A Metabolomics-Based Investigation of the Effects of a Short-Term Body Weight Reduction Program in a Cohort of Adolescents with Obesity: A Prospective Interventional Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Body Weight Reduction Program (BWRP)
2.4. Resting Energy Expenditure
2.5. Anthropometric Measurements
2.6. Biological Sample Collection
2.7. Metabolic, Biochemical and Hormonal Evaluation
2.8. Evaluation of Blood Pressure
2.9. Definition of Metabolic Syndrome
2.10. Metabolomics Analyses
2.11. Data Elaboration and Statistical Analyses
3. Results
3.1. Descriptive Statistics of the Population
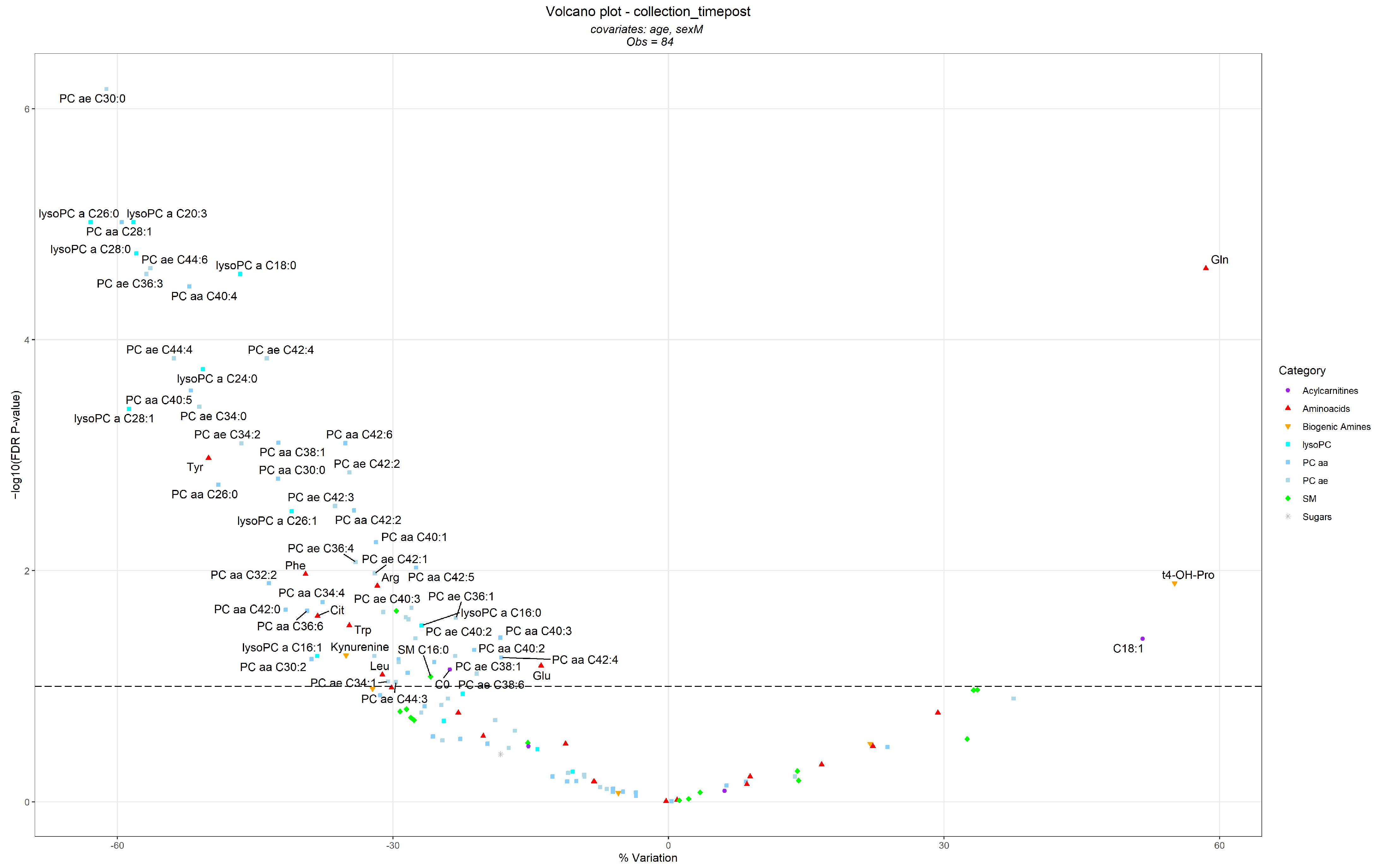
3.2. Differences in Metabolite Levels Following the BWRP
3.3. Differences in Metabolite Levels Associated with Other Variables
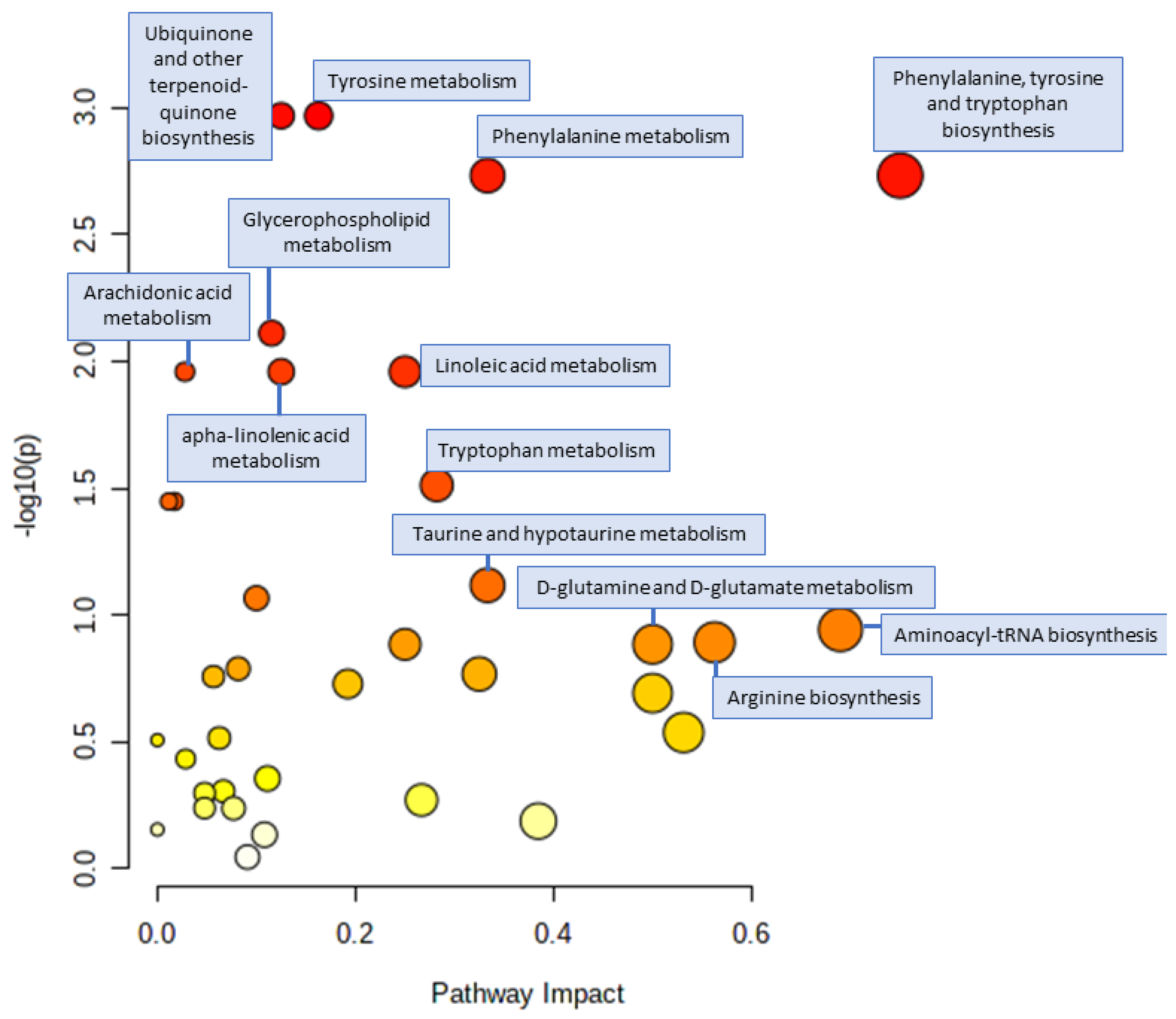
3.4. Pathway Analysis
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Bondesan, A.; Rondinelli, E.; Cella, S.G.; Sartorio, A. The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents. Metabolites 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Caroli, D.; Grugni, G.; Cella, S.G.; Sartorio, A. Frequent Medical Supervision Increases the Effectiveness of a Longitudinal Multidisciplinary Body Weight Reduction Program: A Real-World Experience in a Population of Children and Adolescents with Obesity. Nutrients 2021, 13, 3362. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Tringali, G.; Micheli, R.; De Col, A.; Tamini, S.; Saezza, A.; Cella, S.G.; Sartorio, A. Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome. Nutrients 2020, 12, 208. [Google Scholar] [CrossRef] [Green Version]
- Rigamonti, A.E.; Bollati, V.; Favero, C.; Albetti, B.; Caroli, D.; Abbruzzese, L.; Cella, S.G.; Sartorio, A. Effect of a 3-Week Multidisciplinary Body Weight Reduction Program on the Epigenetic Age Acceleration in Obese Adults. J. Clin. Med. 2022, 11, 4677. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Hurtado, A.M.D.; Acosta, A. Precision Medicine and Obesity. Gastroenterol. Clin. N. Am. 2021, 50, 127–139. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Perng, W.; Peterson, K.E. Precision Nutrition and Childhood Obesity: A Scoping Review. Metabolites 2020, 10, 235. [Google Scholar] [CrossRef]
- Handakas, E.; Lau, C.H.; Alfano, R.; Chatzi, V.L.; Plusquin, M.; Vineis, P.; Robinson, O. A systematic review of metabolomic studies of childhood obesity: State of the evidence for metabolic determinants and consequences. Obes. Rev. 2022, 23 (Suppl. S1), e13384. [Google Scholar] [CrossRef] [PubMed]
