Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
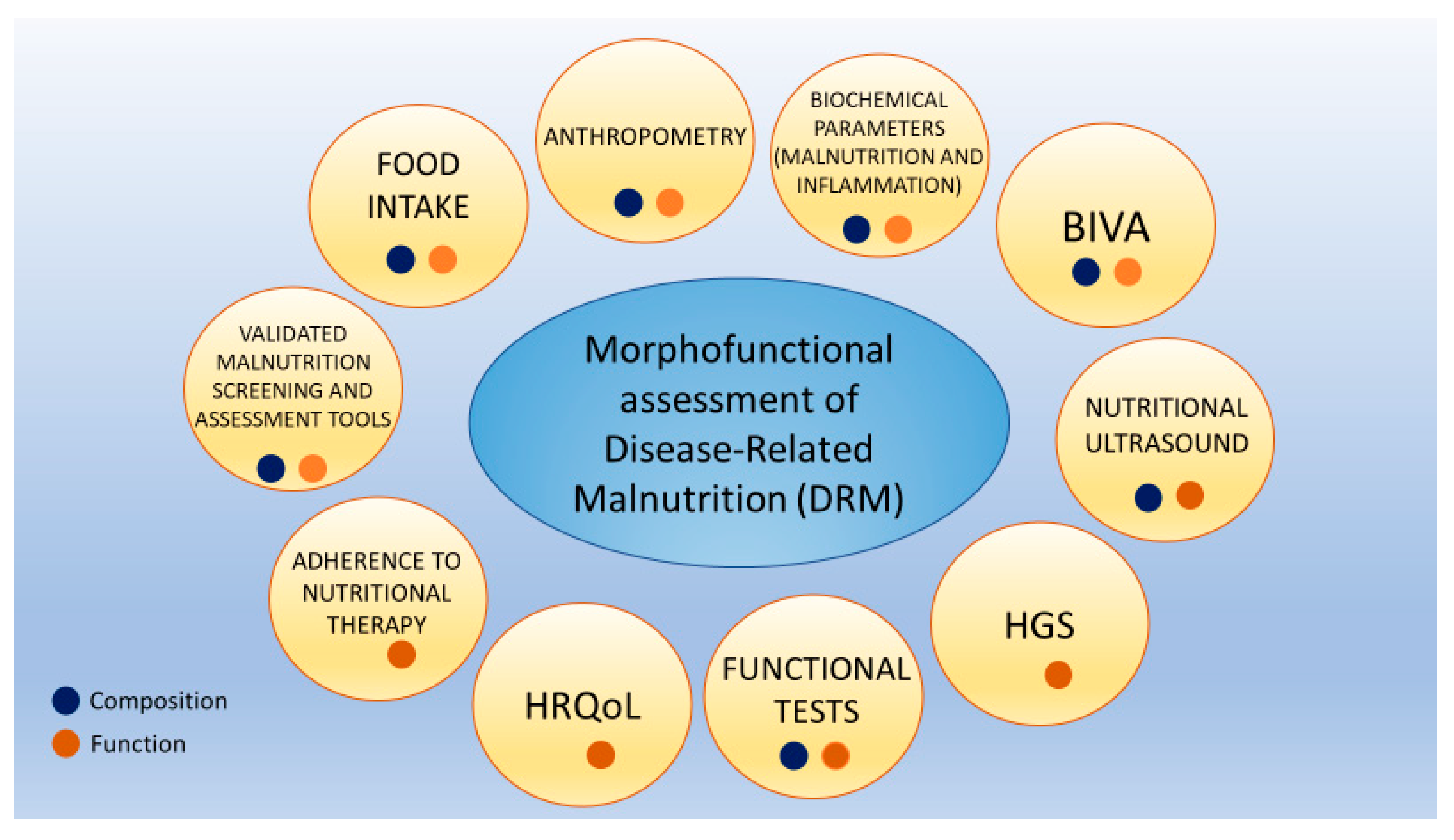
2.2. Morphofunctional Assessment of Disease-Related Malnutrition
2.2.1. Clinical, Anthropometric, and Nutritional Data
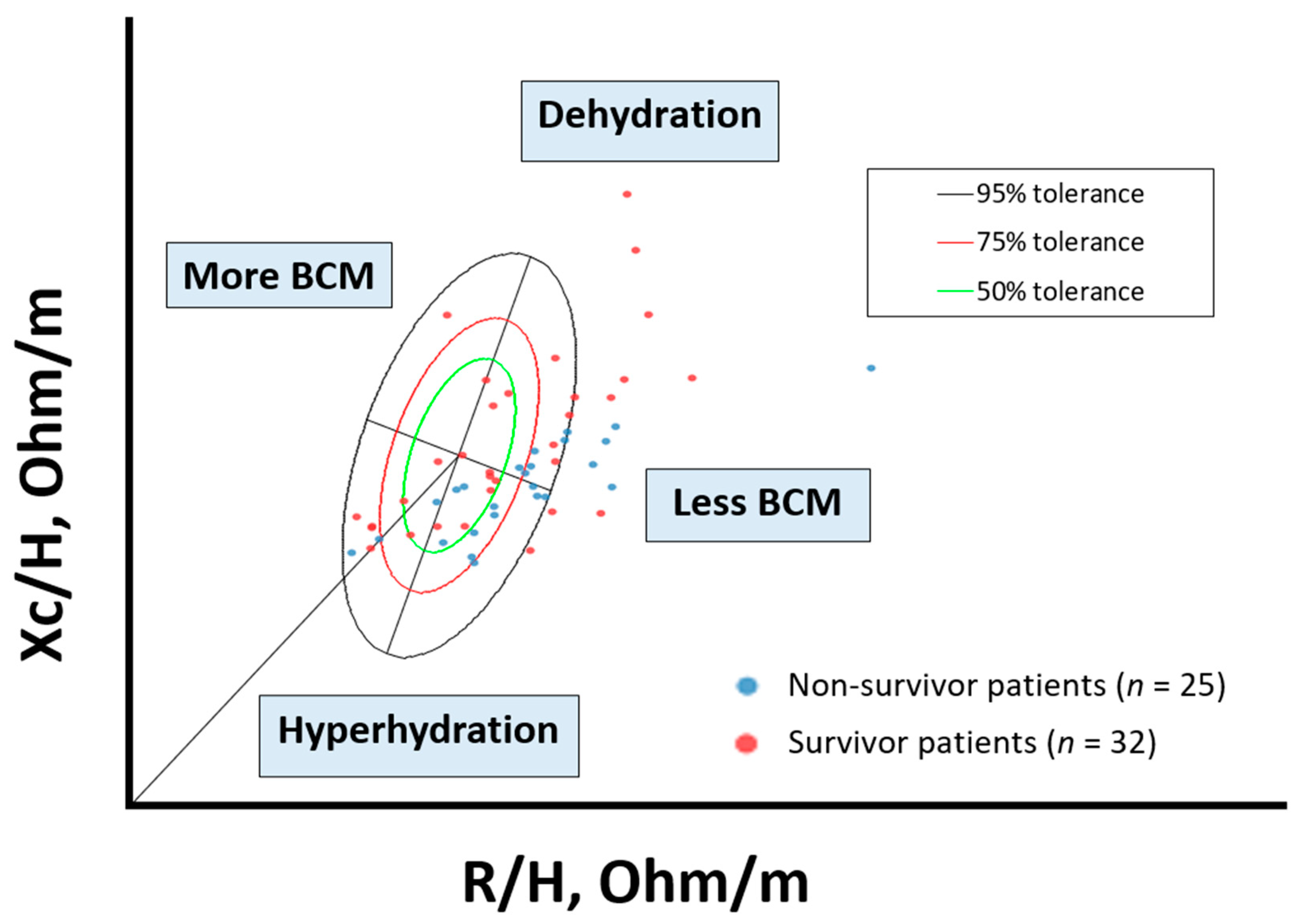
2.2.2. Bioelectrical Impedance Analysis Assessment
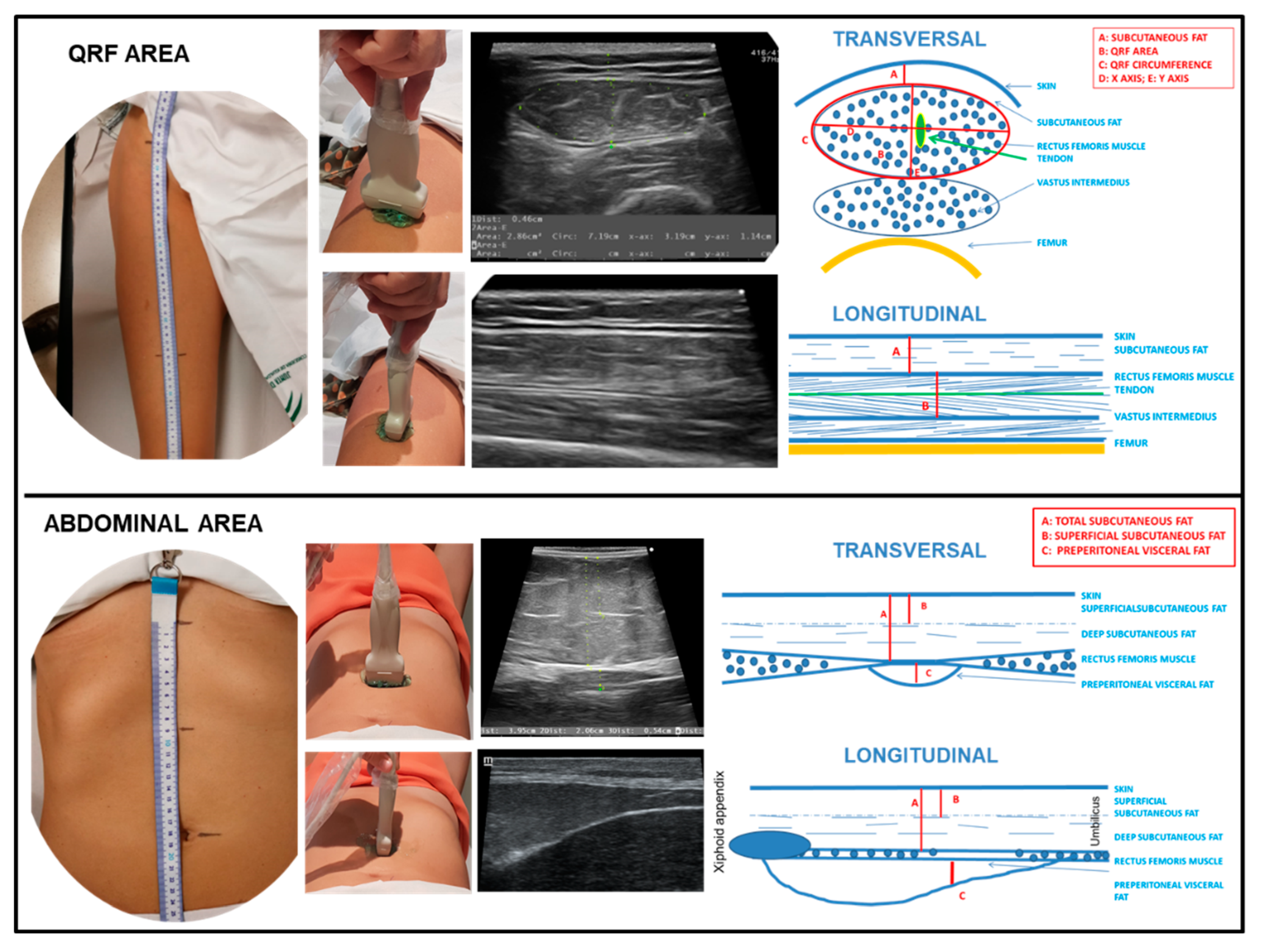
2.2.3. Nutritional Ultrasound®
2.2.4. Hand Grip Strength
2.2.5. Functional Tests: Timed Up and Go Test
2.3. Biochemical Parameters (Malnutrition and Inflammation)
2.4. HRQoL and Adherence
2.5. Follow-Up and Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Morphofunctional Assessment Measurements between Survivor and Non-Survivor Patients at 12 Months
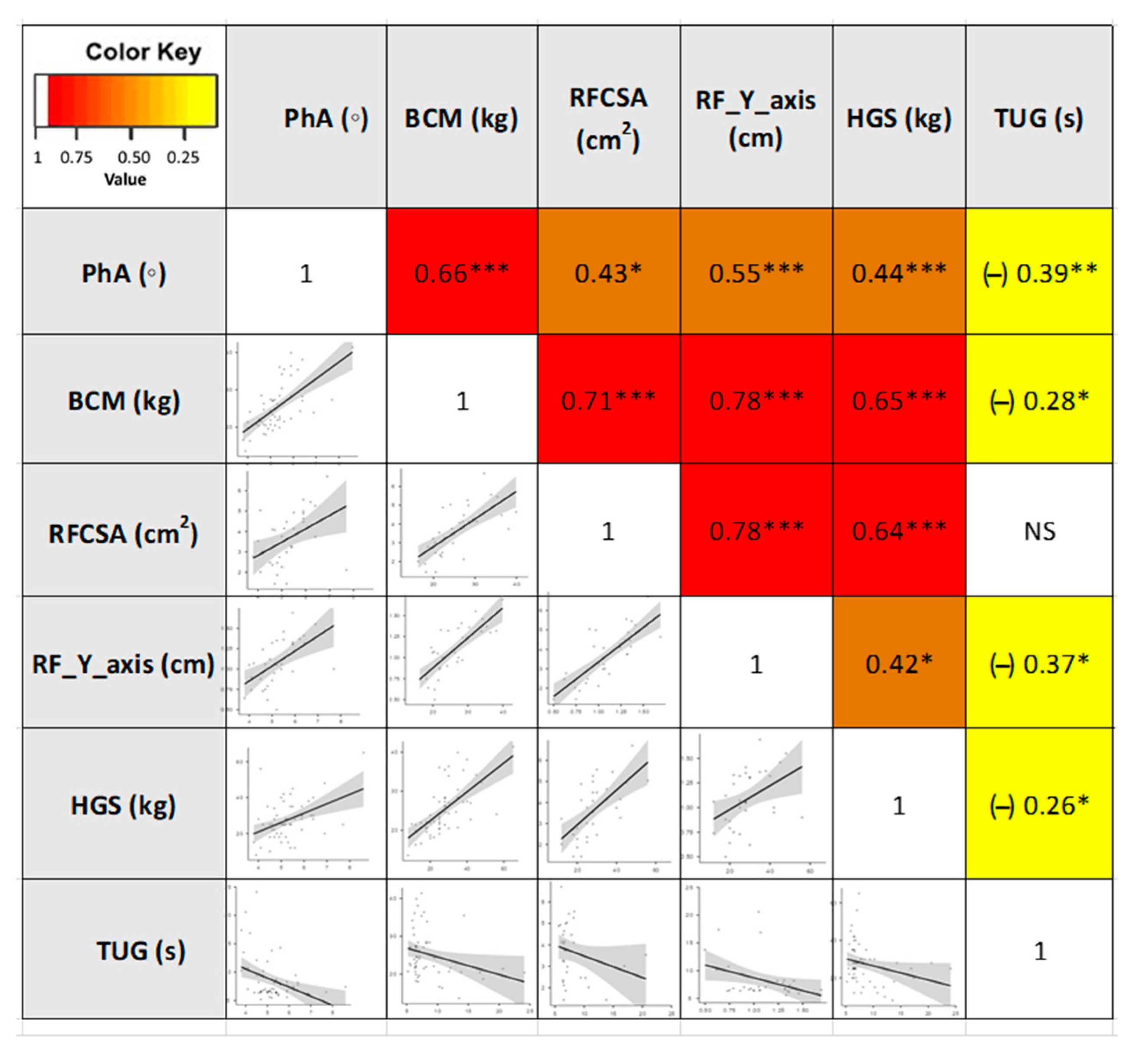
3.3. Correlations between the Different Parameters of Morphofunctional Assessment of DRM
3.4. Optimal Morphofunctional Parameters of DRM Cut-Off Value and 12-Months Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. Edinb. Scotl. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. Edinb. Scotl. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, X.; Shi, H.; Zhang, K.; Zhang, Q.; Tang, M.; Li, W.; Zhou, F.; Liu, M.; Cong, M.; et al. Association of the fat-free mass index with mortality in patients with cancer: A multicenter observational study. Nutr. Burbank Los. Angel Cty. C. Calif. 2022, 94, 111508. [Google Scholar] [CrossRef]
- García Almeida, J.M.; García García, C.; Vegas Aguilar, I.M.; Bellido Castañeda, V.; Bellido Guerrero, D. Morphofunctional assessment of patient´s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar]
- Prado, C.M.; Sawyer, M.B.; Ghosh, S.; Lieffers, J.R.; Esfandiari, N.; Antoun, S.; Baracos, V.E. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential? Am. J. Clin. Nutr. 2013, 98, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. Edinb. Scotl. 2007, 26, 389–399. [Google Scholar] [CrossRef]
- Surov, A.; Pech, M.; Gessner, D.; Mikusko, M.; Fischer, T.; Alter, M.; Wienke, A. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis. Clin. Nutr. Edinb. Scotl. 2021, 40, 5298–5310. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Faria, S.; Alvim Moravia, R.; Howell, D.; Eluf Neto, J. Adherence to nutritional interventions in head and neck cancer patients: A systematic scoping review of the literature. J. Hum. Nutr. Diet. 2021, 34, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, E1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Lammersfeld, C.A.; Burrows, J.L.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Hoffman, S.; Lis, C.G. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in advanced colorectal cancer. Am. J. Clin. Nutr. 2004, 80, 1634–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arribas, L.; Hurtós, L.; Sendrós, M.J.; Peiró, I.; Salleras, N.; Fort, E.; Sánchez-Migallón, J.M. NUTRISCORE: A new nutritional screening tool for oncological outpatients. Nutr. Burbank. Los Angel Cty Calif. 2017, 33, 297–303. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enteral. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y. Overview Of The Mna®–Its History And Challenges. J. Nutr. Health Aging 2006, 10, 456–465. [Google Scholar]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. Edinb. Scotl. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Alaustré, A.; Rull, M.; Camps, I.; Ginesta, C.; Melus, M.; Salvá, J. Nuevas normas y consejos en la valoración de los parámetros en nuestra población: Índice adiposo-muscular, índice ponderales y tablas de percentiles de los datos antropométricos útiles en una valoración nutricional. Med. Clin. 1988, 23, 223–236. [Google Scholar]
- Gonzalez, M.C.; Mehrnezhad, A.; Razaviarab, N.; Barbosa-Silva, T.G.; Heymsfield, S.B. Calf circumference: Cutoff values from the NHANES 1999-2006. Am. J. Clin. Nutr. 2021, 113, 1679–1687. [Google Scholar] [CrossRef]
- Fernández García-Salazar, R.; García-Almeida, J.M. Valoración del estado nutricional y concepto de desnutrición. In Olveira G. Manual de Nutrición Clínica Y Dietética; Madrid: Díazz de Santos, Spain, 2016; pp. 179–214. [Google Scholar]
- Villalobos Gámez, J.; García-Almeida, J.; del Río Mata, J.; Rioja Vázquez, R.; Barranco Pérez, J.; Bernal Losada, O. Proceso INFORNUT®. In Minivademecum Nutricional; García-Almeida, J.M., Villalobos Gámez, J., Eds.; Villalobos Gámez and García Almeida: Málaga, Spain, 2012; pp. 27–50. [Google Scholar]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunbar, C.C.; Melahrinides, E.; Michielli, D.W.; Kalinski, M.I. Effects of small errors in electrode placement on body composition assessment by bioelectrical impedance. Res. Q. Exerc. Sport 1994, 65, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.B.; LoVallo, S.J.; Andreacci, J.L.; Goss, F.L. The effect of acute fluid consumption on measures of impedance and percent body fat using leg-to-leg bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2006, 60, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney. Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- De Palo, T.; Messina, G.; Edefonti, A.; Perfumo, F.; Pisanello, L.; Peruzzi, L.; Di Iorio, B.; Mignozzi, M.; Vienna, A.; Conti, G.; et al. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutr. Burbank. Los Angel Cty Calif. 2000, 16, 417–424. [Google Scholar] [CrossRef]
