Matcha Tea Powder’s Antidepressant-like Effect through the Activation of the Dopaminergic System in Mice Is Dependent on Social Isolation Stress
Abstract
:1. Introduction
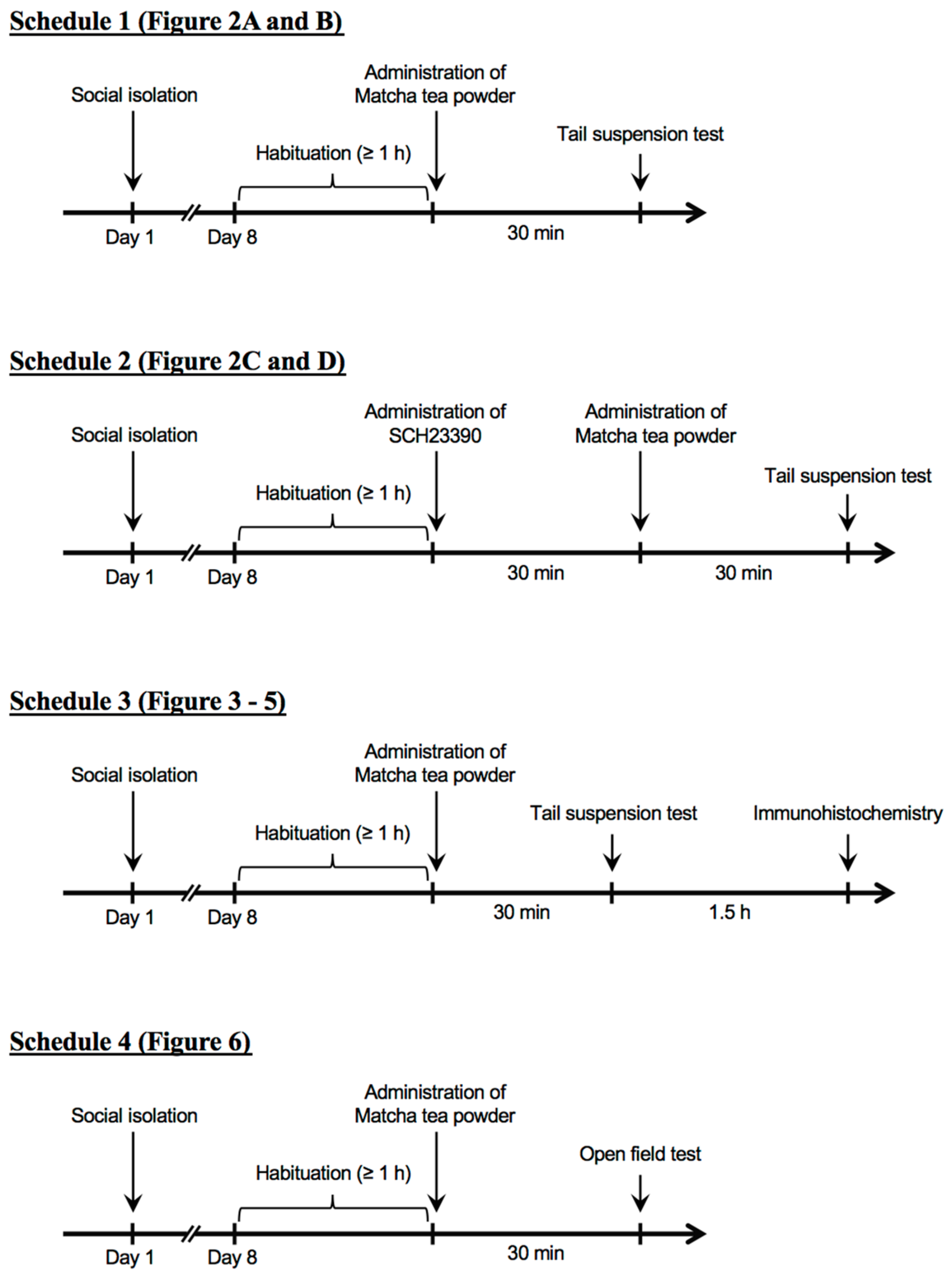
2. Materials and Methods
2.1. Animals
