Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts
Abstract
1. Introduction
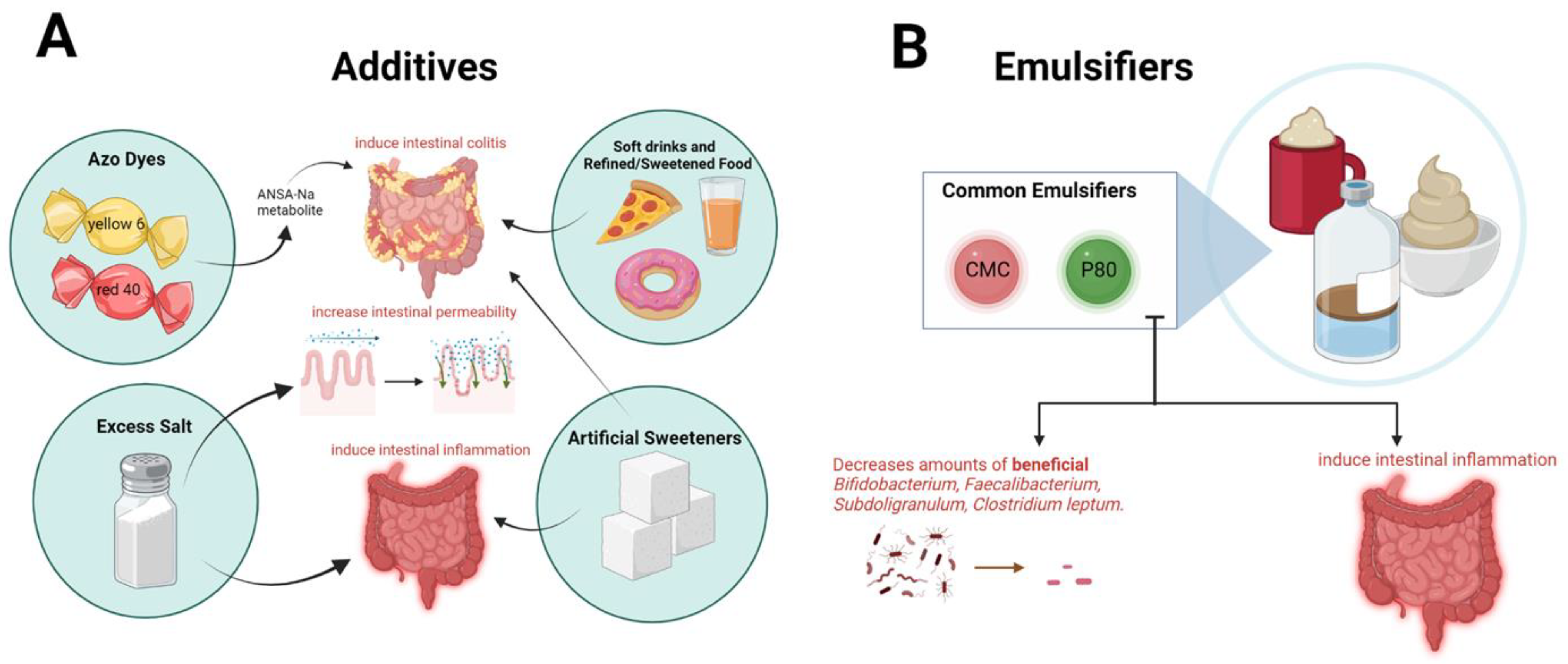
2. Food Processing and Additives and Risk of IBD
2.1. Ultra-Processed Foods in Inflammatory Bowel Disease
2.2. Emulsifiers in Inflammatory Bowel Disease
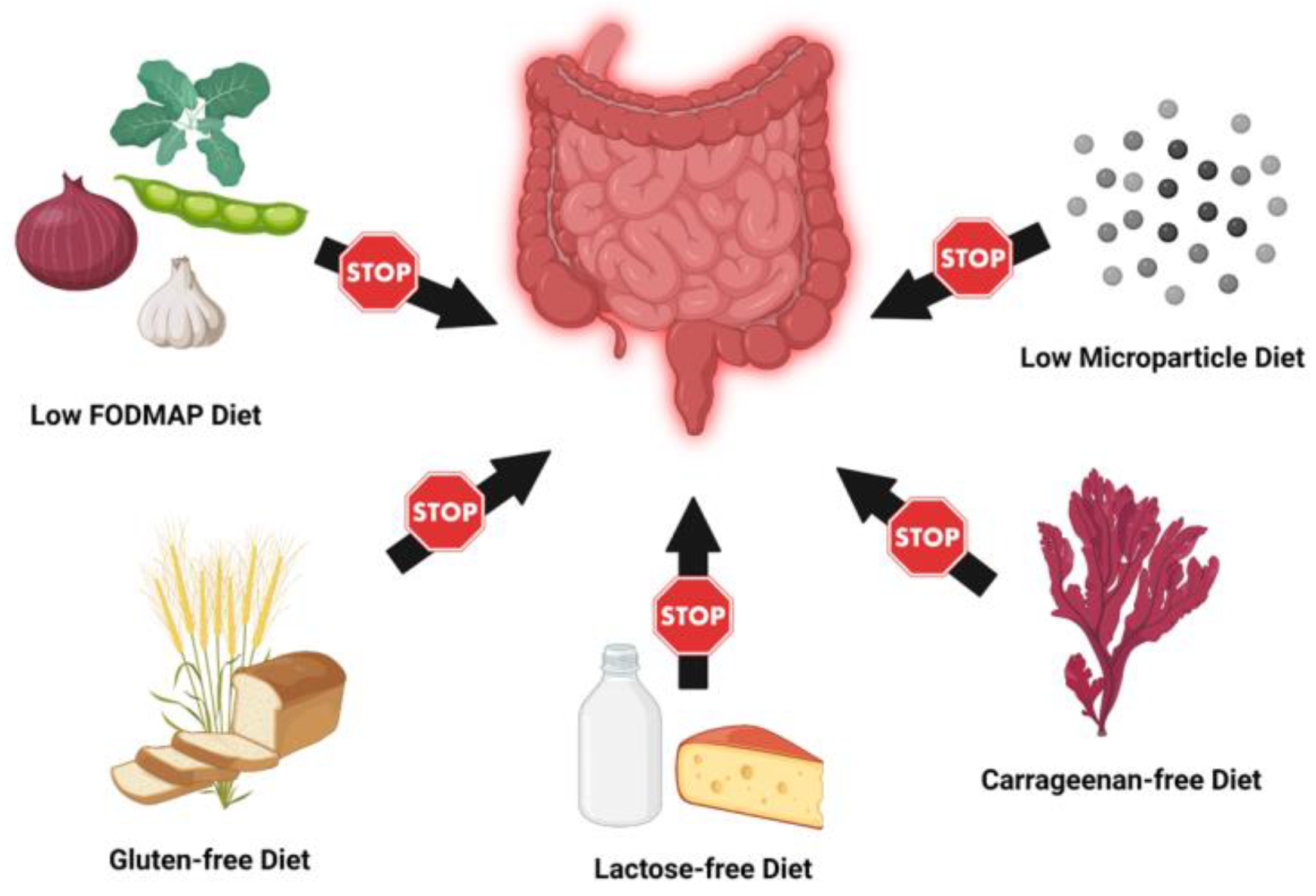
3. Elimination and Exclusion Diets
3.1. Low FODMAP Diet
3.2. Carrageenan-Free Diet
3.3. Lactose-Free Diet
3.4. Gluten-Free Diet
3.5. Low Microparticle Diet
4. Specific Dietary Interventions
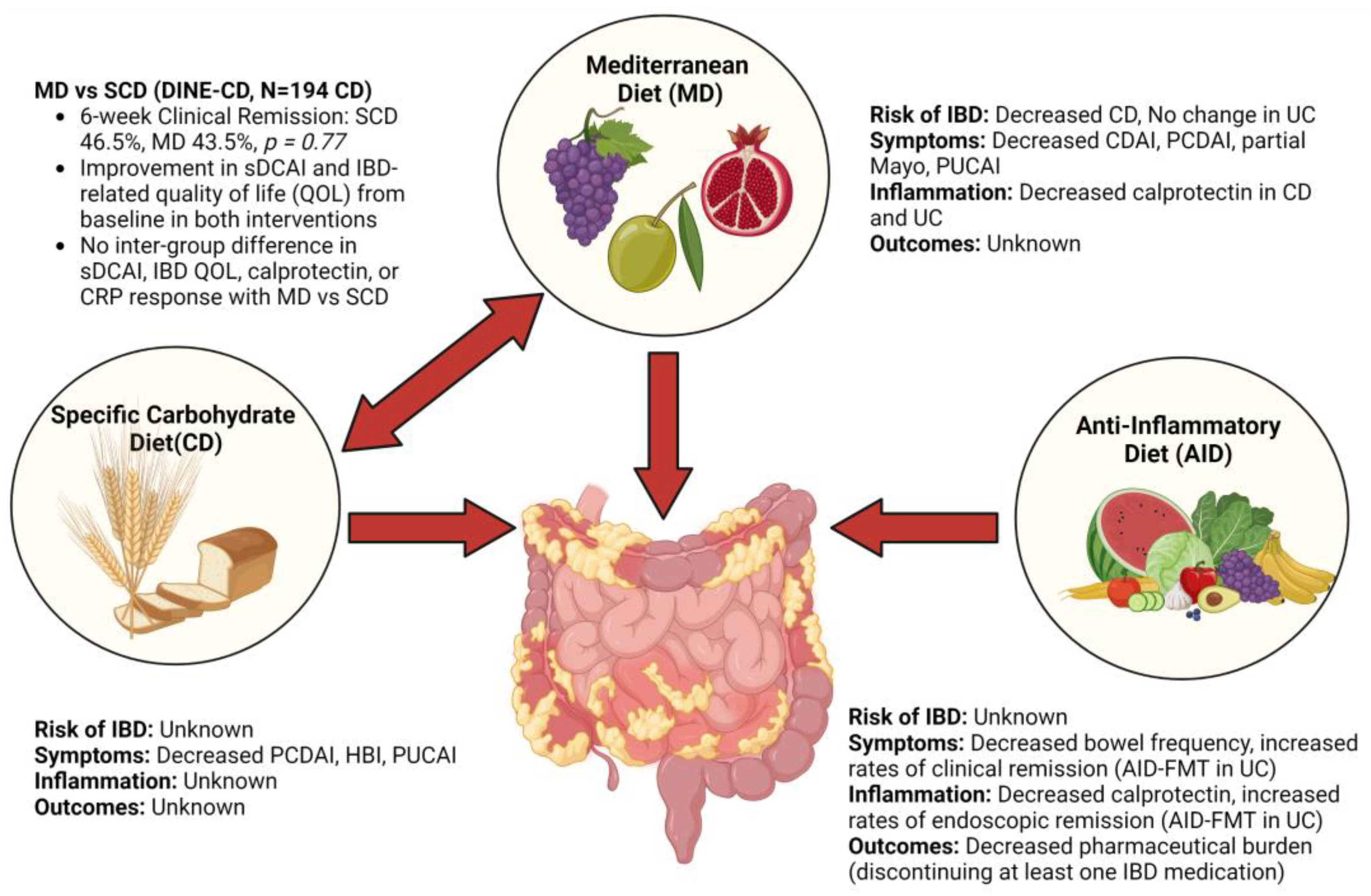
4.1. Mediterranean Diet
4.2. Specific Carbohydrate Diet
4.3. Comparison of Mediterranean Diet and Specific Carbohydrate Diet
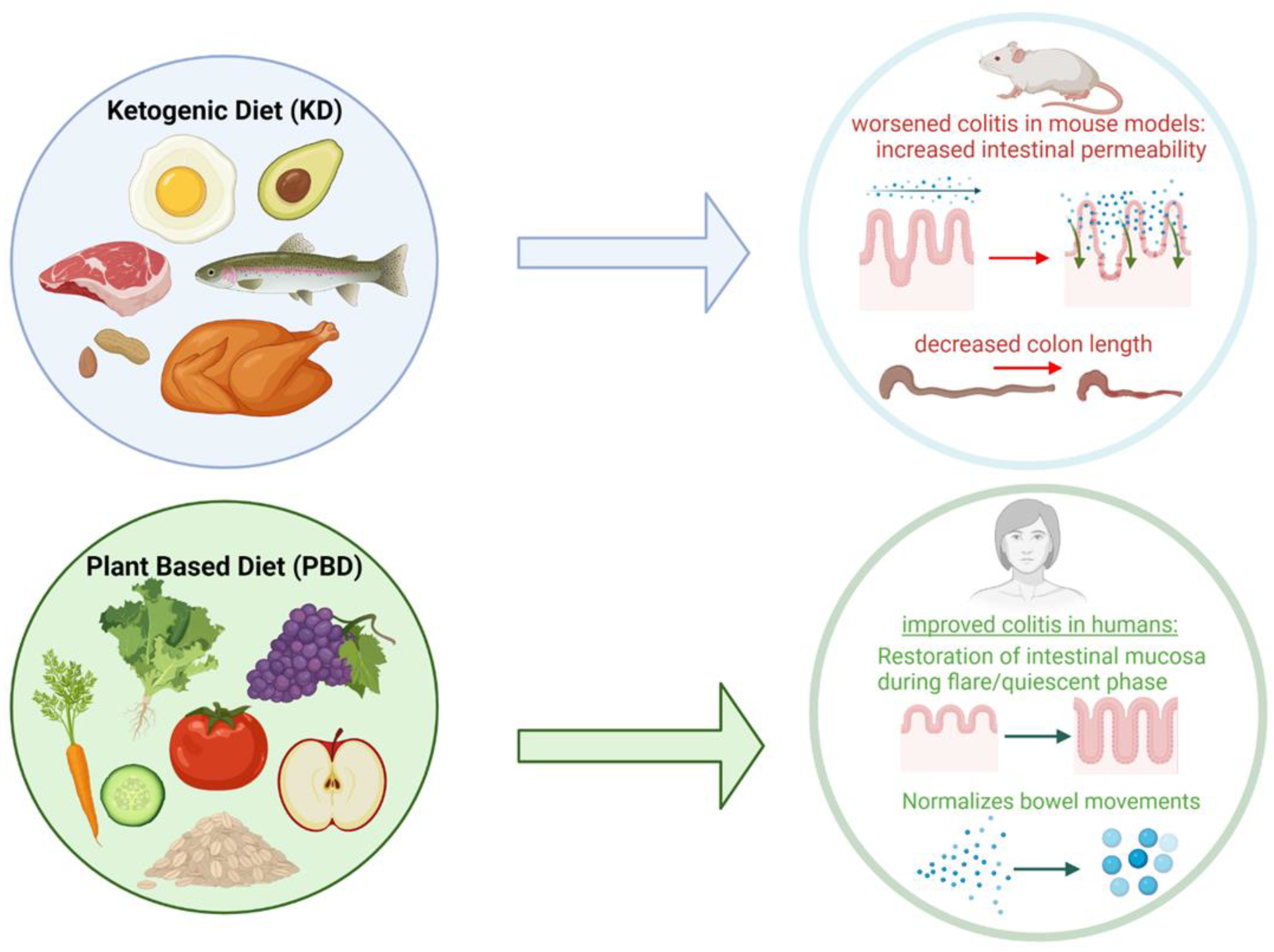
4.4. Ketogenic Diet
4.5. Plant-Based Diet
4.6. Anti-Inflammatory Diet
4.7. High Fiber Diet
4.8. Exclusive Enteral Nutrition (EEN)
5. Emerging Dietary Interventions in IBD
5.1. Reduced Sulfur Diet in UC
5.2. Personalized Fiber Diet in UC
5.3. Fermented Food Diet in UC
5.4. Caloric Restriction, Intermittent Fasting, Time Restricted Feeding Diets in IBD
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Shouval, D.S.; Rufo, P.A. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017, 171, 999–1005. [Google Scholar] [CrossRef]
- Cohen, N.A.; Rubin, D.T. New targets in inflammatory bowel disease therapy: 2021. Curr. Opin. Gastroenterol. 2021, 37, 357–363. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022, 7, 1128–1140. [Google Scholar] [CrossRef]