- Paiva, S.I.; Borges, L.R.; Halpern-Silveira, D.; Assunção, M.C.F.; Barros, A.J.D.; Gonzalez, M.C. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support. Care Cancer 2010, 19, 187–192. [Google Scholar] [CrossRef]
- Schutz, Y.; Kyle, U.U.G.; Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Moore, F.D.; Boyden, C.M. Body Cell Mass And Limits Of Hydration Of The Fat-Free Body: Their Relation To Estimated Skeletal Weight. Ann. NY Acad. Sci. USA 1963, 110, 62–71. [Google Scholar] [CrossRef]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Ruiz-Santana, S. Assessment of Muscle Wasting in Long-Stay ICU Patients Using a New Ultrasound Protocol. Nutrients 2018, 10, 1849. [Google Scholar] [CrossRef] [Green Version]
- Perkisas, S.; Bastijns, S.; Sanchez-Rodriguez, D.; Piotrowicz, K.; De Cock, A.-M. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update: Reply to the letter to the editor: SARCUS working group on behalf of the Sarcopenia Special Interest Group of the European Geriatric Medicine Society. Eur. Geriatr. Med. 2021, 12, 427–428. [Google Scholar] [CrossRef]
- Hamagawa, K.; Matsumura, Y.; Kubo, T.; Hayato, K.; Okawa, M.; Tanioka, K.; Yamasaki, N.; Kitaoka, H.; Yabe, T.; Nishinaga, M.; et al. Abdominal visceral fat thickness measured by ultrasonography predicts the presence and severity of coronary artery disease. Ultrasound. Med. Biol. 2010, 36, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Pérez Miguelsanz, M.J.; Cabrera Parra, W.; Varela Moreiras, G.; Garaulet, M. Regional distribution of the body fat: Use of image techniques as tools for nutritional diagnosis. Nutr. Hosp. 2010, 25, 207–223. [Google Scholar] [PubMed]
- Sanz-Paris, A.; González-Fernandez, M.; Hueso-Del Río, L.E.; Ferrer-Lahuerta, E.; Monge-Vazquez, A.; Losfablos-Callau, F.; Sanclemente-Hernández, T.; Sanz-Arque, A.; Arbones-Mainar, J.M. Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture. Nutrients 2021, 13, 2401. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Torralvo, F.J.; Porras, N.; Abuín Fernández, J.; García Torres, F.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Rojo Martínez, G.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podsiadlo, D.; Richardson, S. The timed ‘Up & Go’: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar]
- Beck, F.K.; Rosenthal, T.C. Prealbumin: A marker for nutritional evaluation. Am. Fam. Physician 2002, 65, 1575–1578. [Google Scholar]
- Pinilla, J.C.; Hayes, P.; Laverty, W.; Arnold, C.; Laxdal, V. The C-reactive protein to prealbumin ratio correlates with the severity of multiple organ dysfunction. Surgery 1998, 124, 799–805, discussion 805–806. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, A.-S.; Choi, H.-I.; Jung, J.-H.; Park, J.Y.; Ko, H.-J. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PloS ONE 2020, 15, e0236445. [Google Scholar] [CrossRef]