- Leal-Witt, M.J.; Ramon-Krauel, M.; Samino, S.; Llobet, M.; Cuadras, D.; Jimenez-Chillaron, J.C.; Yanes, O.; Lerin, C. Untargeted metabolomics identifies a plasma sphingolipid-related signature associated with lifestyle intervention in prepubertal children with obesity. Int. J. Obes. 2018, 42, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Leal-Witt, M.J.; Llobet, M.; Samino, S.; Castellano, P.; Cuadras, D.; Jimenez-Chillaron, J.C.; Yanes, O.; Ramon-Krauel, M.; Lerin, C. Lifestyle Intervention decreases urine trimethylamine N-oxide levels in prepubertal children with obesity. Obesity 2018, 26, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Pathmasiri, W.; Pratt, K.J.; Collier, D.N.; Lutes, L.D.; McRitchie, S.; Sumner, S.C.J. Integrating metabolomic signatures and psychosocial parameters in responsivity to an immersion treatment model for adolescent obesity. Metabolomics 2012, 8, 1037–1051. [Google Scholar] [CrossRef]
- Wahl, S.; Holzapfel, C.; Yu, Z.H.; Breier, M.; Kondofersky, I.; Fuchs, C.; Singmann, P.; Prehn, C.; Adamski, J.; Grallert, H.; et al. Metabolomics reveals determinants of weight loss during lifestyle intervention in obese children. Metabolomics 2013, 9, 1157–1167. [Google Scholar] [CrossRef] [Green Version]
- Reinehr, T.; Wolters, B.; Knop, C.; Lass, N.; Hellmuth, C.; Harder, U.; Peissner, W.; Wahl, S.; Grallert, H.; Adamski, J.; et al. Changes in the serum metabolite profile in obese children with weight loss. Eur. J. Nutr. 2014, 54, 173–181. [Google Scholar] [CrossRef]
- Reinehr, T.; Kulle, A.; Rothermel, J.; Knop-Schmenn, C.; Lass, N.; Bosse, C.; Holterhus, P.M. Longitudinal analyses of the steroid metabolome in obese PCOS girls with weight loss. Endocr. Connect. 2017, 6, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, K.R.; Chadwick, J.Q.; Teague, A.M.; Tullier, M.A.; Wolbert, L.; Coleman, C.; Copeland, K.C. Effect of obesity and exercise training on plasma amino acids and amino metabolites in American Indian adolescents. J. Clin. Endocrinol. Metab. 2019, 104, 3249–3261. [Google Scholar] [CrossRef]
- Sohn, M.J.; Chae, W.; Ko, J.S.; Cho, J.Y.; Kim, J.E.; Choi, J.Y.; Jang, H.B.; Lee, H.J.; Park, S.I.; Park, K.H.; et al. Metabolomic Signatures for the Effects of Weight Loss Interventions on Severe Obesity in Children and Adolescents. Metabolites 2021, 12, 27. [Google Scholar] [CrossRef]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- Ramos Salas, X.; Alberga, A.S.; Cameron, E.; Estey, L.; Forhan, M.; Kirk, S.F.L.; Russell-Mayhew, S.; Sharma, A.M. Addressing weight bias and discrimination: Moving beyond raising awareness to creating change. Obes. Rev. 2017, 18, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Puhl, R.M.; Cummings, D.E.; Eckel, R.H.; Ryan, D.H.; Mechanick, J.I.; Nadglowski, J.; Ramos Salas, X.; Schauer, P.R.; Twenefour, D.; et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020, 26, 485–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- McCharty, H.D.; Jarret, K.V.; Crawley, H.F. The development of waist circumference percentiles in British children aged 5.0–16.9 y. Eur. J. Clin. Nutr. 2001, 55, 902–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biocrates-Life-Sciences-AG AbsoluteIDQ® p180 Kit. Available online: https://biocrates.com/absoluteidq-p180-kit/ (accessed on 31 January 2022).
