The Effect of Hydrolyzed and Fermented Arabinoxylan-Oligo Saccharides (AXOS) Intake on the Middle-Term Gut Microbiome Modulation and Its Metabolic Answer
Abstract
:1. Introduction
2. Materials and Methods
2.1. AXOS Formulations
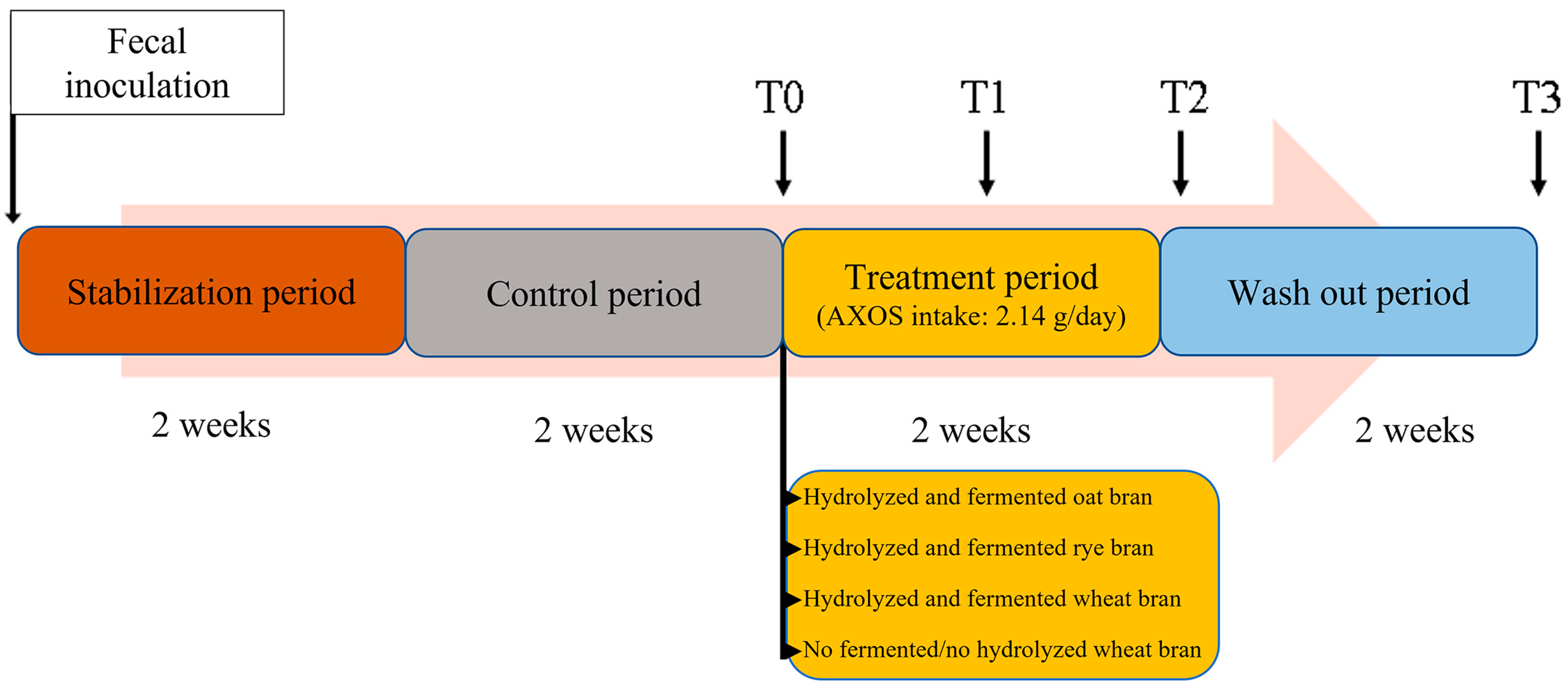
2.2. SHIME® Four-Line Configuration and Experimental Design
2.3. Fecal Donor Selection
2.4. SCFA Quantification
2.5. Microbial Community Analysis
2.6. Statistical Analyses
3. Results
3.1. Fecal Donor Representative of High Adherence to Mediterranean Diet
3.2. SCFA Profiles
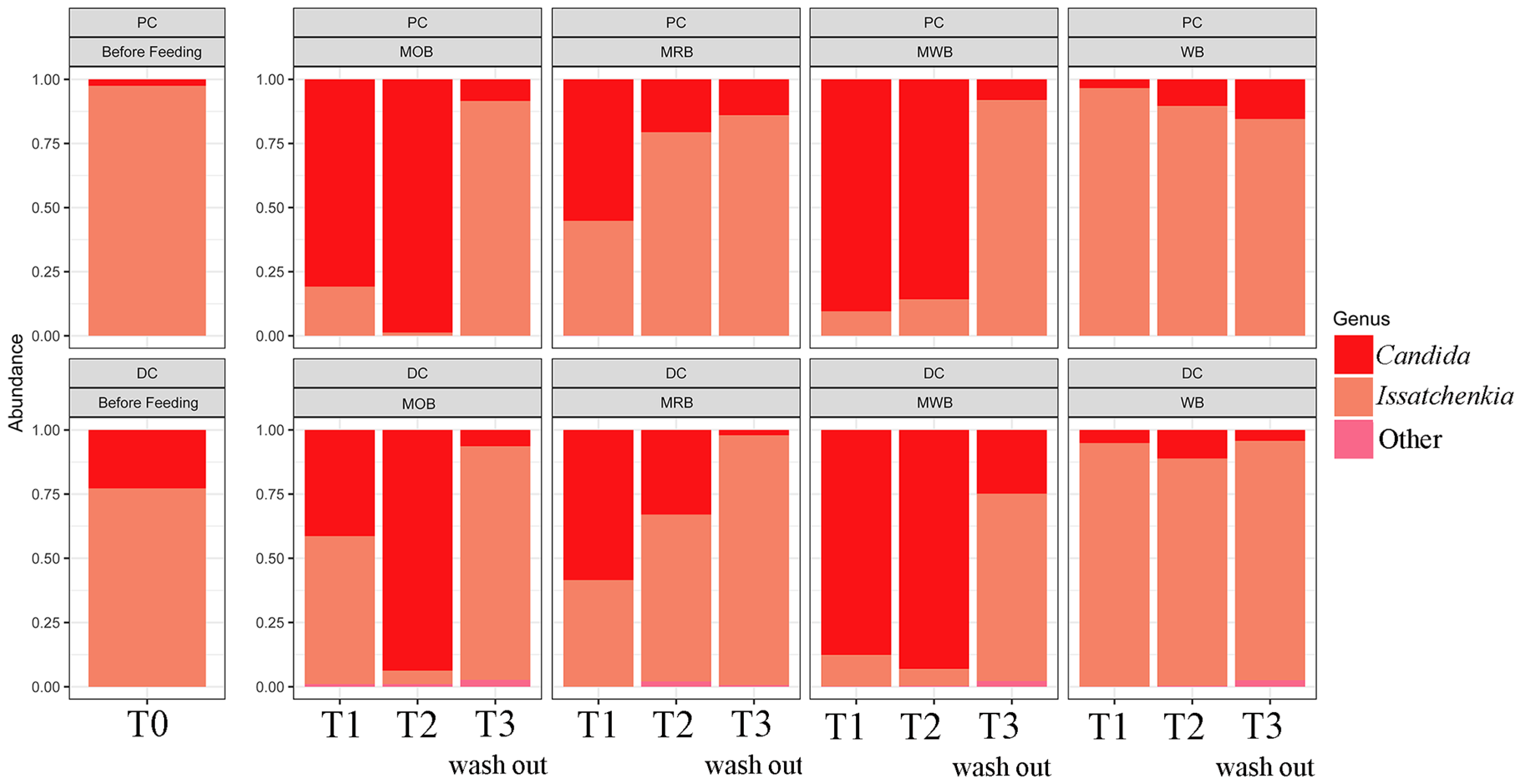
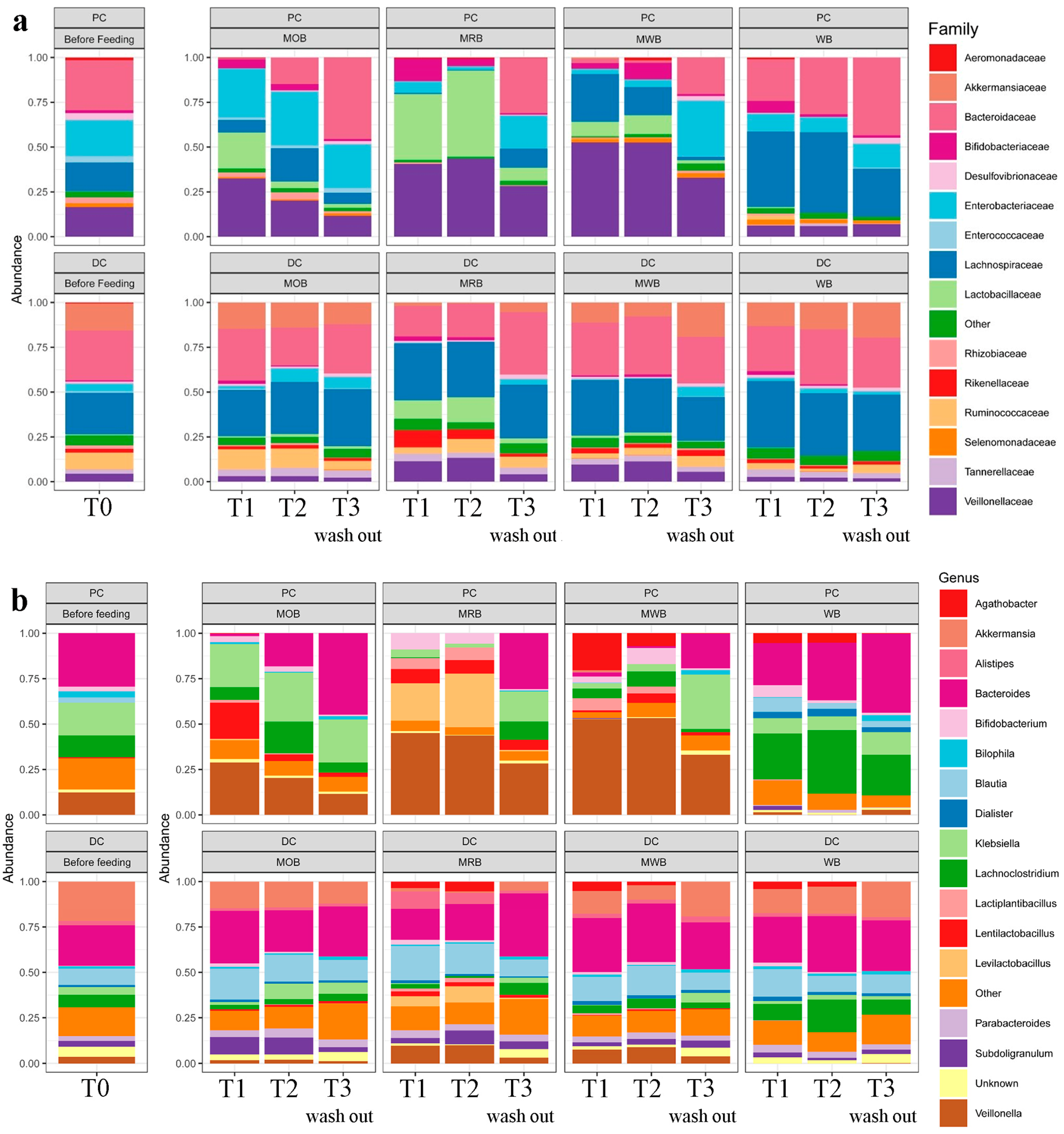
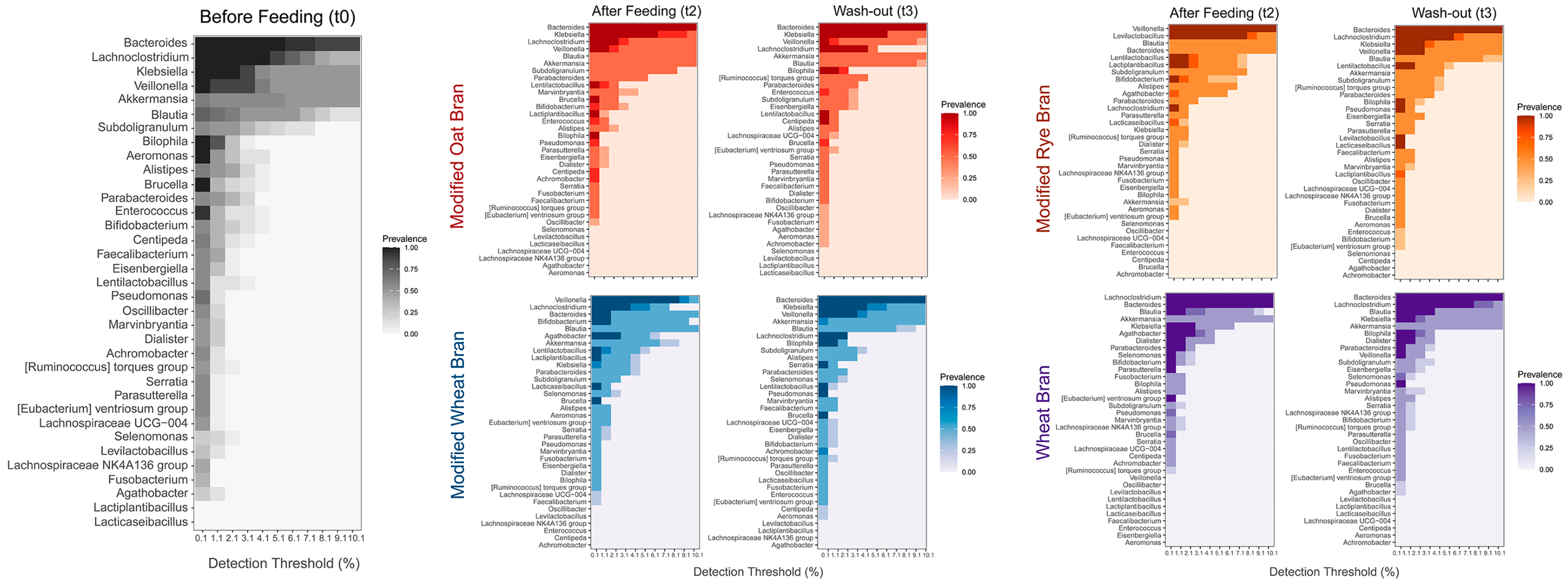
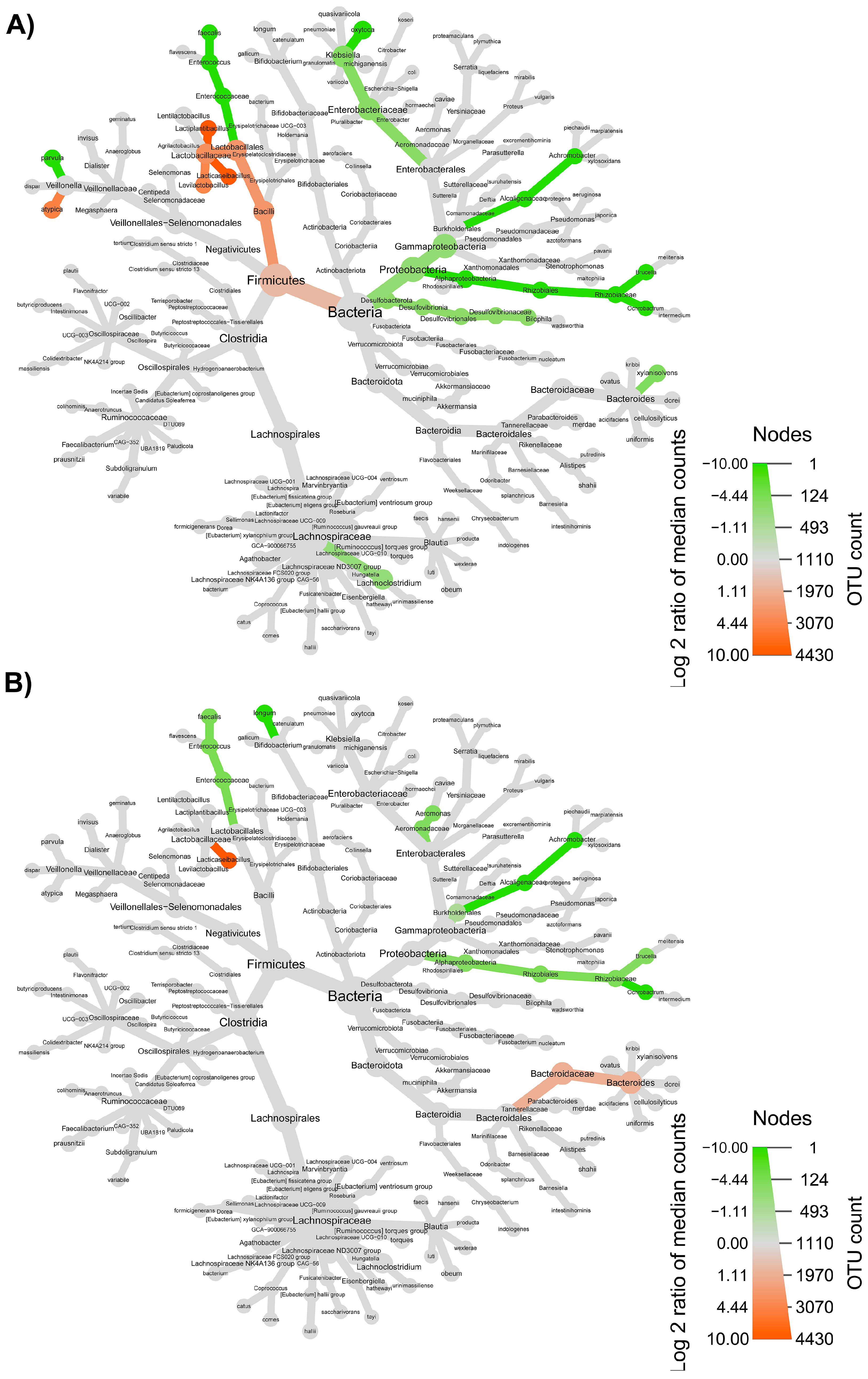
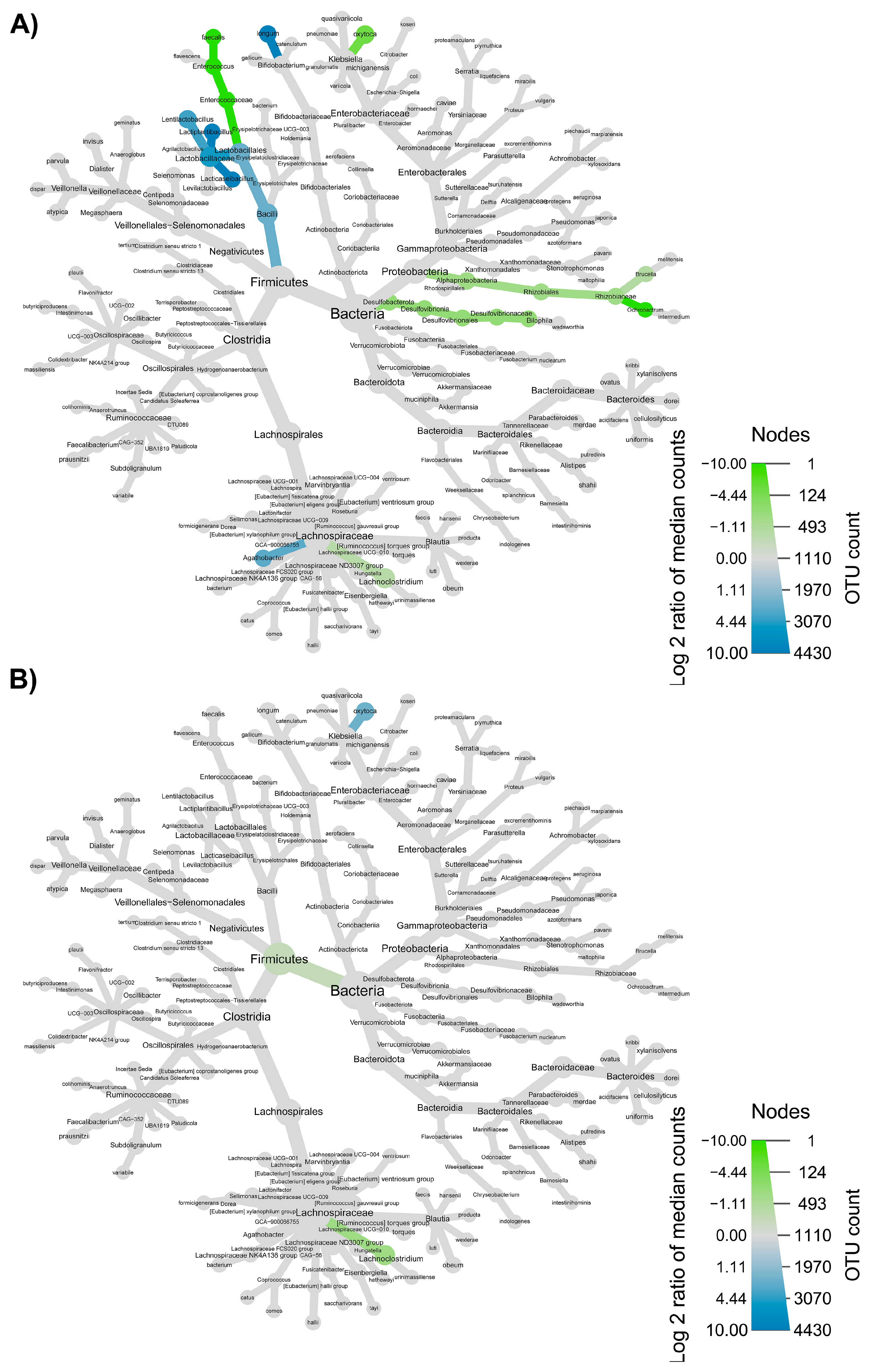
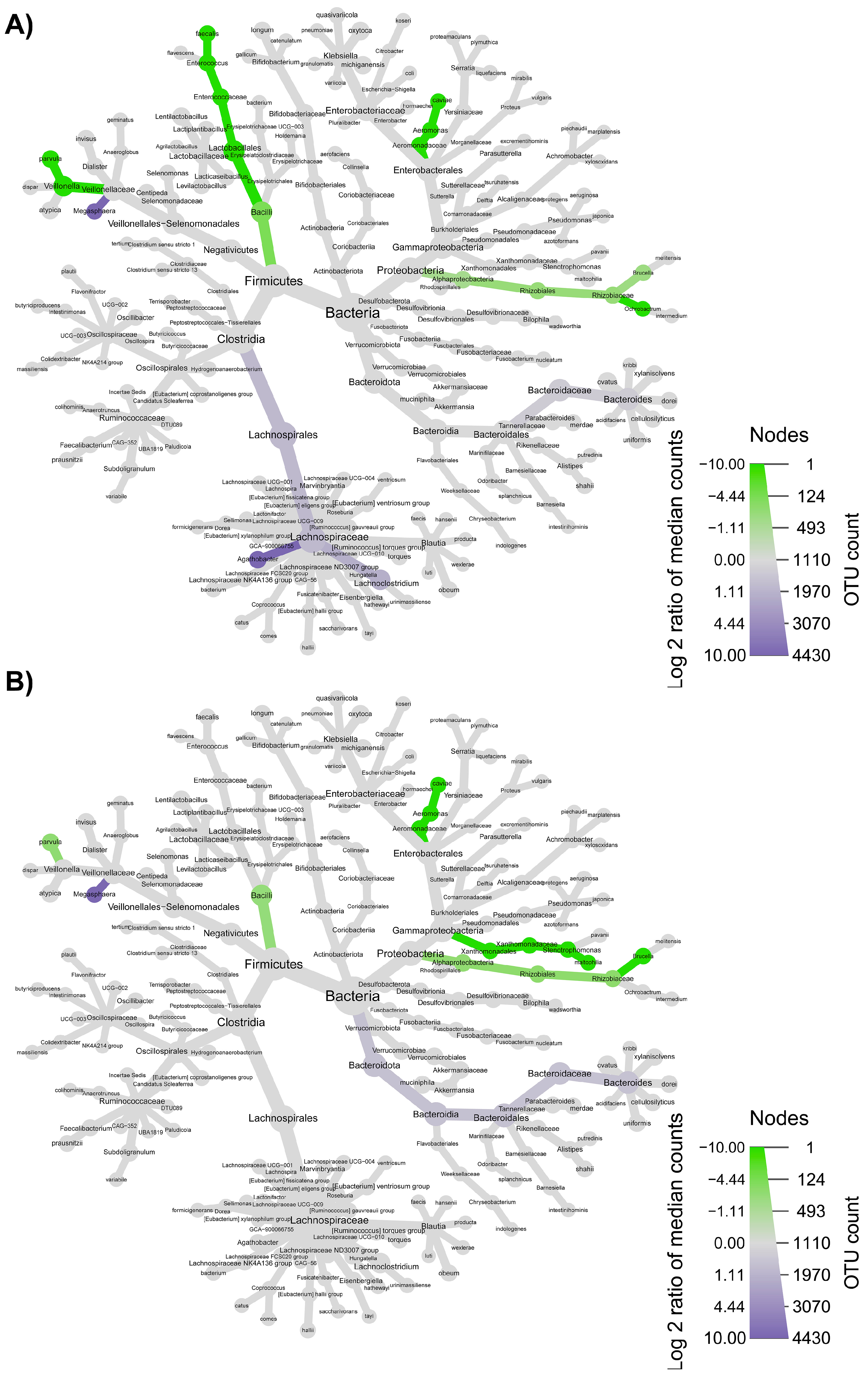
3.3. Colon Microbiome
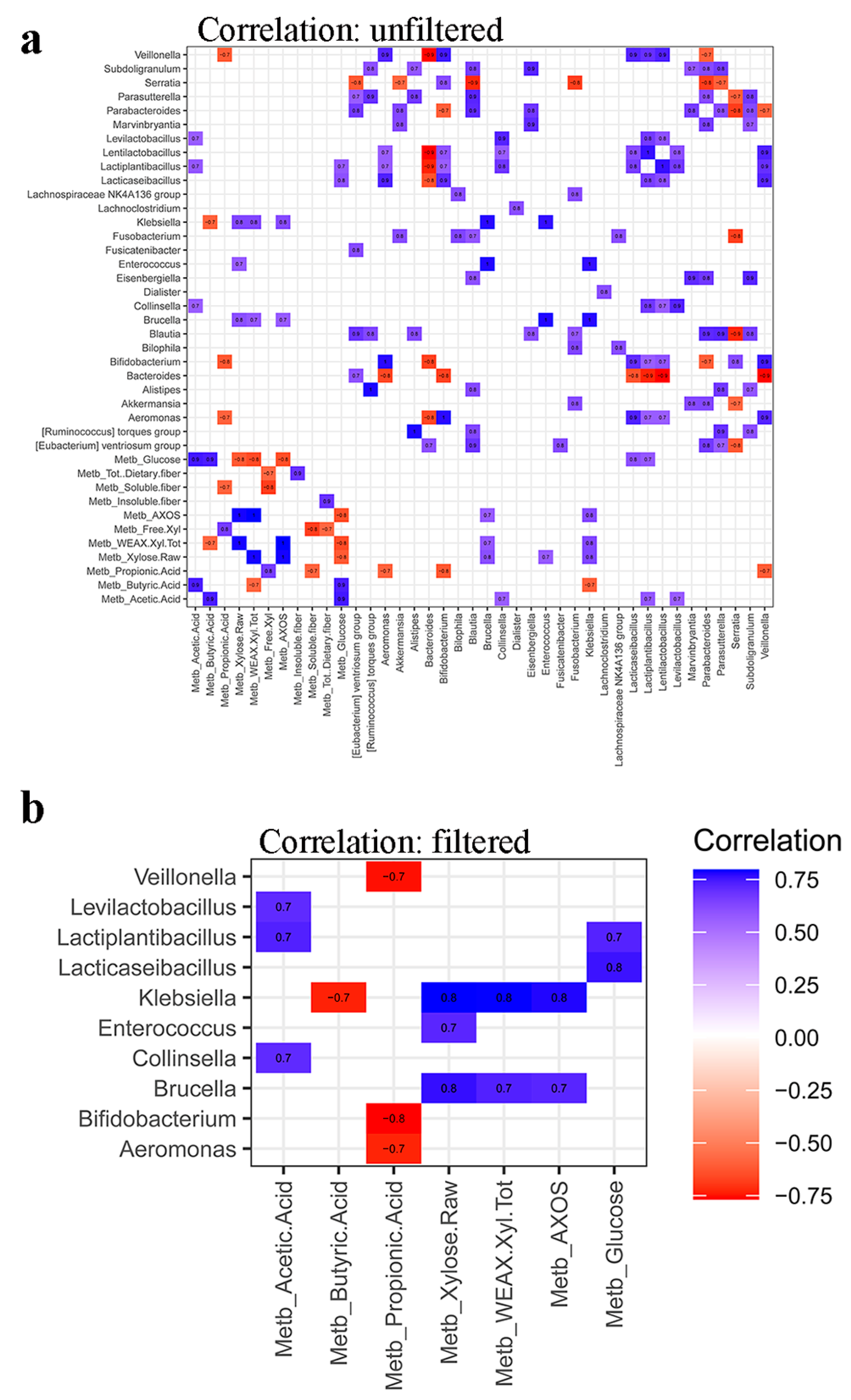
3.4. Synergy among AXOS Composition, Microbiome, SCFA and Dietary Fibers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantu-Jungles, T.M.; Hamaker, B.R. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio 2020, 11, e02179-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 117. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Sánchez, B.; Margolles, A.; González, S. Mediterranean diet and faecal microbiota: A transversal study. Food Funct. 2016, 7, 2347–2356. