L-Fucose Suppresses Lipid Accumulation via the AMPK Pathway in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Adipocyte Differentiation
2.3. Cell Viability Assay
2.4. Oil Red O Staining
2.5. Western Blot Analysis
2.6. Glucose Uptake Assay
2.7. Statistical Analysis
3. Results
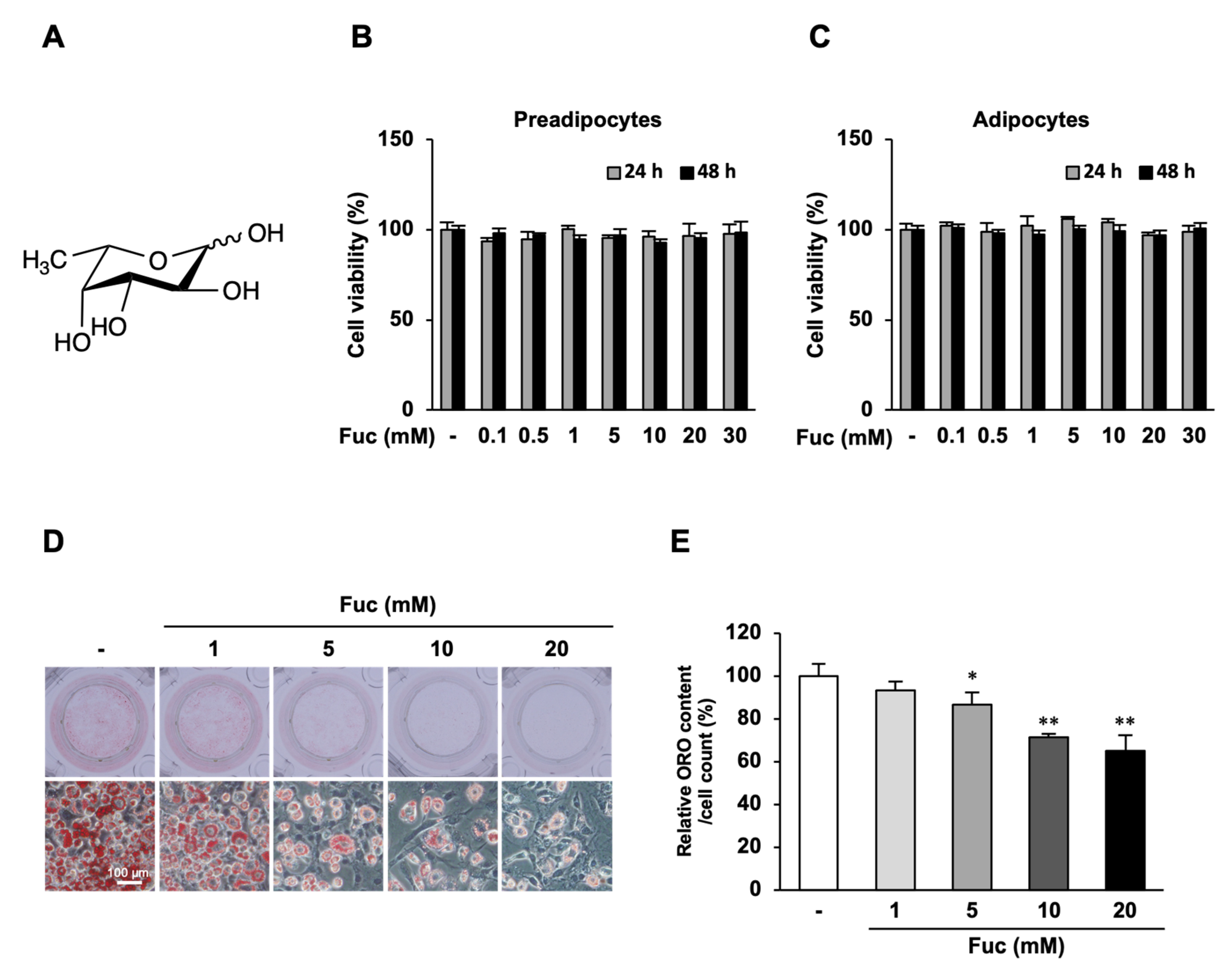
3.1. Effects of Fuc on Cell Viability and Lipid Accumulation
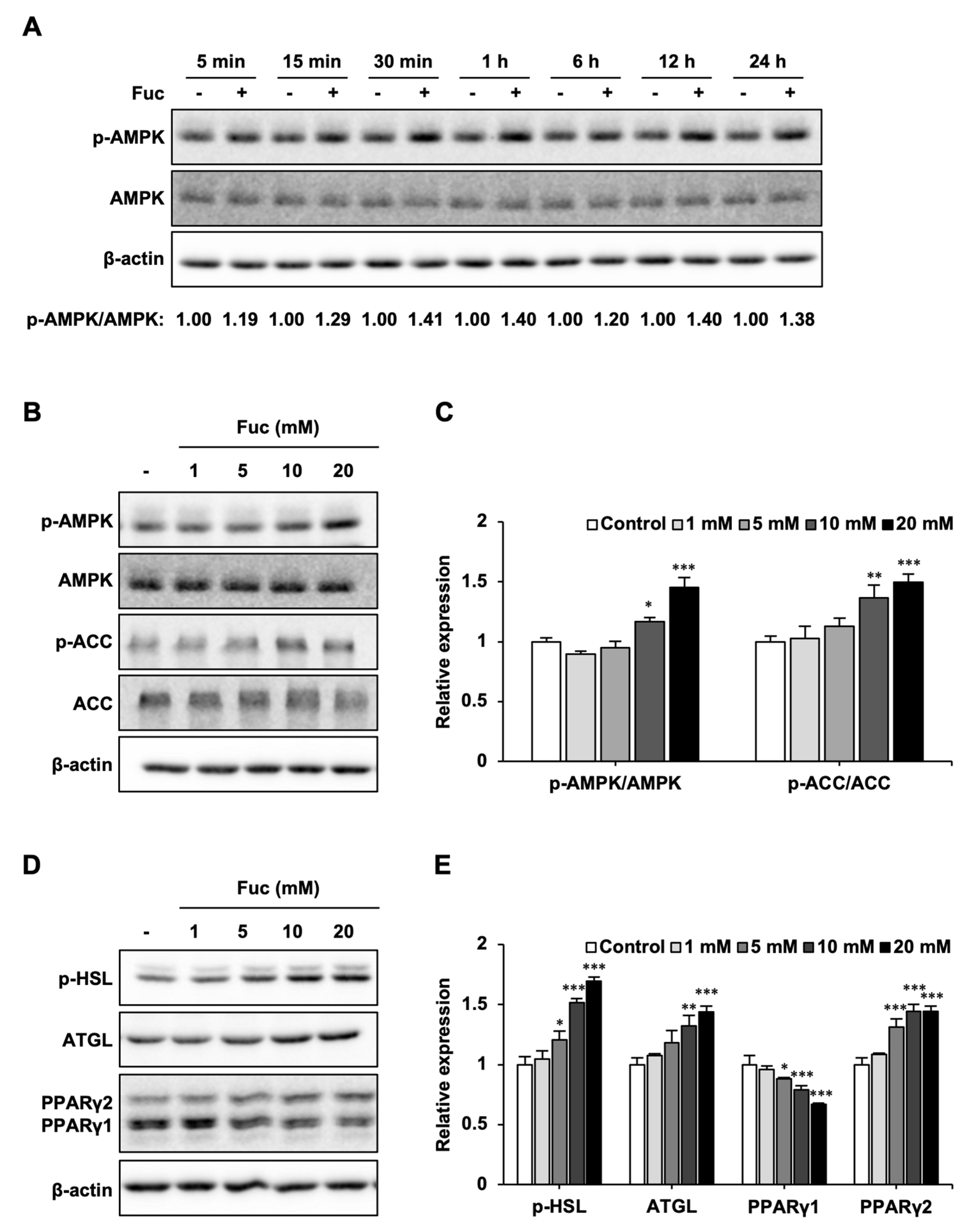
3.2. Effects of Fuc on Lipid Metabolism-Related Proteins
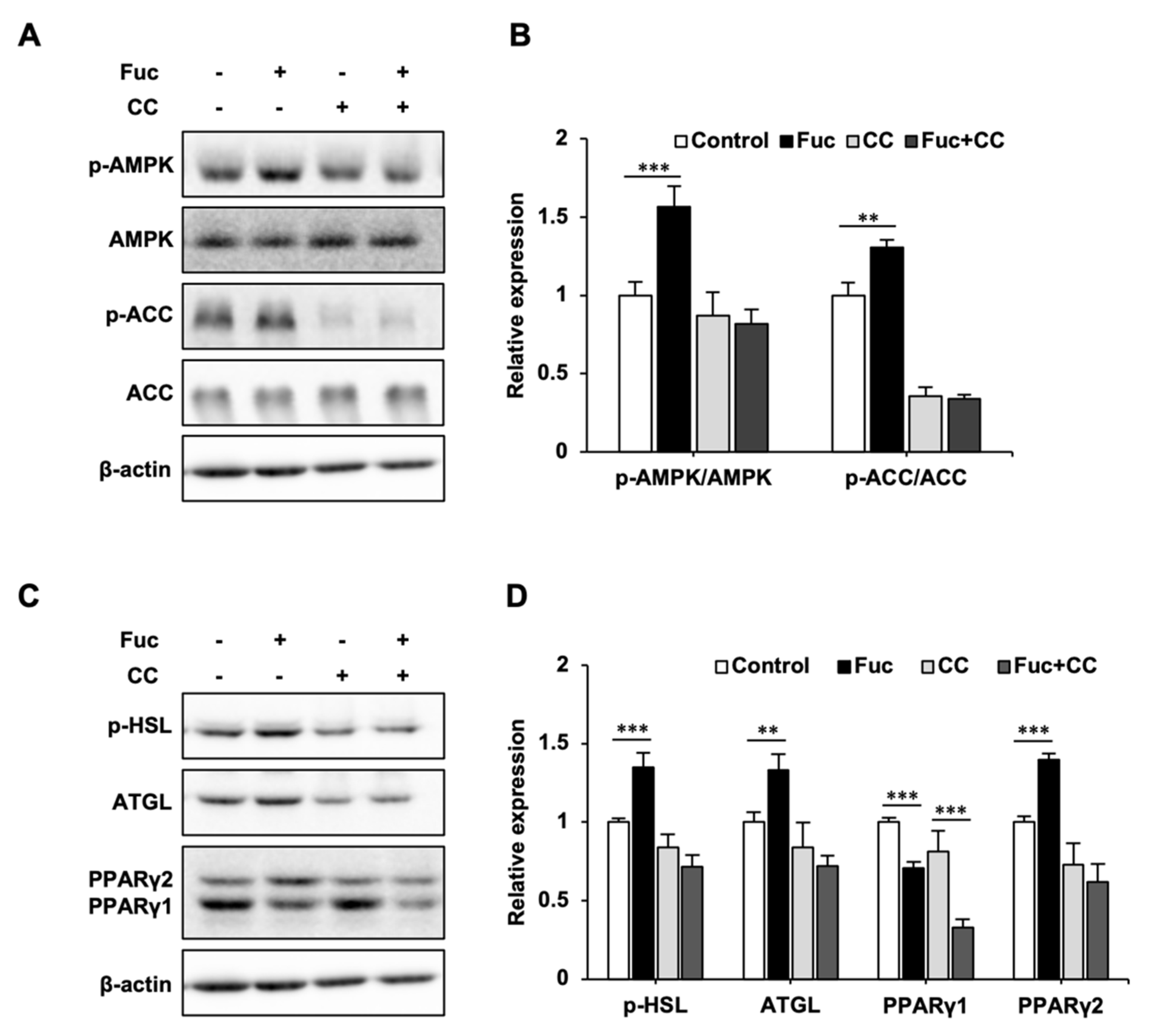
3.3. Effects of Fuc and Compound C on Lipid Metabolism-Related Proteins
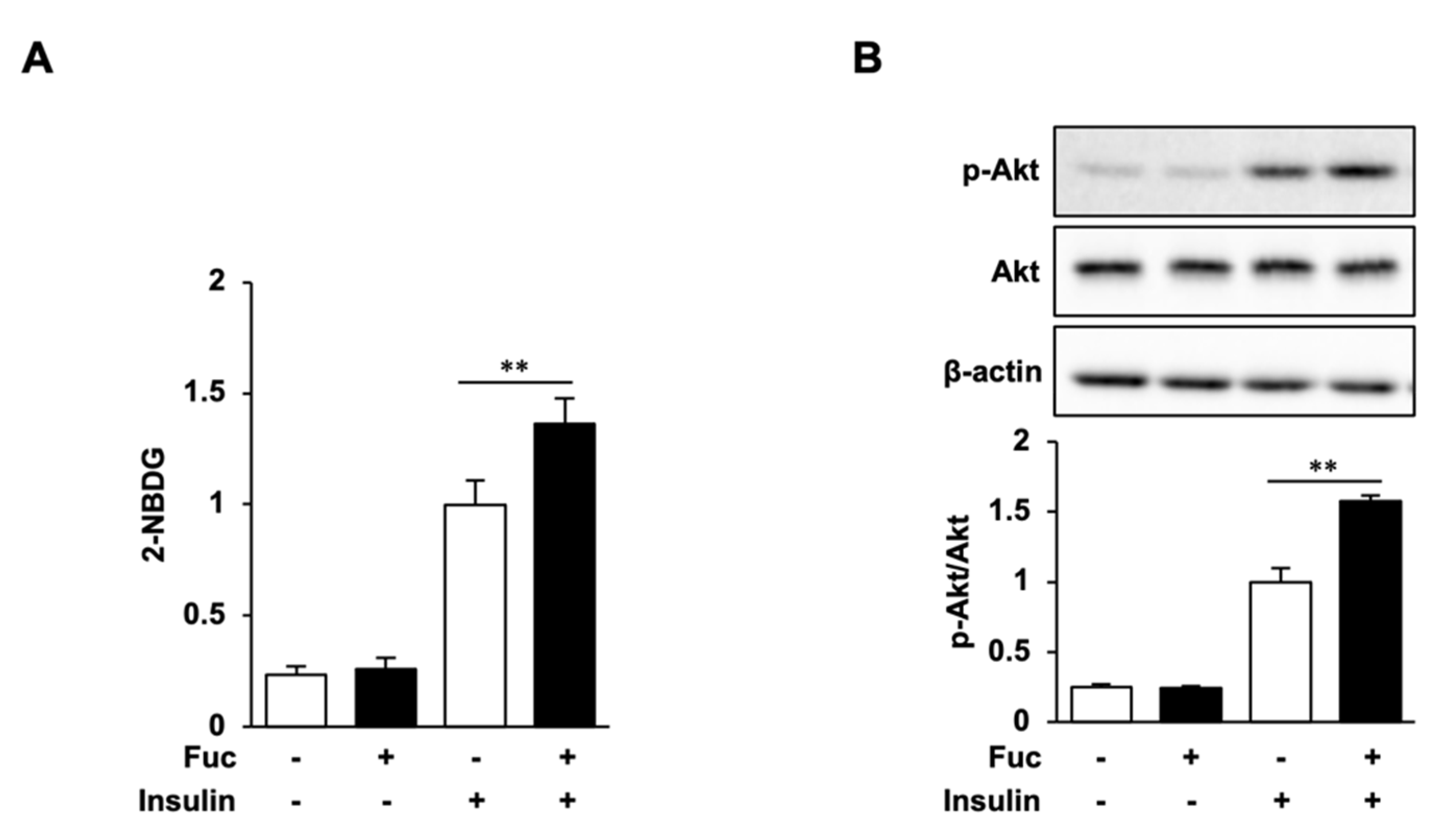
3.4. Glucose Uptake and Akt Phosphorylation in Adipocytes Treated with Fuc and Insulin
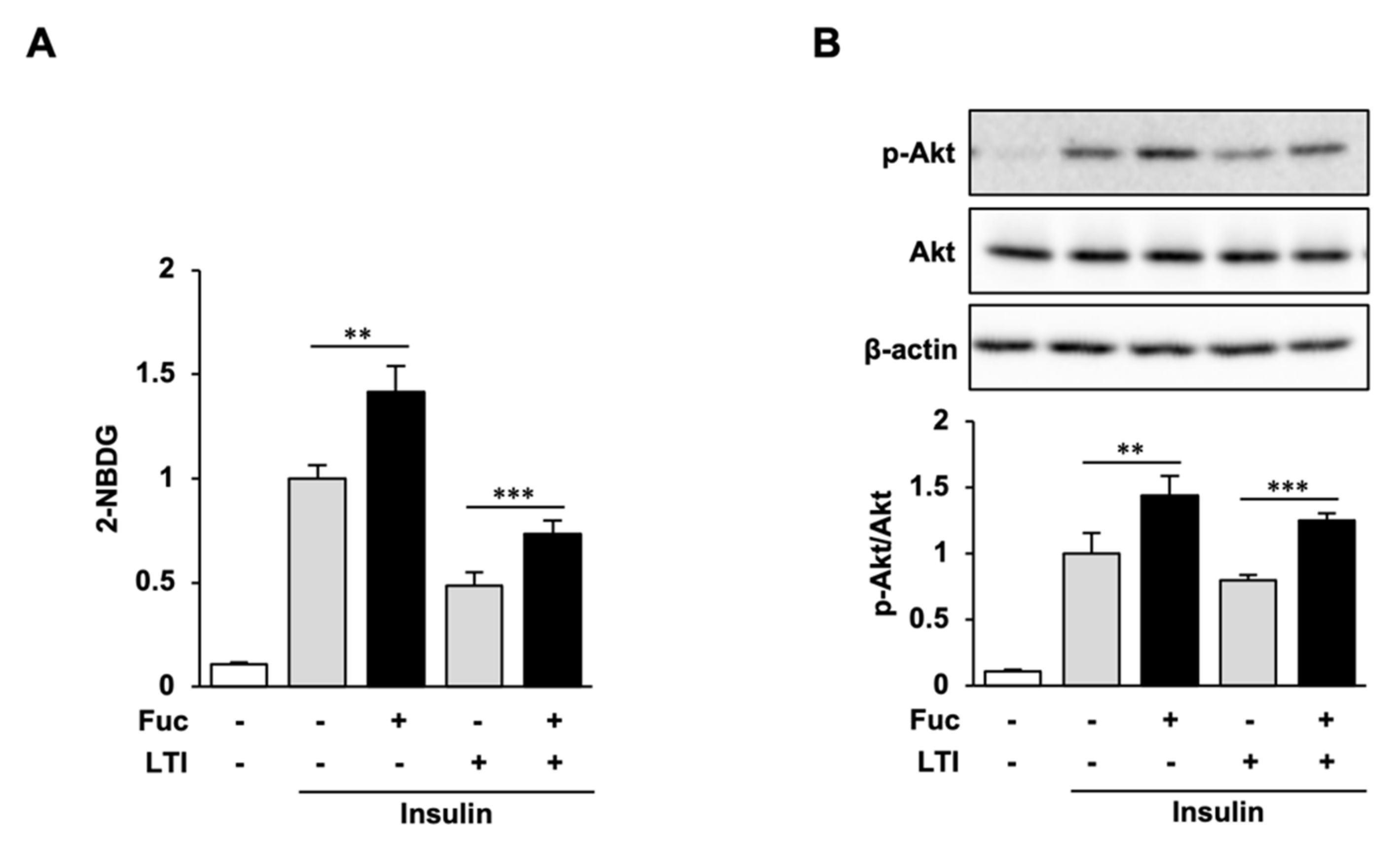
3.5. Glucose Uptake and Akt Phosphorylation in Insulin-Resistant Adipocytes Treated with Fuc and Insulin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Tetens, I.; Bügel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. The environmental foodprint of obesity. Obesity 2020, 28, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Stein, S.C.; Woods, A.; Jones, N.A.; Davison, M.D.; Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 2000, 345, 437–443. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Tang, T.; Abbott, M.; Viscarra, J.A.; Wang, Y.; Sul, H.S. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase to Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol. Cell Biol. 2016, 36, 1961–1976. [Google Scholar] [CrossRef] [Green Version]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL Is Regulated by AMPK and Is Required for a Brown Adipose Phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef]
- Fredrikson, G.; Tornqvist, H.; Belfrage, P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1986, 876, 288–293. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Giri, S.; Rattan, R.; Haq, E.; Khan, M.; Yasmin, R.; Won, J.S.; Key, L.; Singh, A.K.; Singh, I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. 2006, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Lee, W.S.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Curcumin exerts antidifferentiation effect through AMPKα-PPAR-γ in 3T3-L1 adipocytes and antiproliferatory effect through AMPKα-COX-2 in cancer cells. J. Agric. Food Chem. 2009, 57, 305–310. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Wu, K.; Wang, X. Dual Effects of Metformin on Adipogenic Differentiation of 3T3-L1 Preadipocyte in AMPK-Dependent and Independent Manners. Int. J. Mol. Sci. 2018, 19, 1547. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, J.; Elmendorf, J.S.; Ozcan, S. Analysis of insulin-stimulated glucose uptake in differentiated 3T3-L1 adipocytes. Methods Mol. Med. 2003, 83, 97–103. [Google Scholar] [CrossRef]
- Kohn, A.D.; Summers, S.A.; Birnbaum, M.J.; Roth, R.A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 1996, 271, 31372–31378. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, G.; Guo, J.; Su, Z.Q. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.E.; Han, J.S. Fucoidan Stimulates Glucose Uptake via the PI3K/AMPK Pathway and Increases Insulin Sensitivity in 3T3-L1 Adipocytes. J. Life Sci. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan Prevents High-Fat Diet-Induced Obesity in Animals by Suppression of Fat Accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Chang, U.-J.; Lee, J.-S. Inhibitory Effects of Fucoidan in 3T3-L1 Adipocyte Differentiation. Mar. Biotechnol. 