2.2. Preparation of Matcha
2.3. Dosage Information
2.4. Administration of SCH23390
2.5. Tail Suspension Test
2.6. Open Field Test
2.7. Immunohistochemistry
2.8. Statistical Analysis
3. Results
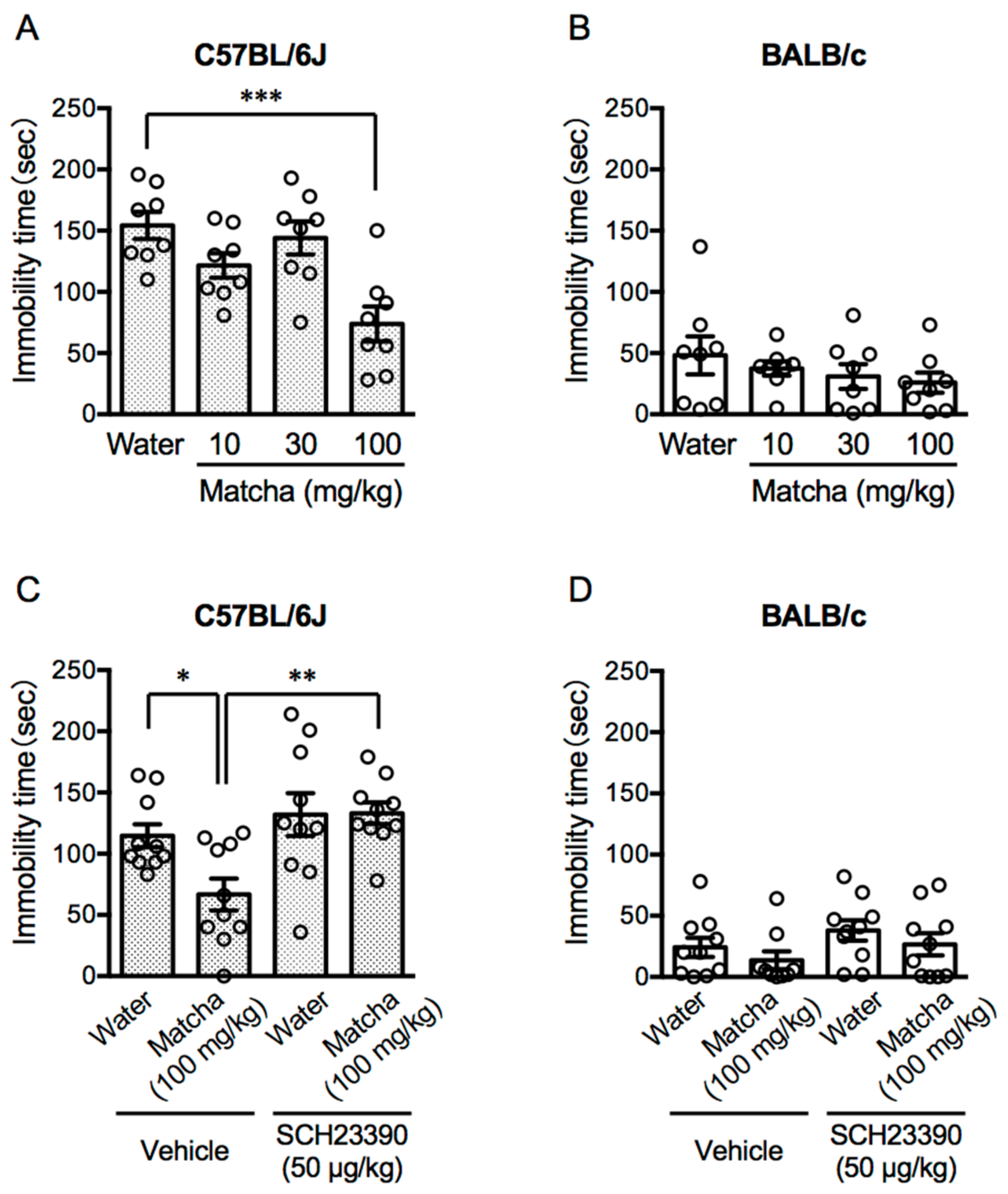