- Frigerio, G.; Favero, C.; Savino, D.; Mercadante, R.; Albetti, B.; Dioni, L.; Vigna, L.; Bollati, V.; Pesatori, A.C.; Fustinoni, S. Plasma Metabolomic Profiling in 1391 Subjects with Overweight and Obesity from the SPHERE Study. Metabolites 2021, 11, 194. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 31 January 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Jeppesen, J.; Kiens, B. Regulation and limitations to fatty acid oxidation during exercise. J. Physiol. 2012, 590, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Simpson, J.A.; Gibson, R.A.; Sinclair, A.J.; Makrides, M.; O’Dea, K.; English, D.R.; Giles, G.G. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Siahanidou, T.; Voyatzis, C.; Pervanidou, P.; Kaminioti, C.; Lazopoulou, N.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Papassotiriou, I. Elevated circulating levels of lipoprotein-associated phospholipase A2 in obese children. Clin. Chem. Lab. Med. 2015, 53, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J. Mol. Cell. Cardiol. 2003, 35, 1375–1384. [Google Scholar] [CrossRef]
- Wahl, S.; Yu, Z.; Kleber, M.; Singmann, P.; Holzapfel, C.; He, Y.; Mittelstrass, K.; Polonikov, A.; Prehn, C.; Römisch-Margl, W.; et al. Childhood obesity is associated with changes in the serum metabolite profile. Obes. Facts 2012, 5, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.J.; Maahs, D.M.; Daniels, S.R.; Eckel, R.H. Childhood obesity and cardiovascular disease: Links and prevention strategies. Nat. Rev. Cardiol. 2011, 8, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Polidori, N.; Grasso, E.A.; Chiarelli, F.; Giannini, C. Amino Acid-Related Metabolic Signature in Obese Children and Adolescents. Nutrients 2022, 14, 1454. [Google Scholar] [CrossRef]
- Palmer, N.D.; Stevens, R.D.; Antinozzi, P.A.; Anderson, A.; Bergman, R.N.; Wagenknecht, L.E.; Newgard, C.B.; Bowden, D.W. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2015, 100, E463–E468. [Google Scholar] [CrossRef]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood. Obesuty 2014, 22, 2570–2578. [Google Scholar] [CrossRef] [Green Version]
- Huffman, K.M.; Shah, S.H.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.; Slentz, C.A.; Tanner, C.J.; Kuchibhatla, M.; Houmard, J.A.; Newgard, C.B.; et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009, 32, 1678–1683. [Google Scholar] [CrossRef]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Tai, E.S.; Tan, M.L.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.M.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019, 29, 417–429.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holecek, M. Branched-chain amino acids and branched-chain keto acids in hyperammonemic states: Metabolism and as supplements. Metabolites 2020, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef] [Green Version]
- Suryawan, A.; Hawes, J.W.; Harris, R.A.; Shimomura, Y.; Jenkins, A.E.; Hutson, S.M. A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 1998, 68, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Wynn, R.M.; Kato, M.; Machius, M.; Chuang, J.L.; Li, J.; Tomchick, D.R.; Chuang, D.T. Molecular mechanism for regulation of the human mitochondrial branched-chain alphaketoacid dehydrogenase complex by phosphorylation. Structure 2004, 12, 2185–2196. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doisaki, M.; Katano, Y.; Nakano, I.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Hayashi, K.; Goto, H.; Fujita, Y.; Kadota, Y.; et al. Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem. Biophys. Res. Commun. 2010, 393, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Shao, J.; Wu, C.Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J.; et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef] [Green Version]
- She, P.; Reid, T.M.; Bronson, S.K.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutsonet, S.M. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; McGarrah, R.W.; Grimsrud, P.A.; Tso, S.C.; Yang, W.H.; Haldeman, J.M.; Grenier-Larouche, T.; Lapworth, A.L.; Astapova, I.; Hannou, S.A.; et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018, 27, 1281. [Google Scholar] [CrossRef] [Green Version]
- Lian, K.; Du, C.; Liu, Y.; Zhu, D.; Yan, W.; Zhang, H.; Hong, Z.; Liu, P.; Zhang, L.; Pei, H.; et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 2015, 64, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Lu, G.; Gao, C.; Wang, Y.; Sun, H. Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm). J. Biol. Chem. 2012, 287, 23397–23406. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Sun, H.; She, P.; Youn, J.Y.; Warburton, S.; Ping, P.; Vondriska, T.M.; Cai, H.; Lynch, C.J.; Wang, Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Investig. 2009, 119, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.; Ren, S.; Liu, Y.; et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef]