- Li, L.; Dai, L.; Wang, X.; Wang, Y.; Zhou, L.; Chen, M.; Wang, H. Predictive value of the C-reactive protein-to-prealbumin ratio in medical ICU patients. Biomark Med. 2017, 11, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30, a quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Apezetxea, A.; Carrillo, L.; Casanueva, F.; Cuerda, C.; Cuesta, F.; Irles, J.; Virgili, M.; Virgili, M.; Lizán, L. The NutriQoL® questionnaire for assessing health-related quality of life (HRQoL) in patients with home enteral nutrition (HEN): Validation and first results. Nutr. Hosp. 2016, 33, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Wanden-Berghe, C.; Cheikh Moussa, K.; Sanz-Valero, J. Adherencia a la Nutrición Enteral Domiciliaria. Hosp. Domic. 2018, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, K.-P.; Zhang, X.; Tang, M.; Song, C.-H.; Cong, M.-H.; Guo, Z.-Q.; Ding, J.-S.; Braga, M.; Cederholm, T.; et al. Scored-GLIM as an effective tool to assess nutrition status and predict survival in patients with cancer. Clin. Nutr. Edinb. Scotl. 2021, 40, 4225–4233. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef]
- Axelsson, L.; Silander, E.; Bosaeus, I.; Hammerlid, E. Bioelectrical phase angle at diagnosis as a prognostic factor for survival in advanced head and neck cancer. Eur Arch. Oto-Rhino-Laryngol. 2018, 275, 2379–2386. [Google Scholar] [CrossRef] [Green Version]
- Pérez Camargo, D.A.; Allende Pérez, S.R.; Verastegui Avilés, E.; Rivera Franco, M.M.; Meneses García, A.; Herrera Gómez, Á.; Urbalejo Ceniceros, V.I. Assessment and Impact of Phase Angle and Sarcopenia in Palliative Cancer Patients. Nutr. Cancer 2017, 69, 1227–1233. [Google Scholar] [CrossRef]
- Hui, D.; Moore, J.; Park, M.; Liu, D.; Bruera, E. Phase Angle and the Diagnosis of Impending Death in Patients with Advanced Cancer: Preliminary Findings. Oncol. 2019, 24, e365–e373. [Google Scholar] [CrossRef] [Green Version]
- Arab, A.; Karimi, E.; Vingrys, K.; Shirani, F. Is phase angle a valuable prognostic tool in cancer patients’ survival? A systematic review and meta-analysis of available literature. Clin. Nutr. Edinb. Scotl. 2021, 40, 3182–3190. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin. Nutr. Edinb. Scotl. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Mueller, T.C.; Reik, L.; Prokopchuk, O.; Friess, H.; Martignoni, M.E. Measurement of body mass by bioelectrical impedance analysis and computed tomography in cancer patients with malnutrition—A cross-sectional observational study. Medicine 2020, 99, e23642. [Google Scholar] [CrossRef] [PubMed]