- Teo, K.; Chow, C.K.; Vaz, M.; Rangarajan, S.; Yusuf, S. PURE Investigators-Writing Group. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am. Heart J. 2009, 158, 1–7.e1. [Google Scholar] [CrossRef]
- Lo, C.-H.; Khandpur, N.; Rossato, S.L.; Lochhead, P.; Lopes, E.W.; Burke, K.E.; Richter, J.M.; Song, M.; Ardisson Korat, A.V.; Sun, Q.; et al. Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1323–e1337. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Meyer, A.; Dong, C.; Casagrande, C.; Chan, S.; Huybrechts, I.; Nicolas, G.; Rauber, F.; Levy, R.B.; Millett, C.; Oldenburg, B.; et al. Food processing and risk of Crohn’s disease and ulcerative colitis: A European Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, in press. [Google Scholar] [CrossRef]
- Vasseur, P.; Dugelay, E.; Benamouzig, R.; Savoye, G.; Lan, A.; Srour, B.; Hercberg, S.; Touvier, M.; Hugot, J.-P.; Julia, C.; et al. Dietary Patterns, Ultra-processed Food, and the Risk of Inflammatory Bowel Diseases in the NutriNet-Santé Cohort. Inflamm. Bowel Dis. 2021, 27, 65–73. [Google Scholar] [CrossRef]
- Rashvand, S.; Behrooz, M.; Samsamikor, M.; Jacobson, K.; Hekmatdoost, A. Dietary patterns and risk of ulcerative colitis: A case-control study. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2018, 31, 408–412. [Google Scholar] [CrossRef]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of ultra-processed foods is associated with an increased risk of Crohn’s disease: A cross-sectional and prospective analysis of 187,154 participants in the UK Biobank. J. Crohn’s Colitis 2022, jjac167. [Google Scholar] [CrossRef]
- Liu, C.; Zhan, S.; Tian, Z.; Li, N.; Li, T.; Wu, D.; Zeng, Z.; Zhuang, X. Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies? Nutrients 2022, 14, 3049. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef]
- Raoul, P.; Cintoni, M.; Palombaro, M.; Basso, L.; Rinninella, E.; Gasbarrini, A.; Mele, M.C. Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis. Microorganisms 2022, 10, 167. [Google Scholar] [CrossRef]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers Impact Colonic Length in Mice and Emulsifier Restriction is Feasible in People with Crohn’s Disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—A pilot study. J. Crohn’s Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef]
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota with Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled, Cross-over, Re-Challenge Trial. J. Crohn’s Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef]
- Kakodkar, S.; Mutlu, E.A. Diet as a Therapeutic Option for Adult Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 745–767. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192. [Google Scholar] [CrossRef]
- Gudmand-Hoyer, E.; Jarnum, S. Incidence and clinical significance of lactose malabsorption in ulcerative colitis and Crohn’s disease. Gut 1970, 11, 338–343. [Google Scholar] [CrossRef]
- Asfari, M.M.; Sarmini, M.T.; Kendrick, K.; Hudgi, A.; Uy, P.; Sridhar, S.; Sifuentes, H. Association between Inflammatory Bowel Disease and Lactose Intolerance: Fact or Fiction. Korean J. Gastroenterol. 2020, 76, 185–190. [Google Scholar] [CrossRef]
- Szilagyi, A.; Galiatsatos, P.; Xue, X. Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr. J. 2016, 15, 67. [Google Scholar] [CrossRef]
- Shah, A.; Walker, M.; Burger, D.; Martin, N.; Von Wulffen, M.; Koloski, N.; Jones, M.; Talley, N.J.; Holtmann, G.J. Link Between Celiac Disease and Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2019, 53, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Bar Yehuda, S.; Axlerod, R.; Toker, O.; Zigman, N.; Goren, I.; Mourad, V.; Lederman, N.; Cohen, N.; Matz, E.; Dushnitzky, D.; et al. The Association of Inflammatory Bowel Diseases with Autoimmune Disorders: A Report from the epi-IIRN. J. Crohn’s Colitis 2019, 13, 324–329. [Google Scholar] [CrossRef]
- Casella, G.; D’Incà, R.; Oliva, L.; Daperno, M.; Saladino, V.; Zoli, G.; Annese, V.; Fries, W.; Cortellezzi, C. Prevalence of celiac disease in inflammatory bowel diseases: An IG-IBD multicentre study. Dig. Liver Dis. 2010, 42, 175–178. [Google Scholar] [CrossRef]