- Joshi, M.; Jeoung, N.H.; Popov, K.M.; Harris, R.A. Identification of a novel PP2C-type mitochondrial phosphatase. Biochem. Biophys. Res. Commun. 2007, 356, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.; Duffley, L.; Pulinilkunnil, T. Role of branched-chain amino acidcatabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB J. 2019, 33, 8711–8731. [Google Scholar] [CrossRef]
- Fisch, S.; Gray, S.; Heymans, S.; Haldar, S.M.; Wang, B.; Pfister, O.; Cui, L.; Kumar, A.; Lin, Z.; Sen-Banerjee, S.; et al. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 7074–7079. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef] [Green Version]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene expression in human NAFLD. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef] [Green Version]
- Sperringer, J.E.; Addington, A.; Hutson, S.M. Branched-chain amino acids and brain metabolism. Neurochem. Res. 2017, 42, 1697–1709. [Google Scholar] [CrossRef]
- Hernandez-Alvarez, M.I.; Diaz-Ramos, A.; Berdasco, M.; Cobb, J.; Planet, E.; Cooper, D.; Pazderska, A.; Wanic, K.; O’Hanlon, D.; Gomez, A.; et al. Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Sci. Rep. 2017, 7, 13850. [Google Scholar]
- Salinas-Rubio, D.; Tovar, A.R.; Torre-Villalvazo, I.; Granados-Portillo, O.; Torres, N.; Pedraza-Chaverri, J.; Noriega, L.G. Interaction between leucine and palmitate catabolism in 3T3-L1 adipocytes and primary adipocytes from control and obese rats. J. Nutr. Biochem. 2018, 59, 29–36. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yahagi, N.; Aita, Y.; Murayama, Y.; Sawada, Y.; Piao, X.; Toya, N.; Oya, Y.; Shikama, A.; Takarada, A.; et al. KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting. Cell Rep. 2016, 16, 2373–2386. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, G.; Chapman, J.; Ofrecio, J.M.; Wilkes, J.; Resnik, J.L.; Thapar, D.; Subramaniam, S.; Searset, D.D. Multi-tissue, selective PPARgamma modulation of insulin sensitivity and metabolic pathways in obese rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E164–E174. [Google Scholar] [CrossRef] [PubMed]
- 0Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Altman, N.S. An introduction to kernel and nearest-neighbor nonparametric regression. Am. Stat. 1992, 46, 175–185. [Google Scholar]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.M.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Lefort, N.; Glancy, B.; Bowen, B.; Willis, W.T.; Bailowitz, Z.; De Filippis, E.A.; Brophy, C.; Meyer, C.; Højlund, K.; Yi, Z.; et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 2010, 59, 2444–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.B.; et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: A Mendelian randomisation analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef] [Green Version]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrère, B.; et al. Branched- chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012, 55, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [Green Version]
- She, P.; Olson, K.C.; Kadota, Y.; Inukai, A.; Shimomura, Y.; Hoppel, C.L.; Adams, S.H.; Kawamata, Y.; Matsumoto, H.; Sakai, R.; et al. Leucine and protein metabolism in obese Zucker rats. PLoS ONE 2013, 8, e59443. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Bradley, D.; Schweitzer, G.G.; Finck, B.N.; Eagon, J.C.; Ilkayeva, O.; Newgard, C.B. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 2013, 62, 2757–2761. [Google Scholar] [CrossRef] [Green Version]
- Fiehn, O.; Garvey, W.T.; Newman, J.W.; Lok, K.H.; Hoppel, C.L.; Adams, S.H. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 2010, 5, e15234. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Um, S.H.; D’Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribo- somal protein S6 kinase 1, S6K1. Cell Metab. 2006, 3, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef]
- Krebs, M.; Brunmair, B.; Brehm, A.; Artwohl, M.; Szendroedi, J.; Nowotny, P.; Roth, E.; Fürnsinn, C.; Promintzer, M.; Anderwald, C.; et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007, 56, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, F.; Brule, S.; Hee Um, S.; Li, Y.; Masuda, K.; Roden, M.; Sun, X.J.; Krebs, M.; Polakiewicz, R.D.; Thomas, G.; et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 14056–14061. [Google Scholar] [CrossRef] [Green Version]
- Gleason, C.E.; Lu, D.; Witters, L.A.; Newgard, C.B.; Birnbaum, M.J. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J. Biol. Chem. 2007, 282, 10341–11051. [Google Scholar] [CrossRef] [Green Version]
- Nagao, H.; Nishizawa, H.; Bamba, T.; Nakayama, Y.; Isozumi, N.; Nagamori, S.; Kanai, Y.; Tanaka, Y.; Kita, S.; Fukuda, S.; et al. Increased dynamics of tricarboxylic acid cycle and glutamate synthesis in obese adipose tissue: In vivo metabolic turnover analysis. J. Biol. Chem. 2017, 292, 4469–4483. [Google Scholar] [CrossRef] [Green Version]