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Kjølbæk, L.; Gómez del Pulgar, E.M.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schött, H.-F.; Krautbauer, S.; Liebisch, G.; Boberska, J.; et al. A Multi-omics Approach to Unraveling the Microbiome-Mediated Effects of Arabinoxylan Oligosaccharides in Overweight Humans. mSystems 2019, 4, e00209-19. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Hermes, G.D.A.; Emanuel, E.C.; Holst, J.J.; Zoetendal, E.G.; Smidt, H.; Troost, F.; Schaap, F.G.; Damink, S.O.; Jocken, J.W.E.; et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: A randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grootaert, C.; van den Abbeele, P.; Marzorati, M.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A.; Verstraete, W.; van de Wiele, T. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2009, 69, 231–242. [Google Scholar] [CrossRef]
- Kjølbæk, L.; Benítez-Páez, A.; Gómez del Pulgar, E.M.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 2013, 14, 950–959. [Google Scholar] [CrossRef]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Walton, G.E.; Lu, C.; Trogh, I.; Arnaut, F.; Gibson, G.R. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan- oligosaccharides enriched bread in healthy volunteers. Nutr. J. 2012, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Arora, K.; Ameur, H.; Polo, A.; di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, e0049421. [Google Scholar] [CrossRef] [PubMed]