- Jandaghi, E.; Hojatnia, M.; Vahedi, H.; Shahbaz-Khani, B.; Kolahdoozan, S.; Ansari, R. Is the Prevalence of Celiac Disease Higher than the General Population in Inflammatory Bowel Diseaese? Middle East J. Dig. Dis. 2015, 7, 82–87. [Google Scholar]
- Leeds, J.S.; Höroldt, B.S.; Sidhu, R.; Hopper, A.D.; Robinson, K.; Toulson, B.; Dixon, L.; Lobo, A.J.; McAlindon, M.E.; Hurlstone, D.P.; et al. Is there an association between coeliac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand. J. Gastroenterol. 2007, 42, 1214–1220. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Sepulveda, R.; Hing, T.; Shah, N.D.; Choe, M.; Limsui, D.; Shah, S. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 147–151. [Google Scholar] [CrossRef]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef]
- Powell, J.J.; Ainley, C.C.; Harvey, R.S.; Mason, I.M.; Kendall, M.D.; Sankey, E.A.; Dhillon, A.P.; Thompson, R.P. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut 1996, 38, 390–395. [Google Scholar] [CrossRef]
- Lomer, M.C.E.; Thompson, R.P.H.; Powell, J.J. Fine and ultrafine particles of the diet: Influence on the mucosal immune response and association with Crohn’s disease. Proc. Nutr. Soc. 2002, 61, 123–130. [Google Scholar] [CrossRef]
- Ashwood, P.; Thompson, R.P.; Powell, J.J. Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Exp. Biol. Med. 2007, 232, 107–117. Available online: http://pubmed.ncbi.nlm.nih.gov/17202591/ (accessed on 31 October 2022).