- Maltais-Payette, I.; Boulet, M.M.; Prehn, C.; Adamski, J.; Tchernof, A. Circulating glutamate concentration as a biomarker of visceral obesity and associated metabolic alterations. Nutr. Metab. 2018, 15, 78. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e11. [Google Scholar] [CrossRef]
- Lewis, B.A.; Hanover, J.A. O-GlcNAc and the epigenetic regulation of gene expression. J. Biol. Chem. 2014, 289, 34440–34448. [Google Scholar] [CrossRef] [Green Version]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Göring, H.; Cole, S.A.; Comuzzie, A.G. Global metabolomic profiling targeting child- hood obesity in the Hispanic population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, J.Y.; Kim, O.Y.; Ham, B.M.; Kim, H.J.; Kwon, D.Y.; Jang, Y.; Lee, J. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS). J. Proteome Res. 2010, 9, 4368–4375. [Google Scholar] [CrossRef]
- Fattuoni, C.; Mandò, C.; Palmas, F.; Anelli, G.M.; Novielli, C.; Parejo Laudicina, E.; Savasi, V.M.; Barberini, L.; Dessì, A.; Pintus, R.; et al. Preliminary metabolomics analysis of placenta in maternal obesity. Placenta 2018, 61, 89–95. [Google Scholar] [CrossRef]
- Wang, S.M.; Yang, R.Y.; Wang, M.; Ji, F.S.; Li, H.X.; Tang, Y.M.; Chen, W.X.; Dong, J. Identification of serum metabolites associated with obesity and traditional risk factors for metabolic disease in Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 112–118. [Google Scholar] [CrossRef]
- Houttu, N.; Mokkala, K.; Laitinen, K. Overweight and obe- sity status in pregnant women are related to intestinal microbiota and serum metabolic and inflammatory profiles. Clin. Nutr. 2018, 37, 1955–1966. [Google Scholar] [CrossRef]
- Yu, H.T.; Fu, X.Y.; Xu, B.; Zuo, L.L.; Ma, H.B.; Wang, S.R. Untargeted metabolomics approach (UPLC-Q-TOF-MS) explores the biomarkers of serum and urine in overweight/obese young men. Asia Pac. J. Clin. Nutr. 2018, 27, 1067–1076. [Google Scholar] [PubMed]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine is associated with insulin resistance in longitudinal metabolomic profiling of obese children. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libert, D.M.; Nowacki, A.S.; Natowicz, M.R. Metabolomic analysis of obesity, metabolic syndrome, and type 2 diabetes: Amino acid and acylcarnitine levels change along a spectrum of metabolic wellness. PeerJ 2018, 6, e5410. [Google Scholar] [CrossRef] [Green Version]
- Buckley, W.T.; Milligan, L.P. Participation of cysteine and cystine in inactivation of tyrosine aminotransferase in rat liver homogenates. Biochem. J. 1978, 176, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Noce, A.; Di Giovamberardino, G.; De Stefano, A.; Callà, C.; Zenobi, R.; Dessì, M.; Di Daniele, N. Homocysteine, cysteine, folate and vitamin B12 status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Wijekoon, E.P.; Brosnan, M.E.; Brosnan, J.T. Homocysteine metabolism in diabetes. Biochem. Soc. Trans. 2007, 35, 1175–1179. [Google Scholar] [CrossRef]
- Ramzan, I.; Taylor, M.; Phillips, B.; Wilkinson, D.; Smith, K.; Hession, K.; Idris, I.; Atherton, P. A Novel Dietary Intervention Reduces Circulatory Branched-Chain Amino Acids by 50%: A Pilot Study of Relevance for Obesity and Diabetes. Nutrients 2020, 13, 95. [Google Scholar] [CrossRef]
- Tam, C.S.; Clément, K.; Baur, L.A.; Tordjman, J. Obesity and low-grade inflammation: A paediatric perspective. Obes. Rev. 2010, 11, 118–126. [Google Scholar] [CrossRef]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Ball, H.J.; Sanchez-Perez, A.; Weiser, S.; Austin, C.J.D.; Astelbauer, F.; Miu, J.; McQuillan, J.A.; Stocker, R.; Jermiin, L.S.; Huntet, N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 2007, 396, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef] [PubMed]
- Wolowczuk, I.; Hennart, B.; Leloire, A.; Bessede, A.; Soichot, M.; Taront, S.; Caiazzo, R.; Raverdy, V.; Pigeyre, M.; ABOS Consortium; et al. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: An attempt to maintain immune homeostasis and vascular tone. AJP Regul. Integr. Comp. Physiol. 2012, 303, R135–R143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangge, H.; Summers, K.L.; Meinitzer, A.; Zelzer, S.; Almer, G.; Prassl, R.; Schnedl, W.J.; Reininghaus, E.; Paulmichl, K.; Weghuber, D.; et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity 2014, 22, 195–201. [Google Scholar] [CrossRef]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Rosen, D.A.; Maengwyn-Davies, G.D.; Becker, B.; Stone, H.H.; Friedenwald, J.S. Xanthurenic acid excretion studies in diabetics with and without retinopathy. Proc. Soc. Exp. Biol. Med. 1955, 88, 321–323. [Google Scholar] [CrossRef]
- Meyramov, G.; Korchin, V.; Kocheryzkina, N. Diabetogenic activity of xanturenic acid determined by its chelating properties? Transplant. Proc. 1998, 30, 2682–2684. [Google Scholar] [CrossRef]