3.1. Activation of the Dopamine D1 Receptor Contributes to the Antidepressant-like Effects of Matcha Tea Powder in C57BL/6J Mice
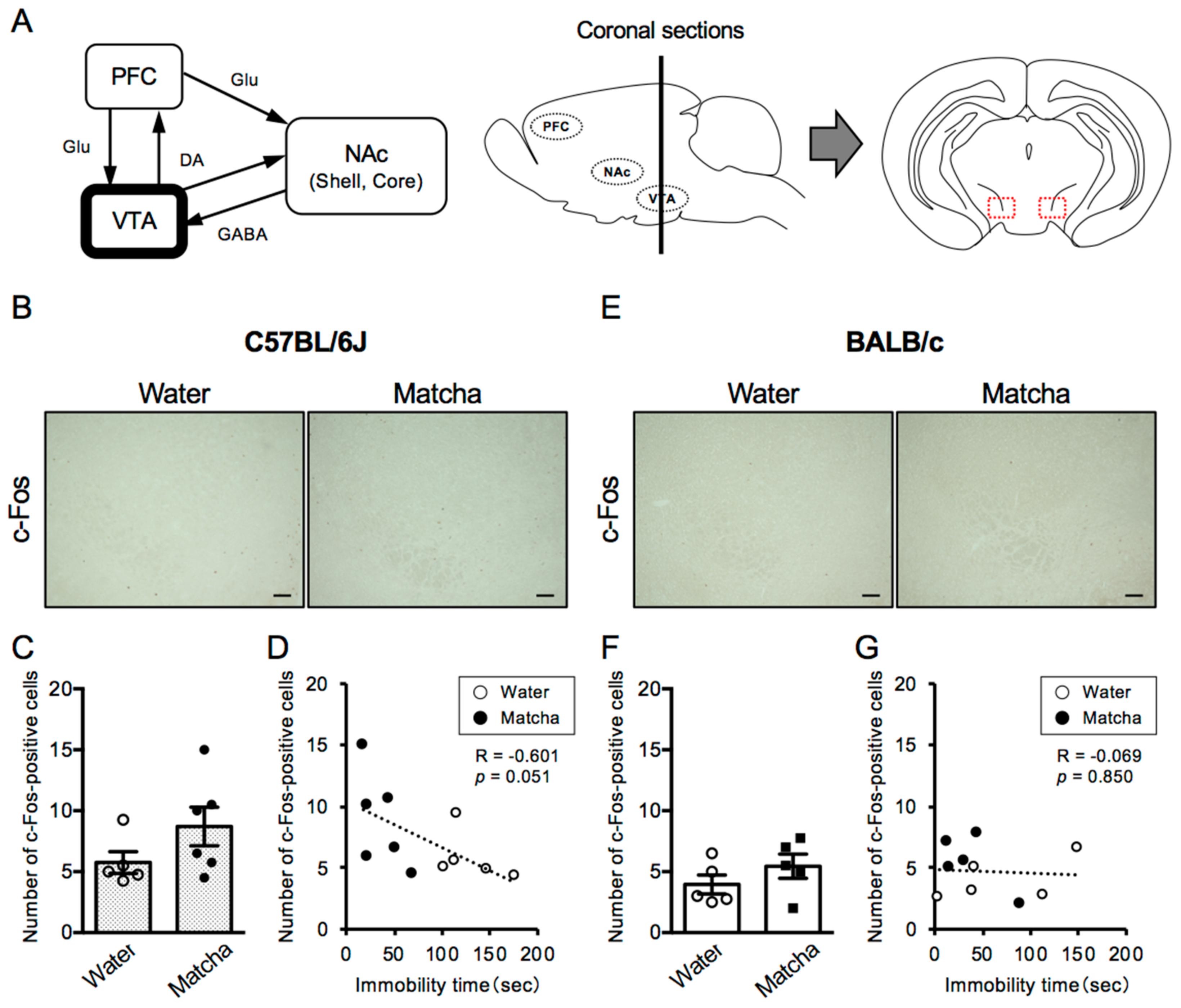
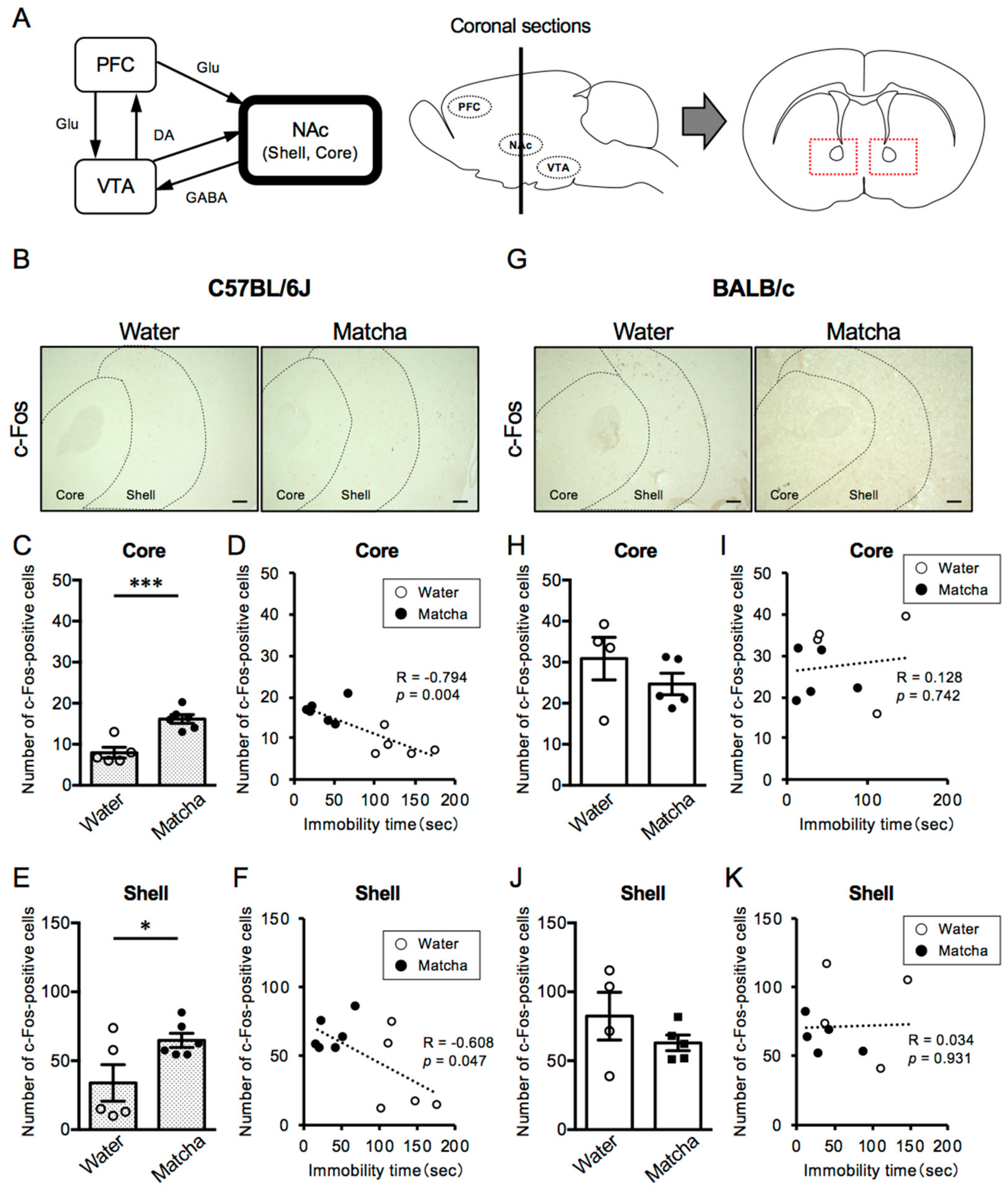
3.2. Antidepressive Effect of Matcha Tea Powder