2009, 11, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Nakao, T.; Satone, H.; Ohara, K.; Kominami, Y.; Ito, M.; Aizawa, T.; Ueno, T.; Ushio, H. The Effects of Brown Algae-Derived Monosaccharide L-Fucose on Lipid Metabolism in C57BL/6J Obese Mice. Nutrients 2020, 12, 3798. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Niu, M.; Tang, W.; Hu, J.; Wei, G.; He, Z.; Chen, Y.; Jiang, Y.; Chen, P. l-Fucose ameliorates high-fat diet-induced obesity and hepatic steatosis in mice. J. Transl. Med. 2018, 16, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobashi, K.; Asayama, K.; Shirahata, A. Differential effects of cyclic AMP on induction of nitric oxide synthase in 3T3-L1 cells and brown adipocytes. Free Radic. Biol. Med. 2003, 35, 94–101. [Google Scholar] [CrossRef]
- Lindholm, C.R.; Ertel, R.L.; Bauwens, J.D.; Schmuck, E.G.; Mulligan, J.D.; Saupe, K.W. A high-fat diet decreases AMPK activity in multiple tissues in the absence of hyperglycemia or systemic inflammation in rats. J. Physiol. Biochem. 2013, 69, 165–175. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Hu, L.; Xie, C.; Fu, J.; Jiang, Y.; Han, N.; Jia, L.; Liu, Y. D-Mannose Inhibits Adipogenic Differentiation of Adipose Tissue-Derived Stem Cells via the miR669b/MAPK Pathway. Stem Cells Int. 2020, 11, 8866048. [Google Scholar] [CrossRef]
- Gonzalez, P.S.; O’Prey, J.; Cardaci, S.; Barthet, V.J.A.; Sakamaki, J.i.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Liou, C.-J.; Lai, X.-Y.; Chen, Y.-L.; Wang, C.-L.; Wei, C.-H.; Huang, W.-C. Ginkgolide C Suppresses Adipogenesis in 3T3-L1 Adipocytes via the AMPK Signaling Pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 298635. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, D.; Zhuang, X.; Wang, Z.; Ni, Y.; Chen, S.; Sun, F. Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis. 2016, 15, 214. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, F.; Zhang, X.; Xue, C.; Namwanje, M.; Fan, L.; Reilly, M.P.; Hu, F.; Qiang, L. Distinct functions of PPARγ isoforms in regulating adipocyte plasticity. Biochem. Biophys. Res. Commun. 2016, 481, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fu, M.; Cui, T.; Xiong, C.; Xu, K.; Zhong, W.; Xiao, Y.; Floyd, D.; Liang, J.; Li, E.; et al. Selective disruption of PPAR2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2004, 101, 10703–10708. [Google Scholar] [CrossRef] [Green Version]