- Evans, S.M.; Ashwood, P.; Warley, A.; Berisha, F.; Thompson, R.P.H.; Powell, J.J. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology 2002, 123, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Harvey, R.S.; Evans, S.M.; Thompson, R.P.; Powell, J.J. Efficacy and tolerability of a low microparticle diet in a double blind, randomized, pilot study in Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2001, 13, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.E.; Grainger, S.L.; Ede, R.; Catterall, A.P.; Greenfield, S.M.; Cowan, R.E.; Vicary, F.R.; Jenkins, A.P.; Fidler, H.; Harvey, R.S.; et al. Lack of efficacy of a reduced microparticle diet in a multi-centred trial of patients with active Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2005, 17, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Turpin, W.; Dong, M.; Sasson, G.; Garay, J.A.; Espin-Garcia, O.; Lee, S.H.; Neustaeter, A.; Smith, M.I.; Leibovitzh, H.; Guttman, D.S.; et al. Mediterranean-Like Dietary Pattern Associations with Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163, 685–698. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Vilović, M.; Živković, P.M.; Hadjina, I.T.; Rušić, D.; Bukić, J.; Borovac, J.A.; Božić, J. Mediterranean Diet Adherence and Dietary Attitudes in Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 3429. [Google Scholar] [CrossRef]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. Available online: https://gut.bmj.com/content/69/9/1637 (accessed on 19 November 2022). [CrossRef] [PubMed]
- Fiorindi, C.; Dinu, M.; Gavazzi, E.; Scaringi, S.; Ficari, F.; Nannoni, A.; Sofi, F.; Giudici, F. Adherence to mediterranean diet in patients with inflammatory bowel disease. Clin. Nutr. ESPEN 2021, 46, 416–423. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; Elashry, H.; Salamah, A.; Maher, S.; Abd-Elsalam, S.M.; Hasan, S. Adherence to the Mediterranean Diet Improved Clinical Scores and Inflammatory Markers in Children with Active Inflammatory Bowel Disease: A Randomized Trial. J. Inflamm. Res. 2022, 15, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, E. Breaking the Vicious Cycle: Intestinal Health through Diet; Kirkton Press: Baltimore, ON, Canada, 1994. [Google Scholar]
- Braly, K.; Williamson, N.; Shaffer, M.L.; Lee, D.; Wahbeh, G.; Klein, J.; Giefer, M.; Suskind, D.L. Nutritional Adequacy of the Specific Carbohydrate Diet in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Gregory, N.; Vendettuoli, H.; Christie, D. Nutritional Therapy in Pediatric Crohn Disease: The Specific Carbohydrate Diet. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 87–91. [Google Scholar] [CrossRef]
- Wahbeh, G.T.; Ward, B.T.; Lee, D.Y.; Giefer, M.J.; Suskind, D.L. Lack of Mucosal Healing from Modified Specific Carbohydrate Diet in Pediatric Patients with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 289–292. [Google Scholar] [CrossRef]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and Mucosal Improvement with Specific Carbohydrate Diet in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef]
- Suskind, D.L.; Lee, D.; Kim, Y.M.; Wahbeh, G.; Singh, N.; Braly, K.; Nuding, M.; Nicora, C.D.; Purvine, S.O.; Lipton, M.S.; et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients 2020, 12, 3749. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Sandler, R.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults with Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Gu, Y.; Hou, H.; Liu, T.; Ding, Y.; Cao, H. Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients 2022, 14, 4003. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The Therapeutic Role of Short-Chain Fatty Acids Mediated Very Low-Calorie Ketogenic Diet–Gut Microbiota Relationships in Paediatric Inflammatory Bowel Diseases. Nutrients 2022, 14, 4113. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M.F.; Goel, A.; Qin, H.; et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct. Target. Ther. 2021, 6, 154. [Google Scholar] [CrossRef]
- Li, S.; Zhuge, A.; Wang, K.; Lv, L.; Bian, X.; Yang, L.; Xia, J.; Jiang, X.; Wu, W.; Wang, S.; et al. Ketogenic diet aggravates colitis, impairs intestinal barrier and alters gut microbiota and metabolism in DSS-induced mice. Food Funct. 2021, 12, 10210–10225. [Google Scholar] [CrossRef]
- Trakman, G.L.; Fehily, S.; Basnayake, C.; Hamilton, A.L.; Russell, E.; Wilson-O’Brien, A.; Kamm, M.A. Diet and gut microbiome in gastrointestinal disease. J. Gastroenterol. Hepatol. 2022, 37, 237–245. [Google Scholar] [CrossRef]
- Tracy, M.; Khalili, H. You Are What You Eat? Growing Evidence That Diet Influences the Risk of Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, jjac025. [Google Scholar] [CrossRef]