- Van Den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; Aidy Ei, S.; Derrien, M.; et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chandran Matheyambath, A.; Ivusic Polic, I.; LaPointe, G. Differential fermentation of raw and processed high-amylose and waxy maize starches in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). J. Funct. Foods 2021, 86, 104735. [Google Scholar] [CrossRef]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef]
- Cloetens, L.; De Preter, V.; Rutgeerts, P.; Verbeke, K.; Swennen, K.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A.; Rutgeerfs, P.; Verbeke, K. Dose-Response Effect of Arabinoxylooligosaccharides on Gastrointestinal Motility and on Colonic Bacterial Metabolism in Healthy Volunteers. J. Am. Coll. Nutr. 2008, 27, 512–518. [Google Scholar] [CrossRef]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Schuit, J.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003, 6, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Lotti, C.; Rubert, J.; Fava, F.; Tuohy, K.; Mattivi, F.; Vrhovsek, U. Development of a fast and cost-effective gas chromatography–mass spectrometry method for the quantification of short-chain and medium-chain fatty acids in human biofluids. Anal. Bioanal. Chem. 2017, 409, 5555–5567. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Pérez, J.C. Fungi of the Human Gut Microbiota: Roles and Significance. Int. J. Med. Microbiol. 2021, 311, 151490. [Google Scholar] [CrossRef]
- Raimondi, S.; Amaretti, A.; Gozzoli, C.; Simone, M.; Righini, L.; Candeliere, F.; Brun, P.; Ardizzoni, A.; Colombari, B.; Paulone, S.; et al. Longitudinal survey of fungi in the human gut: ITS profiling, phenotyping, and colonization. Front. Microbiol. 2019, 10, 1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paepe, K.; Verspreet, J.; Courtin, C.M.; Van de Wiele, T. Microbial succession during wheat bran fermentation and colonisation by human faecal microbiota as a result of niche diversification. ISME J. 2020, 14, 584–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Q.H.; Liu, S.J.; He, T.F.; Liu, H.S.; Mahfuz, S.; Ma, X.K.; Piao, X.S. Effects of wheat bran in comparison to antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens. Poult. Sci. 2020, 99, 4929–4938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Manoil, D.; Belibasakis, G.N.; Kotsakis, G.A. Veillonellae: Beyond Bridging Species in Oral Biofilm Ecology. Front. Oral Health 2021, 2, 774115. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.C. The interplay between gut bacteria and the yeast Candida albicans. Gut Microbes 2021, 13, 1979877. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, E.J.E.; Chaharlangi, D.; Wong, E.O.Y.; Kim, D.; Navarre, W.W. Acids produced by lactobacilli inhibit the growth of commensal Lachnospiraceae and S24-7 bacteria. Gut Microbes 2022, 14, 2046452. [Google Scholar] [CrossRef]
- Sanchez, J.I.; Marzorati, M.; Grootaert, C.; Baran, M.; van Craeyveld, V.; Courtin, C.M.; Broekaert, W.F.; Delcour, J.A.; Verstraete, W.; van de Wiele, T. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb. Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Iversen, K.N.; Johan, D.; Camille, Z.; Rikard, F.; Pelve, A.E.; Langton, M.; Rikard, L. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef]
- Damen, B.; Cloetens, L.; Broekaert, W.F.; François, I.; Lescroart, O.; Trogh, I.; Arnaut, F.; Welling, G.W.; Wijffels, J.; Delcour, J.A.; et al. Consumption of breads containing in situ-produced arabinoxylan oligosaccharides alters gastrointestinal effects in healthy volunteers. J. Nutr. 2012, 142, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; de Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Ciaula, A.D. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; de la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.M.; Huang, H.L.; Xu, J.; He, J.; Zhao, C.; Peng, Y.; Zhao, H.-L.; Huang, W.-Q.; Cao, C.-Y.; Zhou, Y.