- Medina-Gomez, G.; Gray, S.L.; Yetukuri, L.; Shimomura, K.; Virtue, S.; Campbell, M.; Curtis, R.K.; Jimenez-Linan, M.; Blount, M.; Yeo, G.S.H.; et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Shintani, H.; Shintani, T.; Ashida, H.; Sato, M. Calorie Restriction Mimetics: Upstream-Type Compounds for Modulating Glucose Metabolism. Nutrients 2018, 10, 1821. [Google Scholar] [CrossRef] [Green Version]
- Wiese, T.J.; Dunlap, J.A.; Yorek, M.A. L-fucose is accumulated via a specific transport system in eukaryotic cells. J. Biol. Chem. 1994, 269, 22705–22711. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.T.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2012, 24, 715–723. [Google Scholar] [CrossRef]
- Tang, J.C.; Taniguchi, H.; Chu, H.; Zhou, Q.; Nagata, S. Isolation and characterization of alginate-degrading bacteria for disposal of seaweed wastes. Lett. Appl. Microbiol. 2009, 48, 38–43. [Google Scholar] [CrossRef]
- Choi, S.S.H.; Lynch, B.S.; Baldwin, N.; Dakoulas, E.W.; Roy, S.; Moore, C.; Thorsrud, B.A.; Röhrig, C.H. Safety evaluation of the human-identical milk monosaccharide, l-fucose. Regul. Toxicol. Pharmacol. 2015, 72, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Kubomura, D.; Akahori, Y.; Ushio, H. Effect of L-Fucose-Containing Diet on Visceral Fat and Bowel Movement, and Its Safety: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Pharmacometrics 2021, 100, 63–70. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakao, T.; Otaki, S.; Kominami, Y.; Watanabe, S.; Ito, M.; Aizawa, T.; Akahori, Y.; Ushio, H. L-Fucose Suppresses Lipid Accumulation via the AMPK Pathway in 3T3-L1 Adipocytes. Nutrients 2023, 15, 503. https://doi.org/10.3390/nu15030503
Nakao T, Otaki S, Kominami Y, Watanabe S, Ito M, Aizawa T, Akahori Y, Ushio H. L-Fucose Suppresses Lipid Accumulation via the AMPK Pathway in 3T3-L1 Adipocytes. Nutrients. 2023; 15(3):503. https://doi.org/10.3390/nu15030503
Chicago/Turabian StyleNakao, Tomohiko, Shiro Otaki, Yuri Kominami, Soichi Watanabe, Miho Ito, Teruki Aizawa, Yusuke Akahori, and Hideki Ushio. 2023. "L-Fucose Suppresses Lipid Accumulation via the AMPK Pathway in 3T3-L1 Adipocytes" Nutrients 15, no. 3: 503. https://doi.org/10.3390/nu15030503