- Wurtman, J.; Wurtman, R. The Trajectory from Mood to Obesity. Curr. Obes. Rep. 2018, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Carraça, E.V.; Encantado, J.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; van Baak, M.; Dicker, D.; Ermolao, A.; Farpour-Lambert, N.; et al. Effect of exercise training on psychological outcomes in adults with overweight or obesity: A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. S4), e13261. [Google Scholar] [CrossRef]
- Lin, T.W.; Kuo, Y.M. Exercise benefits brain function: The monoamine connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018, 93, 741–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppe, L.; Nyam, E.; Vivot, K.; Manning Fox, J.E.; Dai, X.Q.; Nguyen, B.N.; Trudel, D.; Attané, C.; Moullé, V.S.; MacDonald, P.E.; et al. Urea impairs beta cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Investig. 2016, 126, 3598–3612. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Tawfik, H.E.; Labazi, M.; El-Remessy, A.B.; Bartoli, M.; Caldwell, R.B.; Caldwell, R.W. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ. Res. 2008, 102, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.R.; Roman, L.J.; Lamont, J.; Masters, B.S.; Bajaj, M.; Suraamornkul, S.; Belfort, R.; Berria, R.; Kellogg, D.L., Jr.; Liu, Y.; et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1100–1105. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, J.; Merino, A.; Briceno, C.; Soriano, S.; Buendia, P.; Calleros, L.; Rodrigue, M.; Martín-Malo, A.; Aljama, P.; Ramírez, R. Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. FASEB J. 2011, 25, 1314–1322. [Google Scholar] [CrossRef]
- Pietzner, M.; Kaul, A.; Henning, A.K.; Kastenmuller, G.; Artati, A.; Lerch, M.M.; Adamski, J.; Nauck, M.; Friedrich, N. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC Med. 2017, 15, 210. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.F.; Li, J.; Zhang, Z.; Liu, J.; Sun, X.Y.; Feng, X.F.; Luo, H.H.; Yang, W.; Li, S.N.; Yang, X.; et al. Plasma Levels of Amino Acids Related to Urea Cycle and Risk of Type 2 Diabetes Mellitus in Chinese Adults. Front. Endocrinol. 2019, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, P.L.; Vilstrup, H.; Rigbolt, K.; Suppli, M.P.; Sørensen, M.; Heebøll, S.; Veidal, S.S.; Knop, F.K.; Thomsen, K.L. Non-alcoholic fatty liver disease alters expression of genes governing hepatic nitrogen conversion. Liver Int. 2019, 39, 2094–2101. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Astbury, N.M.; Tudor, K.E.; Morris, E.; Henry, J.A.; Noreik, M.; Jebb, S.A.; Aveyard, P. Association of Weight Loss Interventions with Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1262–1271. [Google Scholar] [CrossRef] [Green Version]
- Sailer, M.; Dahlhoff, C.; Giesbertz, P.; Eidens, M.K.; de Wit, N.; Rubio-Aliaga, I.; Boekschoten, M.V.; Müller, M.; Daniel, H. Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS ONE 2013, 8, e63950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Brown, H.; Milner, A.; Kennedy, J.; Delena, S. Hydroxyproline excretion during starvation of obese subjects. Metabolism 1968, 17, 345–351. [Google Scholar] [CrossRef]
- Tofas, T.; Jamurtas, A.Z.; Fatouros, I.; Nikolaidis, M.G.; Koutedakis, Y.; Sinouris, E.A.; Papageorgakopoulou, N.; Theocharis, D.A. Plyometric exercise increases serum indices of muscle damage and collagen breakdown. J. Strength Cond. Res. 2008, 22, 490–496. [Google Scholar] [CrossRef]
- Dolan, E.; Dumas, A.; Keane, K.M.; Bestetti, G.; Freitas, L.H.M.; Gualano, B.; Kohrt, W.M.; Kelley, G.A.; Pereira, R.M.R.; Sale, C.; et al. The Bone Biomarker Response to an Acute Bout of Exercise: A Systematic Review with Meta-Analysis. Sports Med. 2022, 52, 2889–2908. [Google Scholar] [CrossRef]

| Parameter | Before BWRP (Mean ± SD) | After BWRP (Mean ± SD) | p-Value |
|---|---|---|---|
| N. | 42 | - | |
| Sex (F/M) | 32/10 | - | |
| Age (years) | 15.94 ± 1.33 | 16.01 ± 1.33 | <0.0001 |
| BMI (kg/m2) | 37.14 ± 4.81 | 35.60 ± 4.73 | <0.0001 |
| BMI SDS | 2.96 ± 0.46 | 2.83 ± 0.52 | <0.0001 |
| WHR | 0.91 ± 0.08 (missing values: 1) | 0.91 ± 0.09 (missing values: 13) | 0.025 |
| FFM (kg) | 54.85 ± 8.53 | 53.29 ± 8.05 | 0.001 |
| FFM % | 54.68 ± 5.70 | 55.41 ± 5.61 | 0.082 |
| FM (kg) | 45.82 ± 9.72 | 43.28 ± 9.46 | <0.0001 |
| FM % | 45.33 ± 5.71 | 44.59 ± 5.61 | 0.114 |
| SBP (mmHg) | 125.95 ± 10.83 | 124.40 ± 52.15 | 0.223 |
| DBP (mmHg) | 77.50 ± 7.01 | 74.17 ± 5.94 | 0.016 |
| HR (bpm) | 80.02 ± 12.29 | 76.74 ± 10.22 | 0.056 |
| REE (kcal/24 h) | 1896.67 ± 447.96 | 1892.27 ± 444.67 (missing values: 1) | 0.948 |
| Glycemia (mg/dL) | 87.52 ± 8.71 | 83.33 ± 6.35 | <0.0001 |
| Glycemia (mM) | 4.86 ± 0.48 | 4.62 ± 0.35 | <0.0001 |
| Insulin (mU/L) | 23.96 ± 13.60 (missing values: 3) | 18.06 ± 8.03 | 0.0001 |
| HOMA-IR | 5.31 ± 3.41 (missing values: 3) | 3.75 ± 1.86 | <0.0001 |
| Total cholesterol (mg/dL) | 157.64 ± 28.02 | 130.00 ± 19.99 | <0.0001 |
| HDL-C (mg/dL) | 45.05 ± 9.22 | 37.29 ± 8.19 | <0.0001 |
| LDL-C (mg/dL) | 100.62 ± 28.51 | 80.98 ± 20.17 | <0.0001 |
| Triglycerides (mg/dL) | 110.86 ± 50.14 | 100.45 ± 34.70 | 0.092 |