-J.; et al. Cross-Talk Between Butyric Acid and Gut Microbiota in Ulcerative Colitis Following Fecal Microbiota Transplantation. Front. Microbiol. 2021, 12, 658292. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maintinguer, S.I.; Fernandes, B.S.; Duarte, I.C.S.; Saavedra, N.K.; Adorno, M.A.T.; Varesche, M.B.A. Fermentative hydrogen production with xylose by Clostridium and Klebsiella species in anaerobic batch reactors. Int. J. Hydrogen Energy 2011, 36, 13508–13517. [Google Scholar] [CrossRef]


| Xyl Tot (g/L) | WEAX Xyl Tot (g/L) | Free Xyl (g/L) | AXOS (g/L) | Insoluble Fibers (g/L) | Soluble Fibers (g/L) | Total Dietary Fibers (g/L) | Glucose (g/L) | Ara/xyl | |
|---|---|---|---|---|---|---|---|---|---|
| MOB | 82.0 ± 11.3 | 77.6 ± 9.3 | 0.6 ± 0.1 | 72.0 ± 8.5 | 4.6 ± 0.3 | 1.2 ± 0.1 | 5.8 ± 0.2 | 1.5 ± 0.1 | 0.06 |
| MRB | 14.7 ± 2.3 | 9.9 ± 0.1 | 1.5 ± 0.3 | 10.1 ± 0.9 | 4.9 ± 0.2 | 1.0 ± 0.1 | 6.0 ± 0.3 | 11.2 ± 0.4 | 0.34 |
| MWB | 20.6 ± 0.5 | 18.6 ± 1.9 | 3.1 ± 0.1 | 16.9 ± 2.7 | 3.2 ± 0.2 | 1.0 ± 0.1 | 4.2 ± 0.2 | 3.5 ± 0.2 | 0.25 |
| WB | 16.0 ± 0.0 | 1.3 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.0 | 3.4 ± 0.2 | 1.9 ± 0.1 | 5.3 ± 0.3 | 9.6 ± 0.3 | 0.48 |
| SCFA (mM) | PC | ||||
|---|---|---|---|---|---|
| MOB | MRB | MWB | WB | ||