- Katsura, N.; Yamashita, M.; Ishihara, T. Extracellular water to total body water ratio may mediate the association between phase angle and mortality in patients with cancer cachexia: A single-center, retrospective study. Clin. Nutr. ESPEN 2021, 46, 193–199. [Google Scholar] [CrossRef]
- Galli, A.; Colombo, M.; Carrara, G.; Lira Luce, F.; Paesano, P.L.; Giordano, L.; Bondi, S.; Tulli, M.; Mirabile, A.; De Cobelli, F.; et al. Low skeletal muscle mass as predictor of postoperative complications and decreased overall survival in locally advanced head and neck squamous cell carcinoma: The role of ultrasound of rectus femoris muscle. Eur Arch. Oto-Rhino-Laryngol 2020, 277, 3489–3502. [Google Scholar] [CrossRef] [PubMed]
- Bril, S.I.; Pezier, T.F.; Tijink, B.M.; Janssen, L.M.; Braunius, W.W.; de Bree, R. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck 2019, 41, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Formenti, P.; Coppola, S.; Umbrello, M.; Froio, S.; Cacioppola, A.; De Giorgis, V.; Galanti, V.; Lusardi, A.C.; Ferrari, E.; Noè, D.; et al. Time course of the Bioelectrical Impedance Vector Analysis and muscular ultrasound in critically ill patients. J. Crit. Care 2021, 68, 89–95. [Google Scholar] [CrossRef]
- Souza, N.C.; Avesani, C.M.; Prado, C.M.; Martucci, R.B.; Rodrigues, V.D.; de Pinho, N.B.; Heymsfield, S.B.; Gonzalez, M.C. Phase angle as a marker for muscle abnormalities and function in patients with colorectal cancer. Clin. Nutr. 2021, 40, 4799–4806. [Google Scholar] [CrossRef]
- da Silva Passos, L.B.; Macedo, T.A.A.; De-Souza, D.A. Nutritional state assessed by ultrasonography, but not by bioelectric impedance, predicts 28-day mortality in critically ill patients. Prospective cohort study. Clin. Nutr. 2021, 40, 5742–5750. [Google Scholar] [CrossRef]
| Variables | Baseline |
|---|---|
| Primary site tumor Lung Hepatobiliary and Pancreatic Upper gastrointestinal tract Lower gastrointestinal tract Other: Urologic Sarcoma Breast cancer Gynecologic cancer Hematologic cancer Oral cancer | Median (IQR) 62 (54–70) |
| Treatment Only chemotherapy Only radiotherapy Only surgery Biological therapy Concomitant chemoradiotherapy Other combination therapies | n (%) 35 (61.4) 22 (38.6) |
| Tumor stage I II III IV | |
| ECOG 0 1 2 | n (%) 13 (22.8) 10 (17.5) 7 (12.3) 7 (12.3) 20 (35.1) 5 (8.8) 5 (8.8) 4 (7) 3 (5.3) 2 (3.5) 1 (1.7) |
| Primary site tumor Lung Hepatobiliary and Pancreatic Upper gastrointestinal tract Lower gastrointestinal tract Other: Urologic Sarcoma Breast cancer Gynecologic cancer Hematologic cancer Oral cancer | n (%) 21 (36.8) 1 (1.8) 3 (5.3) 5 (8.8) 12 (21.1) 15 (26.2) |