Correlates with Neural Activity in the VTA Region in C57BL/6J Mice
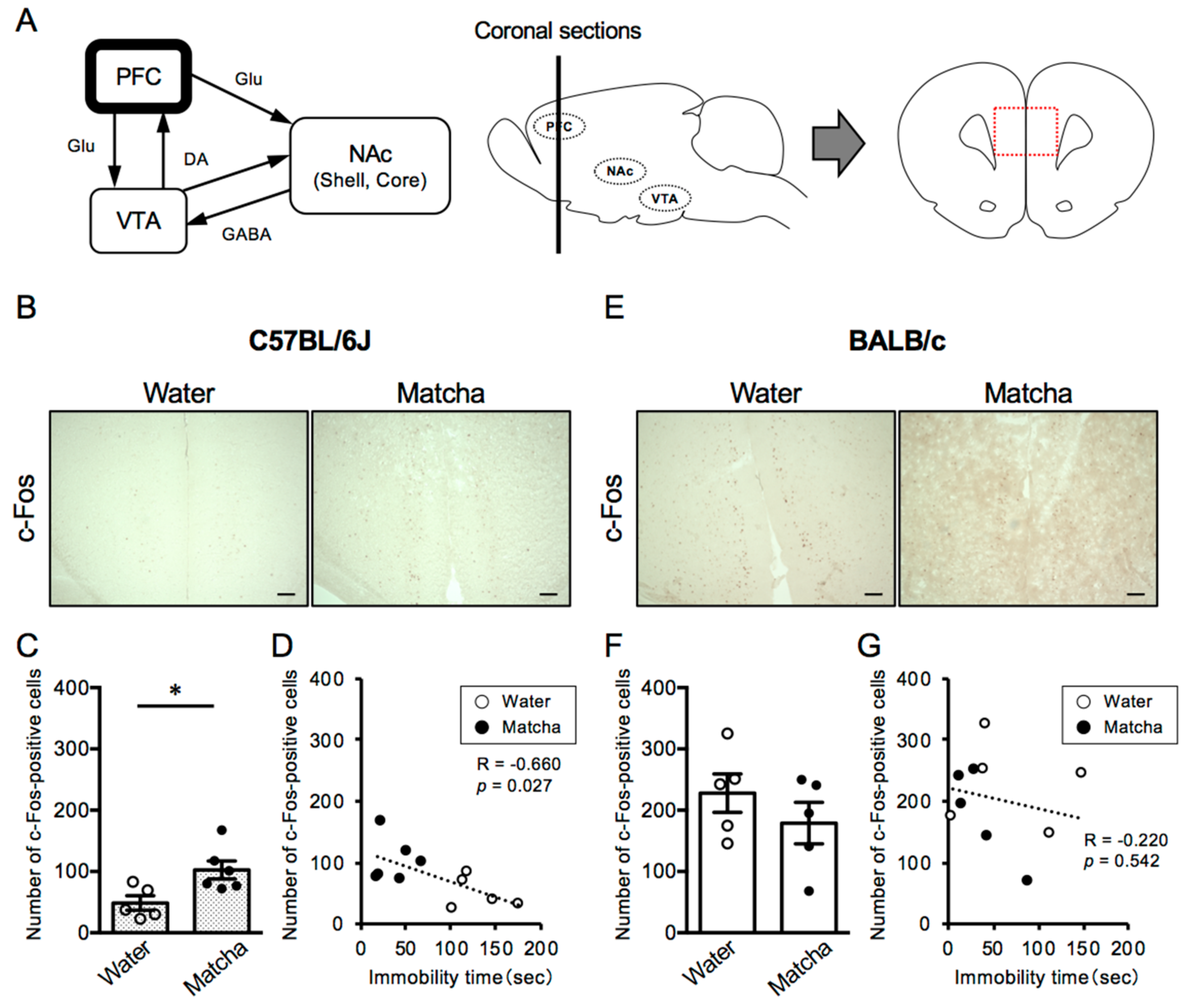
3.3. Matcha Tea Powder Increased Neural Activity in the PFC Region in C57BL/6J Mice
3.4. Matcha Tea Powder Increased Neural Activity in the NAc Region in C57BL/6J Mice