| Acetic acid | T0 T1 T2 T3 | 32.3 ± 1.1 a/c 41.9 ± 11.2 c/b 38.5 ± 10.9 d/b 53.1 ± 2.7 a/a | 33.8 ± 0.9 a/c 135.7 ± 14.0 a/a 127.1 ± 12.9 a/a 48.3 ± 2.0 b/b | 32.4 ± 1.0 a/d 83.4 ± 0.3 b/b 90.2 ± 9.3 b/a 42.3 ± 1.9 c/c | 32.5 ± 1.3 a/c 79.6 ± 12.0 b/a 63.5 ± 13.6 c/b 36.9 ± 5.2 d/c |
| Butyric acid | T0 T1 T2 T3 | 2.1 ± 0.3 a/b 3.3 ± 0.3 c/a 3.5 ± 0.2 d/a 4.0 ± 0.1 a/a | 2.5 ± 0.2 a/d 6.8 ± 0.1 a/b 7.7 ± 0.3 a/a 3.2 ± 0.0 c/c | 2.4 ± 0.7 a/d 5.4 ± 0.0 b/b 7.0 ± 0.3 b/a 3.5 ± 0.1 b/c | 2.3 ± 0.3 a/c 4.0 ± 0.4 c/a 4.6 ± 1.0 c/a 3.1 ± 0.0 c/b |
| Propionic acid | T0 T1 T2 T3 | 9.2 ± 1.0 a/d 13.9 ± 0.1 c/c 15.1 ± 0.1 c/a 14.2 ± 0.2 a/b | 9.1 ± 0.9 a/d 13.1 ± 0.7 c/b 16.3 ± 3.1 b/a 11.7 ± 0.4 c/c | 9.1 ± 1.2 a/c 15.9 ± 1.3 b/a 12.9 ± 0.6 d/b 12.9 ± 0.5 b/b | 9.4 ± 0.5 a/d 20.3 ± 1.0 a/a 17.5 ± 0.1 a/b 14.4 ± 0.3 a/c |
| DC | |||||
| MOB | MRB | MWB | WB | ||
| Acetic acid | T0 T1 T2 T3 | 22.1 ± 0.9 a/d 76.4 ± 4.3 b/a 59.4 ± 10.6 c/b 45.5 ± 1.8 b/c | 21.7 ± 0.1 a/d 118.1 ± 3.2 a/b 127.6 ± 2.8 a/a 48.3 ± 4.0 b/c | 22.0 ± 1.1 a/d 76.2 ± 4.8 b/b 92.0 ± 5.9 b/a 52.3 ± 2.5 a/c | 22.4 ± 1.3 a/d 82.0 ± 5.3 b/a 57.4 ± 4.3 c/b 45.2 ± 4.0 b/c |
| Butyric acid | T0 T1 T2 T3 | 2.0 ± 0.1 a/c 5.4 ± 0.6 b/a 4.8 ± 0.4 c/a 3.6 ± 0.3 b/b | 2.0 ± 0.1 a/d 6.1 ± 0.4 a/b 9.9 ± 0.4 a/a 3.3 ± 0.1 b/c | 2.0 ± 0.2 a/d 5.7 ± 1.1 b/b 8.1 ± 0.7 b/a 4.4 ± 0.1 a/c | 2.1 ± 0.2 a/c 5.1 ± 0.4 c/a 5.1 ± 0.2 c/a 3.9 ± 0.1 b/b |