| NEFA (mg/dL) | 1.01 ± 0.95 (missing values: 21) | 0.72 ± 0.19 (missing values: 22) | 0.197 |
| HbA1c (mmol/L) | 5.25 ± 0.48 | 5.08 ± 0.37 | <0.0001 |
| CRP (mg/dL) | 0.52 ± 0.53 | 0.33 ± 0.42 | <0.0001 |
| Metabolic syndrome | Yes = 11 | Yes = 5 | 0.095 |
| No = 31 | No = 37 |
| Metabolites | Metabolite Category | T0—Before BWRP | T1—After BWRP | ||
|---|---|---|---|---|---|
| Median (5th, 95th) (µM) | N > LOQ (%) | Median (5th, 95th) (µM) | N > LOQ (%) | ||
| Metabolites significantly increased | |||||
| Glutamine (Gln) | Amino Acids | 460.0 (147.3, 771.2) | 42 (100) | 558.0 (245.8, 919.8) | 42 (100) |
| Hydroxyproline (t4-OH-Pro) | Biogenic Amines | 14.75 (10.00, 23.51) | 35 (83.3) | 15.20 (10.61, 29.36) | 40 (95.2) |
| Octadecenoylcarnitine (C18:1) | Acylcarnitines | 0.146 (0.076, 0.262) | 27 (64.3) | 0.181 (0.076, 0.293) | 32 (76.2) |
| Metabolites significantly decreased | |||||
| PC ae C30:0 | PC ae | 0.228 (0.108, 0.339) | 39 (92.9) | 0.158 (0.108, 0.210) | 37 (88.1) |
| lysoPC a C20:3 | lysoPC | 1.63 (0.662, 3.26) | 41 (97.6) | 1.200 (0.302, 1.588) | 39 (92.9) |
| lysoPC a C26:0 | lysoPC | 0.204 (0.096, 0.316) | 34 (81) | 0.118 (0.078, 0.204) | 35 (83.3) |
| PC aa C28:1 | PC aa | 1.94 (1.25, 2.884) | 42 (100) | 1.455 (1.003, 2.106) | 42 (100) |
| lysoPC a C28:0 | lysoPC | 0.245 (0.163, 0.483) | 32 (76.2) | 0.187 (0.082, 0.340) | 30 (71.4) |
| PC ae C44:6 | PC ae | 0.838 (0.596, 1.289) | 42 (100) | 0.671 (0.470, 1.058) | 42 (100) |
| lysoPC a C18:0 | lysoPC | 20.9 (12.42, 44.33) | 42 (100) | 15.90 (10.22, 36.24) | 42 (100) |
| PC ae C36:3 | PC ae | 4.315 (2.480, 7.717) | 42 (100) | 3.275 (2.471, 5.144) | 42 (100) |
| PC aa C40:4 | PC aa | 4.360 (2.062, 6.650) | 42 (100) | 3.020 (1.424, 5.292) | 42 (100) |
| PC ae C42:4 | PC ae | 0.961 (0.374, 2.075) | 42 (100) | 0.752 (0.325, 1.338) | 42 (100) |
| PC ae C44:4 | PC ae | 0.278 (0.173, 0.355) | 42 (100) | 0.220 (0.111, 0.324) | 42 (100) |
| lysoPC a C24:0 | lysoPC | 0.354 (0.168, 0.512) | 30 (71.4) | 0.206 (0.168, 0.418) | 27 (64.3) |
| PC aa C40:5 | PC aa | 5.595 (3.165, 10.570) | 42 (100) | 4.055 (2.406, 6.841) | 42 (100) |
| PC ae C34:0 | PC ae | 0.716 (0.405, 1.140) | 42 (100) | 0.589 (0.334, 0.902) | 42 (100) |
| lysoPC a C28:1 | lysoPC | 0.225 (0.130, 0.479) | 33 (78.6) | 0.130 (0.119, 0.351) | 29 (69) |
| PC aa C38:1 | PC aa | 1.935 (0.309, 4.628) | 41 (97.6) | 1.250 (0.014, 2.957) | 38 (90.5) |
| PC aa C42:6 | PC aa | 0.336 (0.236, 0.663) | 24 (57.1) | 0.236 (0.236, 0.485) | 21 (50) |
| PC ae C34:2 | PC ae | 6.110 (3.852, 11.530) | 42 (100) | 5.270 (3.811, 7.327) | 42 (100) |
| Tyrosine (Tyr) | Amino Acids | 101.2 (70.61, 145.9) | 42 (100) | 86.80 (56.09, 127.9) | 42 (100) |
| PC ae C42:2 | PC ae | 0.607 (0.226, 1.503) | 42 (100) | 0.496 (0.178, 1.109) | 42 (100) |
| PC aa C30:0 | PC aa | 1.920 (1.234, 3.438) | 42 (100) | 1.575 (1.092, 2.932) | 42 (100) |
| PC aa C26:0 | PC aa | 0.801 (0.522, 0.903) | 25 (59.5) | 0.732 (0.387, 0.801) | 23 (54.8) |
| PC ae C42:3 | PC ae | 0.910 (0.327, 1.823) | 42 (100) | 0.693 (0.256, 1.309) | 41 (97.6) |
| PC aa C42:2 | PC aa | 0.424 (0.102, 0.988) | 41 (97.6) | 0.329 (0.086, 0.692) | 41 (97.6) |
| lysoPC a C26:1 | lysoPC | 0.114 (0.083, 0.229) | 24 (57.1) | 0.092 (0.080, 0.166) | 26 (61.9) |
| PC aa C40:1 | PC aa | 0.563 (0.317, 2.020) | 27 (64.3) | 0.528 (0.382, 1.219) | 23 (54.8) |
| PC ae C36:4 | PC ae | 11.00 (6.113, 19.60) | 42 (100) | 9.930 (6.230, 15.19) | 42 (100) |
| PC aa C42:5 | PC aa | 0.277 (0.033, 0.642) | 38 (90.5) | 0.227 (0.033, 0.483) | 36 (85.7) |
| PC ae C42:1 | PC ae | 0.716 (0.172, 1.433) | 41 (97.6) | 0.462 (0.134, 1.049) | 42 (100) |
| Phenylalanine (Phe) | Amino Acids | 98.85 (67.33, 158.1) | 42 (100) | 89.60 (62.80, 119.0) | 42 (100) |
| PC aa C32:2 | PC aa | 1.980 (0.650, 3.246) | 42 (100) | 1.455 (0.848, 2.378) | 42 (100) |
| Arginine (Arg) | Amino Acids | 103.0 (65.02, 259.7) | 42 (100) | 101.5 (46.80, 193.0) | 42 (100) |
| PC aa C34:4 | PC aa | 0.667 (0.280, 0.999) | 42 (100) | 0.482 (0.287, 0.834) | 42 (100) |
| PC ae C40:3 | PC ae | 5.265 (0.534, 12.920) | 42 (100) | 3.720 (0.429, 9.230) | 42 (100) |
| PC aa C42:0 | PC aa | 0.649 (0.390, 1.174) | 42 (100) | 0.570 (0.276, 0.879) | 42 (100) |
| PC aa C36:6 | PC aa | 0.404 (0.189, 0.643) | 42 (100) | 0.314 (0.217, 0.490) | 42 (100) |
| SM (OH) C14:1 | SM | 4.665 (3.021, 6.995) | 42 (100) | 4.255 (3.070, 6.379) | 42 (100) |
| PC ae C42:5 | PC ae | 2.780 (1.224, 4.480) | 42 (100) | 2.380 (1.091, 3.523) | 42 (100) |
| Citrulline (Cit) | Amino Acids | 24.25 (13.73, 39.48) | 42 (100) | 21.05 (12.09, 32.42) | 42 (100) |
| PC ae C38:2 | PC ae | 5.260 (0.049, 17.32) | 38 (90.5) | 3.860 (0.049, 10.49) | 36 (85.7) |
| PC ae C36:1 | PC ae | 10.85 (3.056, 33.05) | 42 (100) | 8.505 (2.695, 24.28) | 42 (100) |
| PC ae C40:4 | PC ae | 3.905 (1.260, 8.295) | 41 (97.6) | 3.245 (0.883, 6.265) | 42 (100) |
| Tryptophan (Trp) | Amino Acids | 92.20 (58.41, 121.6) | 42 (100) | 81.30 (59.16, 108.0) | 42 (100) |