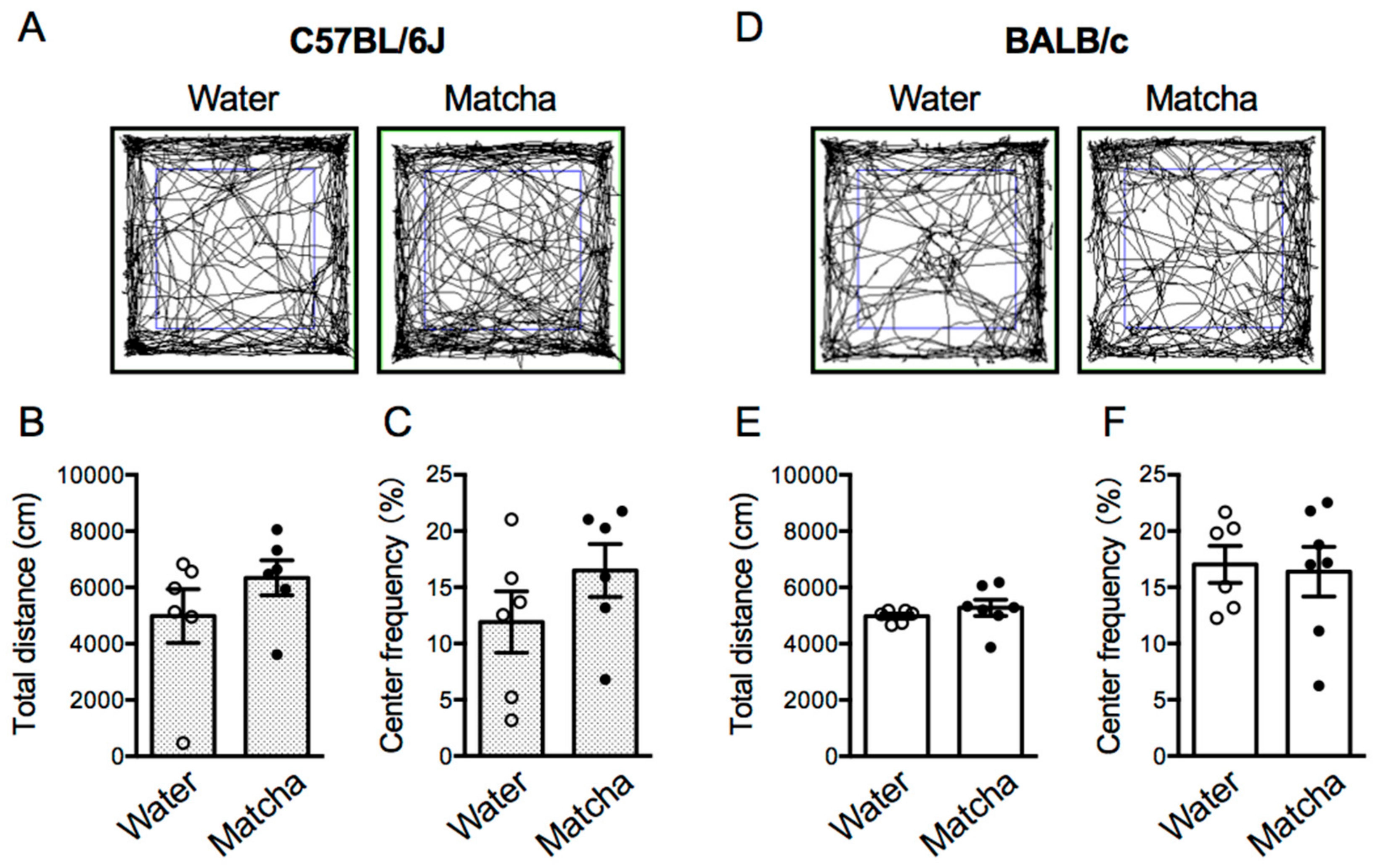
3.5. Matcha Tea Powder Did Not Increase Locomotor Activity in Mice
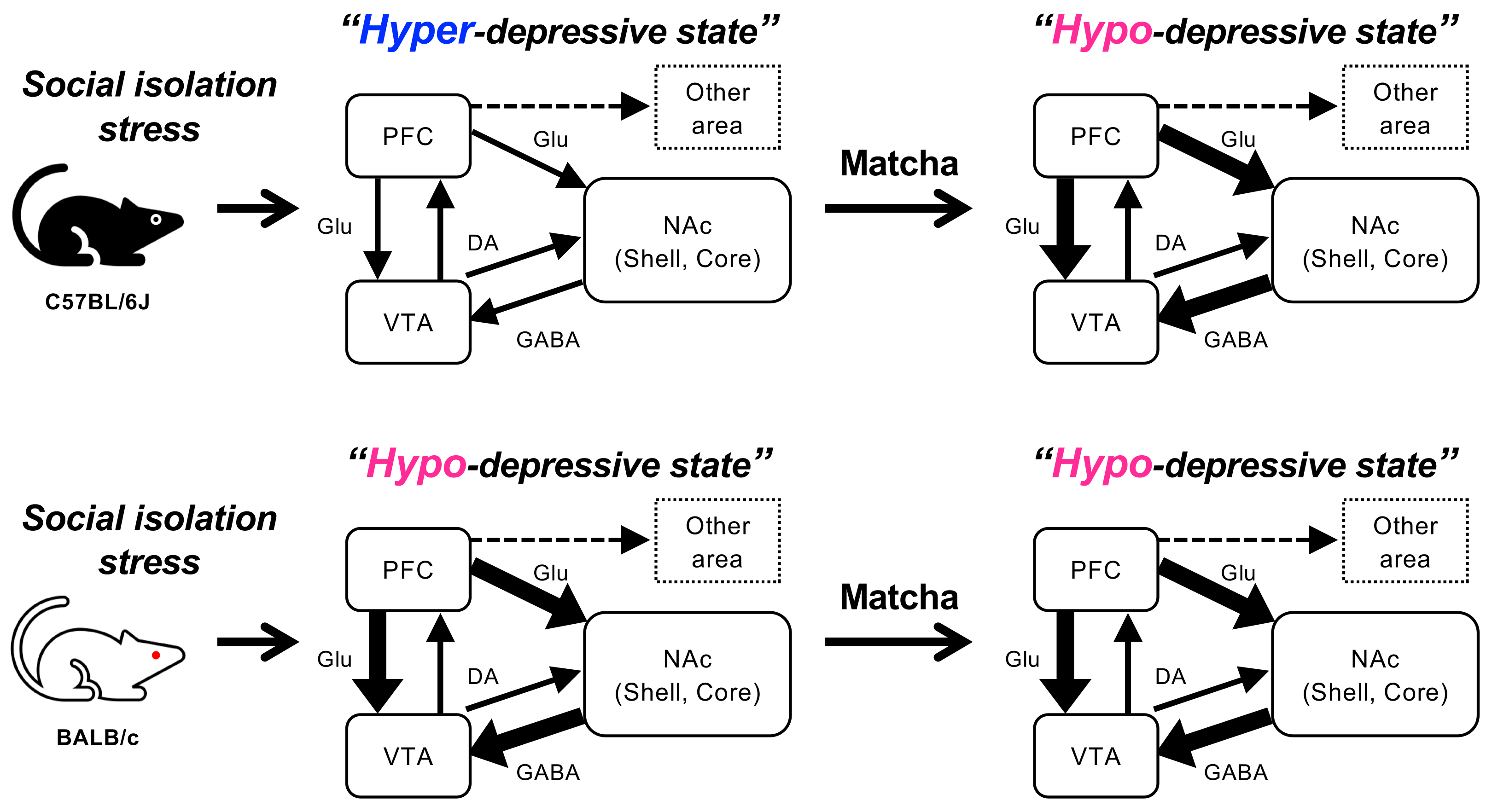
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 5 December 2022).