| Propionic acid | T0 T1 T2 T3 | 8.5 ± 1.1 a/c 16.2 ± 0.4 b/a 16.1 ± 0.5 c/a 14.8 ± 0.5 a/b | 8.8 ± 0.9 a/d 15.3 ± 1.2 b/b 17.3 ± 0.4 b/a 12.8 ± 0.2 c/c | 8.2 ± 1.5 a/c 16.1 ± 1.3 b/a 15.9 ± 0.8 c/a 13.9 ± 0.4 b/b | 8.7 ± 1.1 a/d 22.9 ± 0.5 a/a 18.9 ± 0.16 a/b 15.8 ± 0.2 a/c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo, A.; Albiac, M.A.; Da Ros, A.; Ardèvol, V.N.; Nikoloudaki, O.; Verté, F.; Di Cagno, R.; Gobbetti, M. The Effect of Hydrolyzed and Fermented Arabinoxylan-Oligo Saccharides (AXOS) Intake on the Middle-Term Gut Microbiome Modulation and Its Metabolic Answer. Nutrients 2023, 15, 590. https://doi.org/10.3390/nu15030590
Polo A, Albiac MA, Da Ros A, Ardèvol VN, Nikoloudaki O, Verté F, Di Cagno R, Gobbetti M. The Effect of Hydrolyzed and Fermented Arabinoxylan-Oligo Saccharides (AXOS) Intake on the Middle-Term Gut Microbiome Modulation and Its Metabolic Answer. Nutrients. 2023; 15(3):590. https://doi.org/10.3390/nu15030590
Chicago/Turabian StylePolo, Andrea, Marta Acin Albiac, Alessio Da Ros, Vimac Nolla Ardèvol, Olga Nikoloudaki, Fabienne Verté, Raffaella Di Cagno, and Marco Gobbetti. 2023. "The Effect of Hydrolyzed and Fermented Arabinoxylan-Oligo Saccharides (AXOS) Intake on the Middle-Term Gut Microbiome Modulation and Its Metabolic Answer" Nutrients 15, no. 3: 590. https://doi.org/10.3390/nu15030590