- Chiba, M.; Ishii, H.; Komatsu, M. Recommendation of plant-based diets for inflammatory bowel disease. Transl. Pediatr. 2019, 8, 23–27. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Tsuji, T.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Obara, Y.; Komatsu, M.; Sugawara, T. Relapse Prevention by Plant-Based Diet Incorporated into Induction Therapy for Ulcerative Colitis: A Single-Group Trial. Perm. J. 2019, 23, 18–220. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Obara, Y.; Komatsu, M.; Tozawa, H. High Remission Rate with Infliximab and Plant-Based Diet as First-Line (IPF) Therapy for Severe Ulcerative Colitis: Single-Group Trial. Perm. J. 2020, 24, 19.166. [Google Scholar] [CrossRef]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Sheil, B.; Shanahan, F.; O’Mahony, L. Probiotic effects on inflammatory bowel disease. J. Nutr. 2007, 137 (Suppl. 2), 819S–824S. [Google Scholar] [CrossRef]
- Schwiertz, A.; Jacobi, M.; Frick, J.S.; Richter, M.; Rusch, K.; Köhler, H. Microbiota in pediatric inflammatory bowel disease. J. Pediatr. 2010, 157, 240–244.e1. [Google Scholar] [CrossRef]
- Osterman, M.T. Mucosal healing in inflammatory bowel disease. J. Clin. Gastroenterol. 2013, 47, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.; Bucci, V.; Cawley, C.; Maserati, R.; McManus, M.; Olednzki, E.; Madziar, C.; Chiang, D.; Ward, D.V.; Pellish, R.; et al. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes 2022, 14, 2046244. [Google Scholar] [CrossRef] [PubMed]
- Keshteli, A.H.; Valcheva, R.; Nickurak, C.; Park, H.; Mandal, R.; van Diepen, K.; Kroeker, K.I.; van Zanten, S.V.; Halloran, B.; Wishart, D.S.; et al. Anti-Inflammatory Diet Prevents Subclinical Colonic Inflammation and Alters Metabolomic Profile of Ulcerative Colitis Patients in Clinical Remission. Nutrients 2022, 14, 3294. [Google Scholar] [CrossRef]
- Kedia, S.; Virmani, S.; K Vuyyuru, S.; Kumar, P.; Kante, B.; Sahu, P.; Kaushal, K.; Farooqui, M.; Singh, M.; Verma, M.; et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: A randomised controlled trial. Gut 2022, 71, 2401–2413. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Yusuf, K.; Saha, S.; Umar, S. Health Benefits of Dietary Fiber for the Management of Inflammatory Bowel Disease. Biomedicines 2022, 10, 1242. [Google Scholar] [CrossRef]
- O’Mahony, C.; Amamou, A.; Ghosh, S. Diet-Microbiota Interplay: An Emerging Player in Macrophage Plasticity and Intestinal Health. Int. J. Mol. Sci. 2022, 23, 3901. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jarr, K.; Layton, C.; Gardner, C.D.; Ashouri, J.F.; Abreu, M.T.; Sinha, S.R. Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases. Nutrients 2021, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Takeda, T.; Takada, Y.; Kishi, M.; Beppu, T.; Takatsu, N.; Miyaoka, M.; Hisabe, T.; Yao, K.; Ueki, T. Efficacy of enteral nutrition in patients with Crohn’s disease on maintenance anti-TNF-alpha antibody therapy: A meta-analysis. J. Gastroenterol. 2020, 55, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Benoist, S.; Maggiori, L.; Zerbib, P.; Lefevre, J.H.; Denost, Q.; Germain, A.; Cotte, E.; Beyer-Berjot, L.; Corte, H.; et al. Impact of preoperative enteral nutritional support on postoperative outcome in patients with Crohn’s disease complicated by malnutrition: Results of a subgroup analysis of the nationwide cohort registry from the GETAID Chirurgie group. Colorectal. Dis. 2021, 23, 1451–1462. [Google Scholar] [CrossRef]
- Meade, S.; Patel, K.V.; Luber, R.P.; O’Hanlon, D.; Caracostea, A.; Pavlidis, P.; Honap, S.; Anandarajah, C.; Griffin, N.; Zeki, S.; et al. A retrospective cohort study: Pre-operative oral enteral nutritional optimisation for Crohn’s disease in a UK tertiary IBD centre. Aliment. Pharm. Ther. 2022, 56, 646–663. [Google Scholar] [CrossRef]
- Heerasing, N.; Thompson, B.; Hendy, P.; Heap, G.A.; Walker, G.; Bethune, R.; Mansfield, S.; Calvert, C.; Kennedy, N.A.; Ahmad, T.; et al. Exclusive enteral nutrition provides an effective bridge to safer interval elective surgery for adults with Crohn’s disease. Aliment. Pharm. Ther. 2017, 45, 660–669. [Google Scholar] [CrossRef]
- Reduced Sulfur Diet in Ulcerative Colitis Patients (UCS). Identifier: NCT04474561. Available online: https://clinicaltrials.gov/ct2/show/NCT04474561 (accessed on 12 December 2022).
- Teigen, L.M.; Geng, Z.; Sadowsky, M.J.; Vaughn, B.P.; Hamilton, M.J.; Khoruts, A. Dietary Factors in Sulfur Metabolism and Pathogenesis of Ulcerative Colitis. Nutrients 2019, 11, 931. [Google Scholar] [CrossRef]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Sung-Ho Kim, J.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented β-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology 2022, in press. [Google Scholar] [CrossRef]
- Personalized B-fructan Diet in Inflammatory Bowel Disease Patients. Identifier: NCT056157791. Available online: https://clinicaltrials.gov/ct2/show/NCT05615779 (accessed on 12 December 2022).