- Cannon, D.M.; Klaver, J.M.; Peck, S.A.; Rallis-Voak, D.; Erickson, K.; Drevets, W.C. Dopamine Type-1 Receptor Binding in Major Depressive Disorder Assessed Using Positron Emission Tomography and [11C]NNC-112. Neuropsychopharmacology 2009, 34, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- D’Aquila, P.S.; Collu, M.; Pani, L.; Gessa, G.L.; Serra, G. Antidepressant-like Effect of Selective Dopamine D1 Receptor Agonists in the Behavioural Despair Animal Model of Depression. Eur. J. Pharmacol. 1994, 262, 107–111. [Google Scholar] [CrossRef]
- Dai, W.; Feng, K.; Sun, X.; Xu, L.; Wu, S.; Rahmand, K.; Jia, D.; Han, T. Natural Products for the Treatment of Stress-Induced Depression: Pharmacology, Mechanism and Traditional Use. J. Ethnopharmacol. 2022, 285, 114692. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shen, C.Y.; Jiang, J.G. Antidepressant Active Ingredients from Herbs and Nutraceuticals Used in TCM: Pharmacological Mechanisms and Prospects for Drug Discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Perviz, S.; Sureda, A.; Nabavi, S.M.; Tejada, S. Current Standing of Plant Derived Flavonoids as an Antidepressant. Food Chem. Toxicol. 2018, 119, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Devkota, H.P.; Gaire, B.P.; Hori, K.; Subedi, L.; Adhikari-Devkota, A.; Belwal, T.; Paudel, K.R.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; et al. The Science of Matcha: Bioactive Compounds, Analytical Techniques and Biological Properties. Trends Food Sci. Technol. 2021, 118, 735–743. [Google Scholar] [CrossRef]
- Bouayed, J. Polyphenols: A Potential New Strategy for the Prevention and Treatment of Anxiety and Depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Lao, L.; Lin, Y.-W.; Litscher, G. Complementary and Alternative Medicine for the Treatment of Central Nervous System Disorders. Evid.-Based Complement. Altern. Med. 2014, 2014, 175152. [Google Scholar] [CrossRef]
- Smith, A. Effects of Caffeine on Human Behavior. Food Chem. Toxicol. 2002, 40, 1243–1255. [Google Scholar] [CrossRef]
- Shi, S.-N.; Shi, J.-L.; Liu, Y.; Wang, Y.-L.; Wang, C.-G.; Hou, W.-H.; Guo, J.-Y. The Anxiolytic Effects of Valtrate in Rats Involves Changes of Corticosterone Levels. Evid.-Based Complement. Altern. Med. 2014, 2014, 325948. [Google Scholar] [CrossRef]
- Trebatická, J.; Ďuračková, Z. Psychiatric Disorders and Polyphenols: Can They Be Helpful in Therapy? Oxid. Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignes, M.; Maurice, T.; Lanté, F.; Nedjar, M.; Thethi, K.; Guiramand, J.; Récasens, M. Anxiolytic Properties of Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG). Brain Res. 2006, 1110, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Stough, C.; Farrimond, J.; Scholey, A.B. Acute Effects of Tea Constituents L-Theanine, Caffeine, and Epigallocatechin Gallate on Cognitive Function and Mood: A Systematic Review and Meta-Analysis. Nutr. Rev. 2014, 72, 507–522. [Google Scholar] [CrossRef]
- Childs, E.; De Wit, H. Subjective, Behavioral, and Physiological Effects of Acute Caffeine in Light, Nondependent Caffeine Users. Psychopharmacology 2006, 185, 514–523. [Google Scholar] [CrossRef]
- Dodd, F.L.; Kennedy, D.O.; Riby, L.M.; Haskell-Ramsay, C.F. A Double-Blind, Placebo-Controlled Study Evaluating the Effects of Caffeine and L-Theanine Both Alone and in Combination on Cerebral Blood Flow, Cognition and Mood. Psychopharmacology 2015, 232, 2563–2576. [Google Scholar] [CrossRef] [Green Version]
- Janet, B. Psychological Effects of Dietary Components of Tea- Caffeine and L-Theanine. Nutr. Rev. 2008, 66, 82–90. [Google Scholar] [CrossRef]
- Rogers, P.J.; Smith, J.E.; Heatherley, S.V.; Pleydell-Pearce, C.W. Time for Tea: Mood, Blood Pressure and Cognitive Performance Effects of Caffeine and Theanine Administered Alone and Together. Psychopharmacology 2008, 195, 569–577. [Google Scholar] [CrossRef]
- Kurauchi, Y.; Devkota, H.P.; Hori, K.; Nishihara, Y.; Hisatsune, A.; Seki, T.; Katsuki, H. Anxiolytic Activities of Matcha Tea Powder, Extracts, and Fractions in Mice: Contribution of Dopamine D1 Receptor- and Serotonin 5-HT1A Receptor-Mediated Mechanisms. J. Funct. Foods 2019, 59, 301–308. [Google Scholar] [CrossRef]