| lysoPC a C16:0 | lysoPC | 70.75 (45.05, 156.3) | 42 (100) | 69.90 (38.92, 121.7) | 42 (100) |
| PC aa C40:3 | PC aa | 0.741 (0.179, 3.099) | 42 (100) | 0.540 (0.171, 2.579) | 42 (100) |
| PC ae C40:2 | PC ae | 2.720 (0.686, 7.567) | 41 (97.6) | 2.075 (0.828, 4.231) | 42 (100) |
| PC aa C40:2 | PC aa | 1.110 (0.125, 4.271) | 42 (100) | 0.697 (0.100, 3.349) | 42 (100) |
| ((Kynurenine | Biogenic Amines | 3.125 (2.414, 4.527) | 42 (100) | 2.820 (1.980, 4.254) | 42 (100) |
| lysoPC a C16:1 | lysoPC | 2.38 (1.096, 4.580) | 41 (97.6) | 2.020 (0.182, 3.628) | 37 (88.1) |
| PC ae C30:2 | PC ae | 0.135 (0.033, 0.287) | 40 (95.2) | 0.098 (0.019, 0.194) | 38 (90.5) |
| PC ae C38:1 | PC ae | 4.720 (0.092, 18.83) | 41 (97.6) | 3.070 (0.046, 10.39) | 37 (88.1) |
| PC aa C42:4 | PC aa | 0.301 (0.052, 1.174) | 42 (100) | 0.276 (0.058, 0.875) | 42 (100) |
| PC aa C30:2 | PC aa | 0.105 (0.007, 0.222) | 39 (92.9) | 0.070 (0.007, 0.153) | 35 (83.3) |
| PC aa C42:1 | PC aa | 0.240 (0.080, 0.512) | 41 (97.6) | 0.177 (0.090, 0.317) | 42 (100) |
| PC aa C24:0 | PC aa | 0.268 (0.066, 0.505) | 30 (71.4) | 0.195 (0.066, 0.392) | 31 (73.8) |
| PC ae C40:1 | PC ae | 1.545 (0.491, 3.167) | 42 (100) | 1.195 (0.498, 2.438) | 42 (100) |
| Glutamic acid (Glu) | Amino Acids | 154.5 (68.47, 471.1) | 42 (100) | 139.5 (62.81, 389.4) | 42 (100) |
| L-Carnitine (C0) | Acylcarnitines | 35.45 (5, 52.57) | 38 (90.5) | 33.15 (5.00, 45.17) | 36 (85.7) |
| PC aa C32:1 | PC aa | 8.125 (4.132, 20.64) | 42 (100) | 7.065 (4.53, 14.32) | 42 (100) |
| PC ae C38:6 | PC ae | 3.300 (1.954, 6.958) | 42 (100) | 3.175 (1.812, 6.681) | 42 (100) |
| Leucine (Leu) | Amino Acids | 240.0 (158.4, 339.8) | 42 (100) | 218.5 (169.2, 304.0) | 42 (100) |
| SM C16:0 | SM | 88.20 (59.06, 122.6) | 42 (100) | 82.85 (64.15, 111.9) | 42 (100) |
| PC ae C34:1 | PC ae | 6.250 (4.400, 9.252) | 42 (100) | 5.720 (4.386, 7.315) | 42 (100) |
| PC ae C44:3 | PC ae | 0.134 (0.058, 0.219) | 36 (85.7) | 0.115 (0.057, 0.155) | 32 (76.2) |
| Difference in Metabolites after the BWRP | Possible Mechanisms or Clinical Implications |
|---|---|
| ↓ glycerophospholipids | ↑ β-oxidation ↓ intake of fat nutrients ↓ dyslipidemia |
| ↓ lysoPCs | ↓ LpPLA2 pathway (related to the decrease of plasma LDL) ↓ atherogenesis |
| ↓ BCAA (in particular leucine) | ↑ BCAA catabolic pathway (particularly in the adipose tissue and skeletal muscle) ↑ reversible transamination by BCAT to form BCKA ↑ irreversible oxidative decarboxylation by the BCKD complex (The end products of BCAA catabolism, i.e., succinyl-CoA and acetyl-CoA, enter the TCA cycle as “anaplerotic” substrates) ↓ activation of mTOR with↓ insulin-resistance |
| ↓ tryptophan ↓ kynurenine | ↓ IDO1 (ensuing diversion of tryptophan in methoxyindole pathway) ↓ pro-inflammatory transcriptional response ↓ chronic inflammatory state ↑ synthesis of serotonin and ↑ psychological wellbeing |
| ↑ t4-OH-proline | ↑ catabolism of collagen proteins (particularly in the connective and bone tissues) |
| ↑ glutamine | ↓ synthesis by adipose tissue ↓ O-GlcNAcylation of nuclear proteins ↓ pro-inflammatory transcriptional response ↓ chronic inflammatory state |
| ↓ phenylalanine ↓ tyrosine | ↑ uptake by LAT1 ↑ activity of tyrosine aminotransferase due to ↓ cystine, a by-product of the oxidative stress ↑ hepatic catabolism (with ↓ NAFLD) |
| ↓ arginine ↓ citrulline | ↑ urea cycle due to ↓ fat accumulation in the liver ↑ NOS activity with ↑ endothelial function and cardiovascular benefits |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.E.; Frigerio, G.; Caroli, D.; De Col, A.; Cella, S.G.; Sartorio, A.; Fustinoni, S. A Metabolomics-Based Investigation of the Effects of a Short-Term Body Weight Reduction Program in a Cohort of Adolescents with Obesity: A Prospective Interventional Clinical Study. Nutrients 2023, 15, 529. https://doi.org/10.3390/nu15030529
Rigamonti AE, Frigerio G, Caroli D, De Col A, Cella SG, Sartorio A, Fustinoni S. A Metabolomics-Based Investigation of the Effects of a Short-Term Body Weight Reduction Program in a Cohort of Adolescents with Obesity: A Prospective Interventional Clinical Study. Nutrients. 2023; 15(3):529. https://doi.org/10.3390/nu15030529
Chicago/Turabian StyleRigamonti, Antonello E., Gianfranco Frigerio, Diana Caroli, Alessandra De Col, Silvano G. Cella, Alessandro Sartorio, and Silvia Fustinoni. 2023. "A Metabolomics-Based Investigation of the Effects of a Short-Term Body Weight Reduction Program in a Cohort of Adolescents with Obesity: A Prospective Interventional Clinical Study" Nutrients 15, no. 3: 529. https://doi.org/10.3390/nu15030529
APA StyleRigamonti, A. E., Frigerio, G., Caroli, D., De Col, A., Cella, S. G., Sartorio, A., & Fustinoni, S. (2023). A Metabolomics-Based Investigation of the Effects of a Short-Term Body Weight Reduction Program in a Cohort of Adolescents with Obesity: A Prospective Interventional Clinical Study. Nutrients, 15(3), 529. https://doi.org/10.3390/nu15030529