| Treatment Only chemotherapy Only radiotherapy Only surgery Biological therapy Concomitant chemoradiotherapy Other combination therapies | n (%) 2 (3.5) 11 (19.3) 14 (24.6) 30 (52.6) |
| Tumor stage I II III IV | n (%) 25 (43.9) 24 (42.1) 8 (14) |
| Nutritional assessment | |
| Nutriscore Without risk At risk | n (%) 16 (28.1) 41 (71.9) |
| Subjective Global Assessment (SGA) Normally nourished (A) Moderate malnutrition (B) Severe malnutrition (C) | n (%) 11 (19.3) 7 (12.3) 39 (68.4) |
| Mini Nutritional Assessment (MNA) Normal nutritional status At risk of malnutrition Malnourished | n (%) 23 (40.4) 29 (50.9) 5 (8.8) |
| GLIM criteria Phenotypic criteria Weight loss (kg; >5% within past 6 months) Low BMI (kg/m2; <20 if <70 years or <22 if >70 years) Reduced muscle mass: By BIA Low FFMI (kg/m2; <17 males/15 females) Low ASMI (kg/m2; <7 males/<5.7 females) Low ALM (kg; <21.4 males/<14.1 females) By anthropometry CC (cm; <34 males/<33 females) AMC (cm; <p5) Etiologic criteria Reduced food intake (≤50% for energetic requirements >1 week) Disease burden/inflammation Diagnosis of malnutrition (1 phenotypic + 1 etiologic criteria) | n (%) 47 (82.5) 20 (35.1) 10 (17.5) 0 28 (49.1) 49 (86.0) 10 (17.5) 12 (21.1) 57 (100) 56 (94.2) |
| Cancer Patients | Cancer Patient Survivors at 1 Year | Cancer Patient Non-Survivors at 1 Year | 1p | ||
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | (* p < 0.05; ** p < 0.01; *** p < 0.001) | ||
| n = 57 | n = 32 | n = 25 | |||
| Anthropometric parameters | |||||
| Age | 62 (54–70) | 62 (52.7–72) | 62 (57–70) | 0.901 | |
| Weight (kg) | 60.3 (54.1–73.1) | 60.6 (54.5–74.5) | 60.3 (50.6–72.2) | 0.469 | |
| Height (cm) | 169 (160–175) | 169 (160–175) | 168 (160–173) | 0.449 | |
| BMI (kg/m2) | 23 (19.9–25.5) | 22.6 (20–25.7) | 23 (20–25.4) | 0.537 | |
| Weight loss (%) | 12.5 (6.5–17.9) | 11.9 (4.9–14.7) | 14.9 (7.4–22) | 0.041 * | |
| CC (cm) | 31 (30–34) | 31.5 (30–35) | 30 (28–32) | 0.232 | |
| AMC (cm) | 22.2 (20.2–23.2) | 22.2 (20.7–23.9) | 20.7 (20–23.2) | 0.442 | |
| Food intake assessment | |||||
| 0–25% | 6 (10.5%) | 1 (3.1%) | 5 (20%) | 0.027 * | |
| 25–50% | 6 (10.5%) | 4 (12.5%) | 2 (8%) | ||
| 50–75% | 14 (24.6%) | 5 (15.6%) | 9 (36%) | ||
| 75–100% | 31 (54.4%) | 22 (68.8%) | 9 (36%) | ||
| Bioelectrical Impedance Analysis (BIA) | |||||
| PhA (◦) | 5.2 (4.7–5.6) | 5.4 (5.07–6.2) | 4.7 (4.5–5.2) | <0.001 *** | |
| SPhA | 0.1 (−0.8–0.9) | 0,3 (−0.2–1.3) | −0,5 (−1.5–0.3) | <0.001 *** | |
| Rz/H (Ω/m) | 319.4 (290.1–356.8) | 310.6 (279–358.8) | 330 (296–355) | 0.803 | |
| Xc/H (Ω/m) | 28.4 (25–32.8) | 29.7 (25.1–36) | 27.5 (25–29.4) | 0.066 | |
| BCM (kg) | 23.9 (20.7–28.2) | 26.25 (22.05–29.35) | 21.6 (20.5–24.7) | 0.040 * | |