- Sugimoto, M.; Watanabe, T.; Takaoka, M.; Suzuki, K.; Murakami, T.; Murakami, N.; Sumikawa, S. Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation. Fermentation 2021, 7, 55. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Fermented Food-Supplemented Diet in Ulcerative Colitis. Identifier: NCT04401605. Available online: https://clinicaltrials.gov/ct2/show/NCT04401605 (accessed on 12 December 2022).
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Noguchi, R.; Takahashi, D.; Morikawa, T.; Koshida, K.; Komiyama, S.; Ishihara, N.; Yamada, T.; Kawamura, Y.I.; Muroi, K.; et al. Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell 2019, 178, 1072–1087.e14. [Google Scholar] [CrossRef] [PubMed]
- The Influence of a Fasting Mimicking Diet on Ulcerative Colitis. Identifier: NCT03615690. Available online: https://clinicaltrials.gov/ct2/show/NCT03615690 (accessed on 12 December 2022).
- Effects of an Intermittent Reduced Calorie Diet on Crohn’s Disease. Identifier: NCT04147585. Available online: https://clinicaltrials.gov/ct2/show/NCT04147585 (accessed on 12 December 2022).
- The Impact of Time Restricted Feeding in Crohn’s Disease (TRF-CD). Identifier: NCT04271748. Available online: https://clinicaltrials.gov/ct2/show/NCT04271748 (accessed on 12 December 2022).
| Dietary Patterns | Description of Diet |
|---|---|
| Ultra-processed foods (UPF) | Ready-to-consume foods, typically created by industrial processes. Rich in additives (sweeteners, emulsifiers, flavors). Examples of UPFs include bacon, brownies, and soda. |
| FODMAP diet | Eliminates short-chain carbohydrates that are poorly absorbed in the small bowel (Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols). Examples of FODMAPs include dairy, beans, cherries, and onions. |
| Carrageenan-free Diet | Eliminates carrageenan, a highly sulfated polysaccharide food additive commonly added to dairy products (ice cream, yogurt) and sandwich meats. |
| Gluten-free diet (GFD) | Eliminates gluten, a structural protein found in grains. Examples of gluten-containing foods include bread, beer, and soy sauce |
| Lactose-free diet | Eliminates lactose, a disaccharide protein found in dairy. Examples of lactose-containing foods include milk, cheese, and butter. |
| Low microparticle diet | Diet that eliminates additives with particle size <1 uM, such as titanium dioxide. Examples of microparticle-containing foods include powdered sugar and processed dairy. |
| Mediterranean diet (MD) | High consumption of olive oil, vegetables, fruits, nuts; moderate consumption of fish and dairy; limited meat and unsaturated fats. |
| Specific carbohydrate diet (SCD) | Allows non-starchy vegetables, fruits, unprocessed meats. No grains, processed meats or sugars, or starchy vegetables (such as yams). |
| Ketogenic diet | Low carbohydrate, high fat diet that induces ketosis. Favors meats, eggs, dairy, and non-starchy vegetables. |
| Plant-based diet | Diet that eliminates or minimizes non-plant-based foods; not necessarily entirely vegetarian or vegan. |
| Anti-inflammatory diet (AID) | Eliminates processed carbohydrates, increases n-3-polyunsaturated fatty acids, and decreases n-6-polyunsaturated fatty acids. Examples of disallowed foods includes cured meats, fruit juices, and many dairy items. |
| High fiber diet | Diet enriched with fruits, vegetables, legumes, and nuts. Generally above 30 g of fiber per day. |
| Exclusive enteral nutrition (EEN) | Nutrition exclusively through liquid formula. Only water is allowed beyond the formula-based diet. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubatan, J.; Kulkarni, C.V.; Talamantes, S.M.; Temby, M.; Fardeen, T.; Sinha, S.R. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients 2023, 15, 579. https://doi.org/10.3390/nu15030579
Gubatan J, Kulkarni CV, Talamantes SM, Temby M, Fardeen T, Sinha SR. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients. 2023; 15(3):579. https://doi.org/10.3390/nu15030579
Chicago/Turabian StyleGubatan, John, Chiraag V. Kulkarni, Sarah Melissa Talamantes, Michelle Temby, Touran Fardeen, and Sidhartha R. Sinha. 2023. "Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts" Nutrients 15, no. 3: 579. https://doi.org/10.3390/nu15030579
APA StyleGubatan, J., Kulkarni, C. V., Talamantes, S. M., Temby, M., Fardeen, T., & Sinha, S. R. (2023). Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients, 15(3), 579. https://doi.org/10.3390/nu15030579