- Kurauchi, Y.; Yoshimaru, Y.; Kajiwara, Y.; Yamada, T.; Matsuda, K.; Hisatsune, A.; Seki, T.; Katsuki, H. Na+, K+-ATPase Inhibition Causes Hyperactivity and Impulsivity in Mice via Dopamine D2 Receptor-Mediated Mechanism. Neurosci. Res. 2019, 146, 54–64. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. J. Vis. Exp. 2012, 59, 3–7. [Google Scholar] [CrossRef]
- Brinks, V.; van der Mark, M.; de Kloet, R.; Oitzl, M. Emotion and Cognition in High and Low Stress Sensitive Mouse Strains: A Combined Neuroendocrine and Behavioral Study in BALB/c and C57BL/6J Mice. Front. Behav. Neurosci. 2007, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.; Aghajanian, G. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauczyciel, C.; Robic, S.; Dondaine, T.; Verin, M.; Robert, G.; Drapier, D.; Naudet, F.; Millet, B. The Nucleus Accumbens: A Target for Deep Brain Stimulation in Resistant Major Depressive Disorder. J. Mol. Psychiatry 2013, 1, 17. [Google Scholar] [CrossRef] [Green Version]
- Lorenzetti, V.; Allen, N.B.; Fornito, A.; Yücel, M. Structural Brain Abnormalities in Major Depressive Disorder: A Selective Review of Recent MRI Studies. J. Affect. Disord. 2009, 117, 1–17. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Holmes, A.J.; Dillon, D.G.; Goetz, E.L.; Birk, J.L.; Bogdan, R.; Dougherty, D.D.; Iosifescu, D.V.; Rauch, S.L.; Fava, M. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals with Major Depressive Disorder. Am. J. Psychiatry 2009, 166, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Voleti, B. Signaling Pathways Underlying the Pathophysiology and Treatment of Depression: Novel Mechanisms for Rapid-Acting Agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Christoffel, D.J.; Golden, S.A.; Dumitriu, D.; Robison, A.J.; Janssen, W.G.; Ahn, H.F.; Krishnan, V.; Reyes, C.M.; Han, M.H.; Ables, J.L.; et al. IκB Kinase Regulates Social Defeat Stress-Induced Synaptic and Behavioral Plasticity. J. Neurosci. 2011, 31, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariante, C.M.; Lightman, S.L. The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eede, F.; Claes, S.J. Mechanisms of Depression: Role of the HPA Axis. Drug Discov. Today Dis. Mech. 2004, 1, 413–418. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Van Acker, S.A.B.E.; Sibug, R.M.; Oitzl, M.S.; Meijer, O.C.; Rahmouni, K.; De Jong, W. Brain Mineralocorticoid Receptors and Centrally Regulated Functions. Kidney Int. 2000, 57, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klok, M.D.; Alt, S.R.; Irurzun Lafitte, A.J.M.; Turner, J.D.; Lakke, E.A.J.F.; Huitinga, I.; Muller, C.P.; Zitman, F.G.; Ronald de Kloet, E.; DeRijk, R.H. Decreased Expression of Mineralocorticoid Receptor MRNA and Its Splice Variants in Postmortem Brain Regions of Patients with Major Depressive Disorder. J. Psychiatr. Res. 2011, 45, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Y.; Mou, X.; Liu, H.; Ming, H.; Chen, Y.; Liu, Y.; Liu, S. Synergistic Effect of Several Neurotransmitters in PFC-NAc-VTA Neural Circuit for the Anti-Depression Effect of Shuganheweitang in a Chronic Unpredictable Mild Stress Model. Nat. Prod. Commun. 2021, 16, 1934578X211002415. [Google Scholar] [CrossRef]
- Sampaio, T.B.; Bilheri, F.N.; Zeni, G.R.; Nogueira, C.W. Dopaminergic System Contribution to the Antidepressant-like Effect of 3-Phenyl-4-(Phenylseleno) Isoquinoline in Mice. Behav. Brain Res. 2020, 386, 112602. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Pan, X.; Wang, Y.; Xu, X.; Wang, R.; Liu, Z. Dopamine-Mediated Major Depressive Disorder in the Neural Circuit of Ventral Tegmental Area-Nucleus Accumbens-Medial Prefrontal Cortex: From Biological Evidence to Computational Models. Front. Cell. Neurosci. 2022, 16, 923039. [Google Scholar] [CrossRef]
- Demchenko, I.; Tassone, V.K.; Kennedy, S.H.; Dunlop, K.; Bhat, V. Intrinsic Connectivity Networks of Glutamate-Mediated Antidepressant Response: A Neuroimaging Review. Front. Psychiatry 2022, 13, 864902. [Google Scholar] [CrossRef]
- Sakaue, M.; Somboonthum, P.; Nishihara, B.; Koyama, Y.; Hashimoto, H.; Baba, A.; Matsuda, T. Postsynaptic 5-Hydroxytryptamine(1A) Receptor Activation Increases in Vivo Dopamine Release in Rat Prefrontal Cortex. Br. J. Pharmacol. 2000, 129, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Egerton, A.; Mehta, M.A.; Montgomery, A.J.; Lappin, J.M.; Howes, O.D.; Reeves, S.J.; Cunningham, V.J.; Grasby, P.M. The Dopaminergic Basis of Human Behaviors: A Review of Molecular Imaging Studies. Neurosci. Biobehav. Rev. 2009, 33, 1109–1132. [Google Scholar] [CrossRef] [Green Version]
