT.58.43894205 | NM_007475(1) | 5–6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biolcatti, C.F.; Bobbo, V.C.; Solon, C.; Morari, J.; Haddad-Tovolli, R.; Araujo, E.P.; Simoes, M.R.; Velloso, L.A. Pregnancy Protects against Abnormal Gut Permeability Promoted via the Consumption of a High-Fat Diet in Mice. Nutrients 2023, 15, 5041. https://doi.org/10.3390/nu15245041
Biolcatti CF, Bobbo VC, Solon C, Morari J, Haddad-Tovolli R, Araujo EP, Simoes MR, Velloso LA. Pregnancy Protects against Abnormal Gut Permeability Promoted via the Consumption of a High-Fat Diet in Mice. Nutrients. 2023; 15(24):5041. https://doi.org/10.3390/nu15245041
Chicago/Turabian StyleBiolcatti, Caio F., Vanessa C. Bobbo, Carina Solon, Joseane Morari, Roberta Haddad-Tovolli, Eliana P. Araujo, Marcela R. Simoes, and Licio A. Velloso. 2023. "Pregnancy Protects against Abnormal Gut Permeability Promoted via the Consumption of a High-Fat Diet in Mice" Nutrients 15, no. 24: 5041. https://doi.org/10.3390/nu15245041
APA StyleBiolcatti, C. F., Bobbo, V. C., Solon, C., Morari, J., Haddad-Tovolli, R., Araujo, E. P., Simoes, M. R., & Velloso, L. A. (2023). Pregnancy Protects against Abnormal Gut Permeability Promoted via the Consumption of a High-Fat Diet in Mice. Nutrients, 15(24), 5041. https://doi.org/10.3390/nu15245041