| BCM/H (kg/m) | 14.1 (12.9–16.8) | 15.3 (13.7–17.5) | 13,3 (12.5–14.8) | 0.015 * | |
| FFMI (kg/m2) | 17.6 (16.3–19.1) | 17.9 (16.4–19.2) | 17.5 (15.9–18.9) | 0.590 | |
| FMI (kg/m2) | 4.5 (3–6.1) | 4.6 (2.9–6.1) | 4.4 (3–6.2) | 0.847 | |
| ASMI (kg/m2) | 8.6 (7.7–9.6) | 8.5 (7.3–9.7) | 8.7 (7.8–9.4) | 0.886 | |
| ALM (kg) | 18.3 (15.7–21.8) | 18.65 (15.92–22.07) | 18 (15.2–20.3) | 0.347 | |
| ECW/TBW | 0.5 (0.47–0.53) | 0.48 (0.44–0.50) | 0.52 (0.5–0.54) | <0.001 *** | |
| TBW/FFM (%) | 73.6 (73.2–73.8) | 73.4 (73.07–73.7) | 73.7 (73.5–73.9) | 0.033 * | |
| Nutritional ultrasound®: rectus femoris muscle | |||||
| RFCSA (cm2) | 3.7 (2.5–4.6) | 4,27 (3.06–4.96) | 2.98 (2.30–4.12) | 0.030 * | |
| RF- Circumference (cm) | 8.8 (7.9–10) | 9.69 (8.08–10.18) | 8.61 (7.59–9.47) | 0.186 | |
| RF-X-axis (cm) | 3.7 (3.5–4.3) | 3.98 (3.42–4.34) | 3,66 (3.48–4.1) | 0.369 | |
| RF-Y-axis (cm) | 1.1 ( 0.9–1.3) | 1,30 (1.06–1.34) | 0.9 (0.74–1.05) | 0.007 ** | |
| RF-Adipose tissue (cm) | 0.5 (0.3–0.7) | 0.52 (0.35–0.74) | 0.5 (0.32–0.67) | 0.553 | |
| Nutritional ultrasound®: abdominal adipose tissue | |||||
| Superficial subcutaneous (cm) | 0.63 (0.38–0.82) | 0.68 (0.51–0.98) | 0.59 (0.29–0.66) | 0.349 | |
| Total subcutaneous (cm) | 1.31 (0.84–1.72) | 1.33 (0.84–1.72) | 1.17 (0.59–1.66) | 0.724 | |
| Preperitoneal visceral (cm) | 0.55 (0.38–0.72) | 0.55 (0.36–0.61) | 0.54 (0.38–0.73) | 1.000 | |
| Hand Grip Strength | |||||
| Hand Grip Strength (kg) | 25 (20–34) | 26.5 (21.5–30) | 25 (18–35) | 0.746 | |
| Functional tests | |||||
| Timed Up and Go Test (s) | 6.83 (6.5–8.7) | 6.82 (6.5–7.8) | 7.1 (6.4–11.9) | 0.192 | |
| Biochemical parameters | |||||
| Prealbumin (mg/dL) | 20 (14.9–25.9) | 20.5 (15.92–28.35) | 16.9 (10.2–22.7) | 0.018 * | |
| CRP/Prealbumin | 0.04 (0.01–0.1) | 0.019 (0.012–0.053) | 0.10 (0.03–0.17) | 0.009 ** | |
| HRQoL | |||||
| EORTC QLQ C30 (global) (%) | 58.3 (41.7–83.3) | 58.33 (41.66–83.33) | 66.66 (50–75) | 0.009 ** | |
| NutriQoL® (total score) (%) | 88.2 (79.4–97) | 91.18 (74.5–97.06) | 84.8 (79.41–93.38) | 0.619 | |
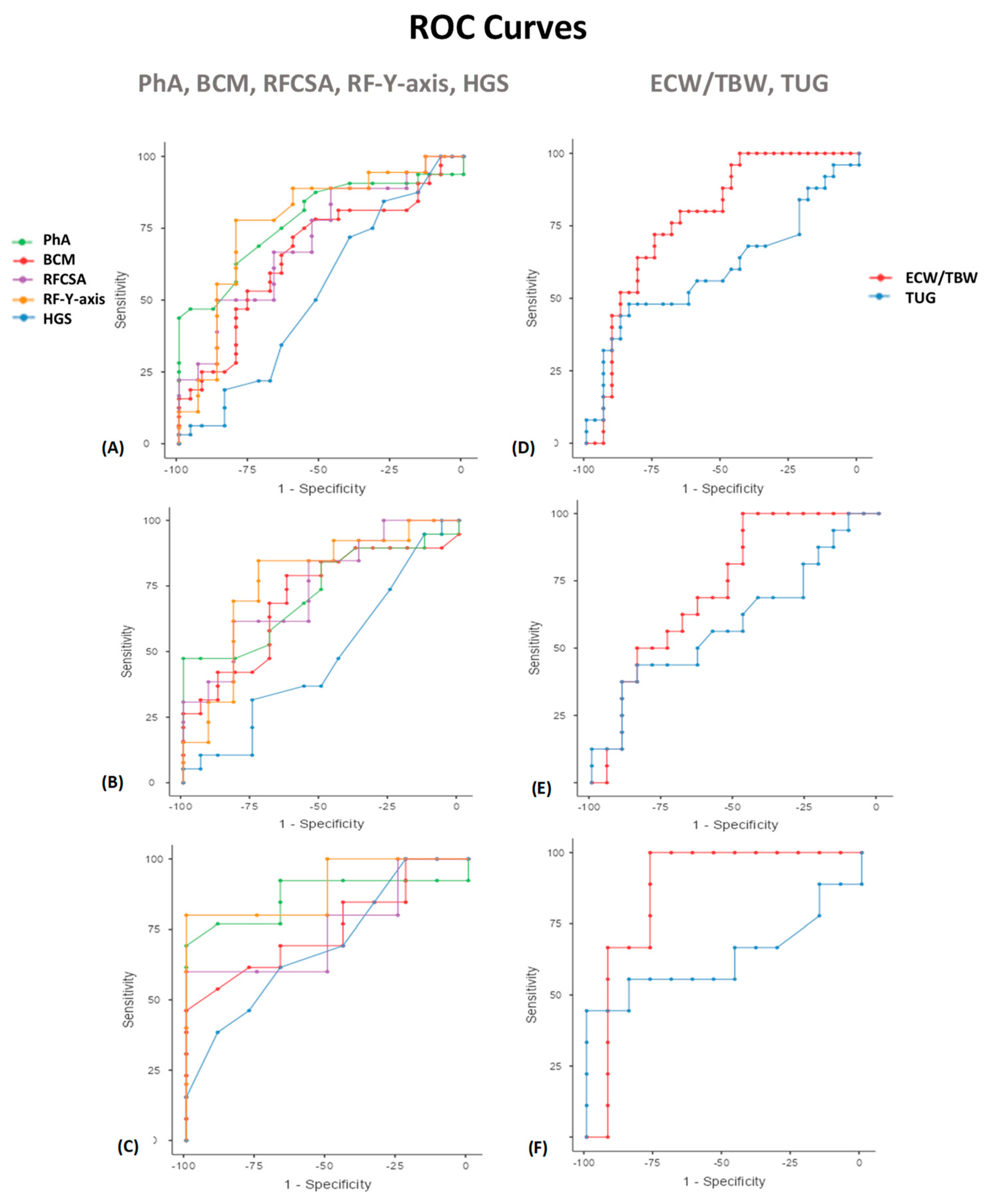
| Variables | Cut Off Point | AUC | Sensitivity | Specificity | PPV (%) | NPV (%) | p | |
|---|---|---|---|---|---|---|---|---|
| PhA (◦) | PhA (◦) | 5.6 | 0.803 | 55.56 | 96.67 | 93.75 | 70.73 | 0.009 ** |
| PhA Men | 5.9 | 0.724 | 47.37 | 100 | 100 | 61.54 | 0.046 * | |
| PhA Women | 5.3 | 0.868 | 69.23 | 100 | 100 | 69.23 | 0.05 * | |
| BCM (kg) | BCM (kg) | 22.3 | 0.661 | 71.88 | 60 | 69.7 | 62.5 | NS |
| BCM Men | 26.2 | 0.702 | 78.95 | 62.5 | 71.43 | 71.43 | 0.05 * | |
| BCM Women | 22.3 | 0.752 | 46.15 | 100 | 100 | 56.25 | 0.05 * | |