- Francis, T.C.; Lobo, M.K. Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol. Psychiatry 2017, 81, 645–653. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; DiLeone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.K.; Huang, K.W.; Grueter, B.A.; Rothwell, P.E.; Malenka, R.C. Anhedonia Requires MC4R-Mediated Synaptic Adaptations in Nucleus Accumbens. Nature 2012, 487, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Francis, T.C.; Chandra, R.; Friend, D.M.; Finkel, E.; Dayrit, G.; Miranda, J.; Brooks, J.M.; Iñiguez, S.D.; O’Donnell, P.; Kravitz, A.; et al. Nucleus Accumbens Medium Spiny Neuron Subtypes Mediate Depression-Related Outcomes to Social Defeat Stress. Biol. Psychiatry 2015, 77, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Khibnik, L.A.; Beaumont, M.; Doyle, M.; Heshmati, M.; Slesinger, P.A.; Nestler, E.J.; Russo, S.J. Stress and Cocaine Trigger Divergent and Cell Type-Specific Regulation of Synaptic Transmission at Single Spines in Nucleus Accumbens. Biol. Psychiatry 2016, 79, 898–905. [Google Scholar] [CrossRef] [Green Version]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A Review of Caffeine’s Effects on Cognitive, Physical and Occupational Performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokogoshi, H.; Terashima, T.; Mochizuki, M.; Terashima, T. Effect of Theanine, r-Glutamylethylamide, on Brain Monoamines, Striatal Dopamine Release and Some Kinds of Behavior in Rats. Neurochem. Res. 1998, 23, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Terashima, T.; Okubo, T.; Juneja, L.R.; Yokogoshi, H. Effects of Theanine, r-Glutamylethylamide, on Neurotransmitter Release and Its Relationship with Glutamic Acid Neurotransmission. Nutr. Neurosci. 2005, 8, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Gray, M.A.; Oliver, C.; Liley, D.T.; Harrison, B.J.; Bartholomeusz, C.F.; Phan, K.L.; Nathan, P.J. The Acute Effects of L-Theanine in Comparison with Alprazolam on Anticipatory Anxiety in Humans. Hum. Psychopharmacol. 2004, 19, 457–465. [Google Scholar] [CrossRef]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. L-Theanine Reduces Psychological and Physiological Stress Responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Nunes, E.J.; Randall, P.A.; Pardo, M. The Behavioral Pharmacology of Effort-Related Choice Behavior: Dopamine, Adenosine and Beyond. J. Exp. Anal. Behav. 2012, 97, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M.; Farrar, A.; Mingote, S.M. Effort-Related Functions of Nucleus Accumbens Dopamine and Associated Forebrain Circuits. Psychopharmacology 2007, 191, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Parise, E.M.; Peña, C.J.; Zhang, H.X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolaños-Guzmán, C.A.; Cheer, J.; et al. Ventral Hippocampal Afferents to the Nucleus Accumbens Regulate Susceptibility to Depression. Nat. Commun. 2015, 6, 7062. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral Animal Models of Depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurauchi, Y.; Ohta, Y.; Matsuda, K.; Sanematsu, W.; Devkota, H.P.; Seki, T.; Katsuki, H. Matcha Tea Powder’s Antidepressant-like Effect through the Activation of the Dopaminergic System in Mice Is Dependent on Social Isolation Stress. Nutrients 2023, 15, 581. https://doi.org/10.3390/nu15030581
Kurauchi Y, Ohta Y, Matsuda K, Sanematsu W, Devkota HP, Seki T, Katsuki H. Matcha Tea Powder’s Antidepressant-like Effect through the Activation of the Dopaminergic System in Mice Is Dependent on Social Isolation Stress. Nutrients. 2023; 15(3):581. https://doi.org/10.3390/nu15030581
Chicago/Turabian StyleKurauchi, Yuki, Yuki Ohta, Keigo Matsuda, Wakana Sanematsu, Hari Prasad Devkota, Takahiro Seki, and Hiroshi Katsuki. 2023. "Matcha Tea Powder’s Antidepressant-like Effect through the Activation of the Dopaminergic System in Mice Is Dependent on Social Isolation Stress" Nutrients 15, no. 3: 581. https://doi.org/10.3390/nu15030581
APA StyleKurauchi, Y., Ohta, Y., Matsuda, K., Sanematsu, W., Devkota, H. P., Seki, T., & Katsuki, H. (2023). Matcha Tea Powder’s Antidepressant-like Effect through the Activation of the Dopaminergic System in Mice Is Dependent on Social Isolation Stress. Nutrients, 15(3), 581. https://doi.org/10.3390/nu15030581