| ECW/TBW | ECW/TBW | 0.5 | 0.780 | 72 | 75 | 69.23 | 77.42 | 0.041 * |
| ECW/TBW Men | 0.47 | 0.730 | 100 | 47.37 | 61.54 | 100 | 0.048 * | |
| ECW/TBW Women | 0.5 | 0.872 | 100 | 76.92 | 75 | 100 | 0.045 * | |
| RFCSA (cm2) | RFCSA (cm2) | 4.47 | 0.722 | 60 | 88.89 | 81.82 | 72.73 | 0.043 * |
| RFCSA Men | 4.47 | 0.741 | 61.54 | 81.82 | 80 | 64.29 | 0.046 * | |
| RFCSA Women | 2.73 | 0.750 | 60 | 100 | 100 | 66.67 | 0.05 * | |
| RF-Y-axis (cm) | RF-Y-axis (cm) | 1.3 | 0.735 | 60 | 83.33 | 75 | 71.43 | 0.007 * |
| RF-Y-axis Men | 1.06 | 0.769 | 84.62 | 72.73 | 78.57 | 80 | 0.026 * | |
| RF-Y-axis Women | 1 | 0.800 | 80 | 100 | 100 | 80 | 0.05 * | |
| HGS (kg) | HGS (kg) | 20 | 0.581 | 88.89 | 30 | 53.33 | 75 | NS |
| HGS Men | 25 | 0.477 | 94.74 | 12.5 | 56.25 | 66.67 | NS | |
| HGS Women | 20 | 0.700 | 61.54 | 66.67 | 72.73 | 54.55 | 0.05 * | |
| TUG (s) | TUG (s) | 8.2 | 0.602 | 48 | 84.38 | 70.59 | 67.5 | NS |
| TUG Men | 8.2 | 0.599 | 43.75 | 84.21 | 70 | 64 | NS | |
| TUG Women | 10.76 | 0.632 | 44.44 | 100 | 100 | 72.22 | 0.05 * | |
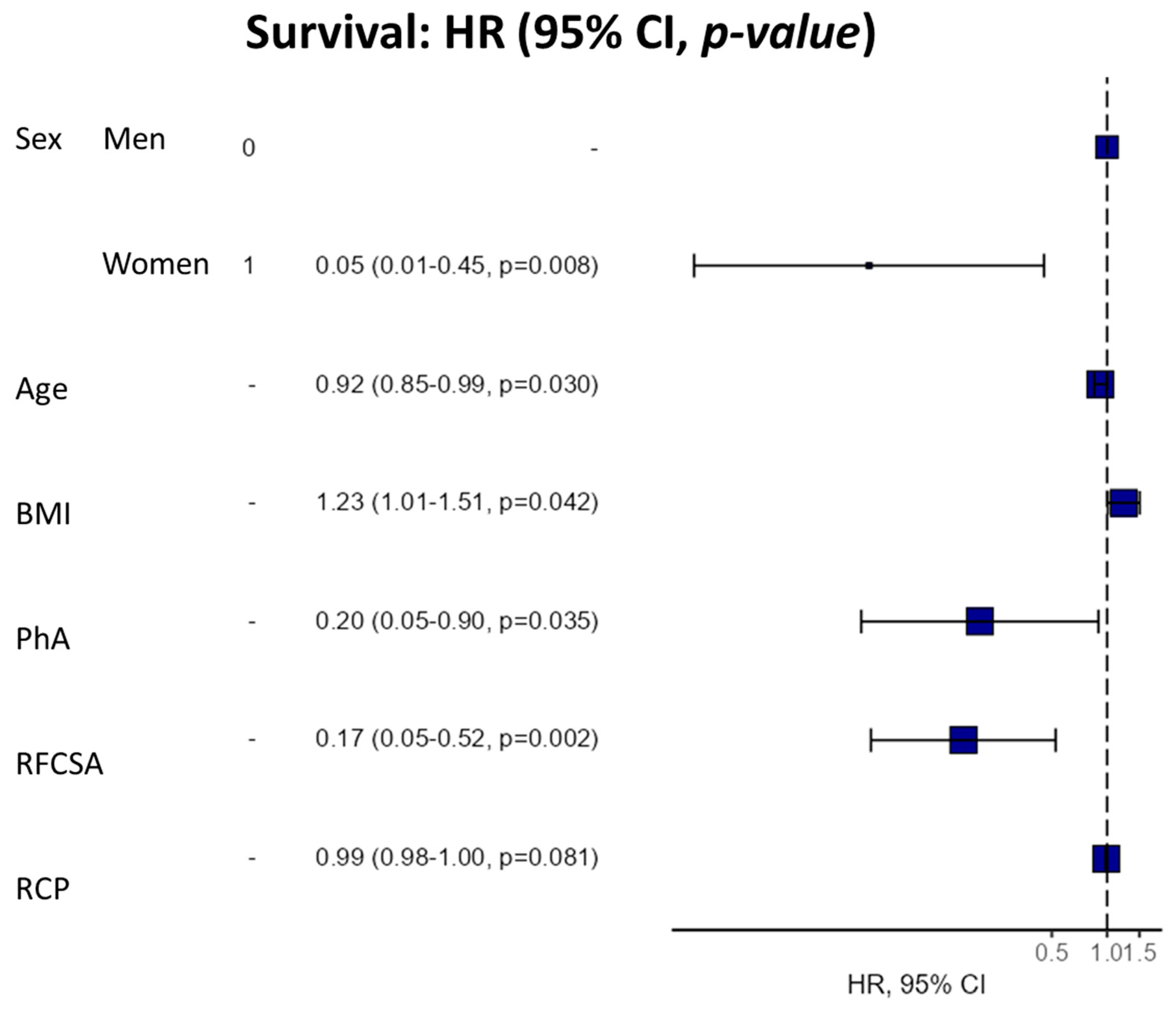
| Dependent | HR (Univariable) | HR (Multivariable) |
|---|---|---|
| Sex (Male-Female) | 0.91 (0.29–2.86, p = 0.870) | 0.05 (0.01-0.45, p = 0.008) |
| Age | 1.00 (0.96–1.04, p = 0.983) | 0.92 (0.85–0.99, p = 0.030) |
| BMI | 0.96 (0.85–1.08, p = 0.459) | 1.23 (1.01–1.51, p = 0.042) |
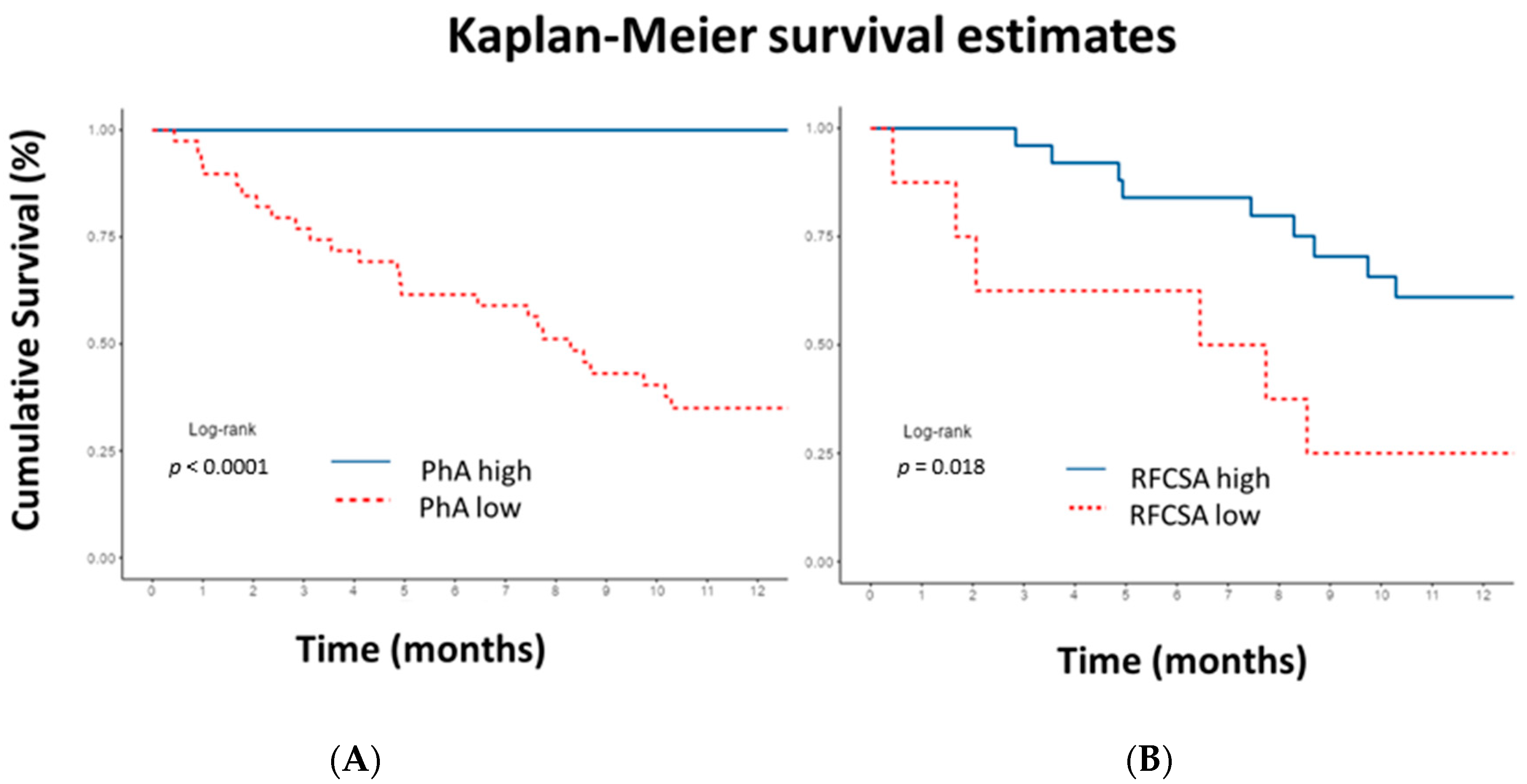
| PhA | 0.42 (0.21–0.84, p = 0.014) | 0.20 (0.05–0.90, p = 0.035) |
| RFCSA | 0.61 (0.39–0.96, p = 0.031) | 0.17 (0.05–0.52, p = 0.002) |
| CRP | 1.00 (1.00–1.01, p = 0.169) | 0.99 (0.98–1.00, p = 0.081) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, C.; Vegas-Aguilar, I.M.; Rioja-Vázquez, R.; Cornejo-Pareja, I.; Tinahones, F.J.; García-Almeida, J.M. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients 2023, 15, 522. https://doi.org/10.3390/nu15030522
García-García C, Vegas-Aguilar IM, Rioja-Vázquez R, Cornejo-Pareja I, Tinahones FJ, García-Almeida JM. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients. 2023; 15(3):522. https://doi.org/10.3390/nu15030522
Chicago/Turabian StyleGarcía-García, Cristina, Isabel María Vegas-Aguilar, Rosalía Rioja-Vázquez, Isabel Cornejo-Pareja, Francisco J. Tinahones, and José Manuel García-Almeida. 2023. "Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial)" Nutrients 15, no. 3: 522. https://doi.org/10.3390/nu15030522
APA StyleGarcía-García, C., Vegas-Aguilar, I. M., Rioja-Vázquez, R., Cornejo-Pareja, I., Tinahones, F. J., & García-Almeida, J. M. (2023). Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients, 15(3), 522. https://doi.org/10.3390/nu15030522





