The Genetic Background Is Shaping Cecal Enlargement in the Absence of Intestinal Microbiota
Abstract
:1. Introduction
2. Material and Methods
2.1. Mice and Sample Collection
2.2. Ethical Statement
2.3. Quantitative Real-Time PCR (qPCR)
2.4. Metabolomics
2.4.1. Untargeted Metabolome Analysis
2.4.2. Data Analysis
2.5. Immunohistology
2.6. Immunofluorescence
2.7. Qualitative Detection of Occult Blood in Intestinal Content
2.8. Determination of Digestive Enzymes in Cecal Content
2.8.1. Quantitative Determination of Luminal Trypsin and Chymotrypsin in Cecal Content
2.8.2. Trypsin Activity Assay
2.9. Lifespan Analysis
2.10. Liver and Pancreatic Enzyme Analysis from Blood
2.11. Statistical Analysis
3. Results
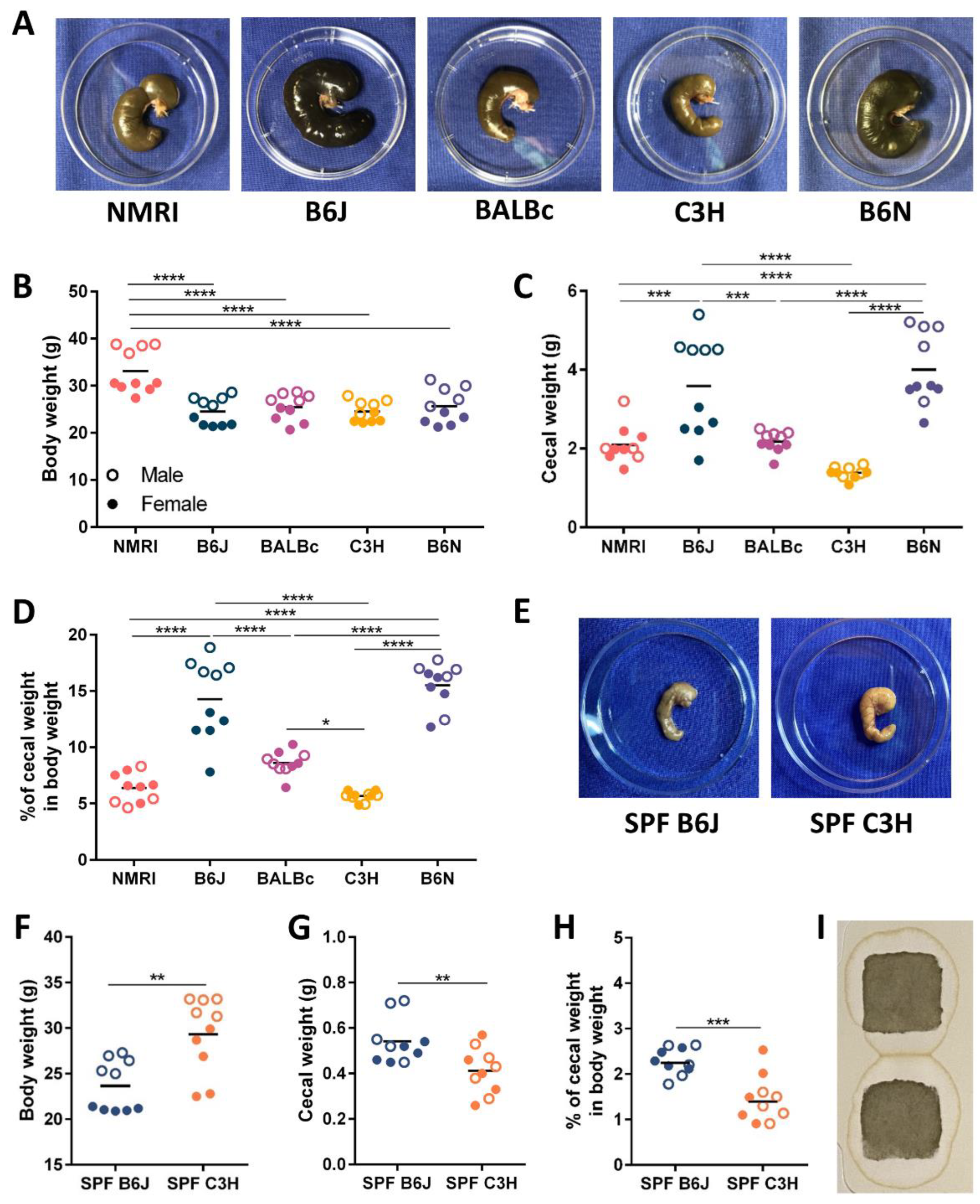
3.1. Cecal Enlargement Varies between Different Germ-Free Strains
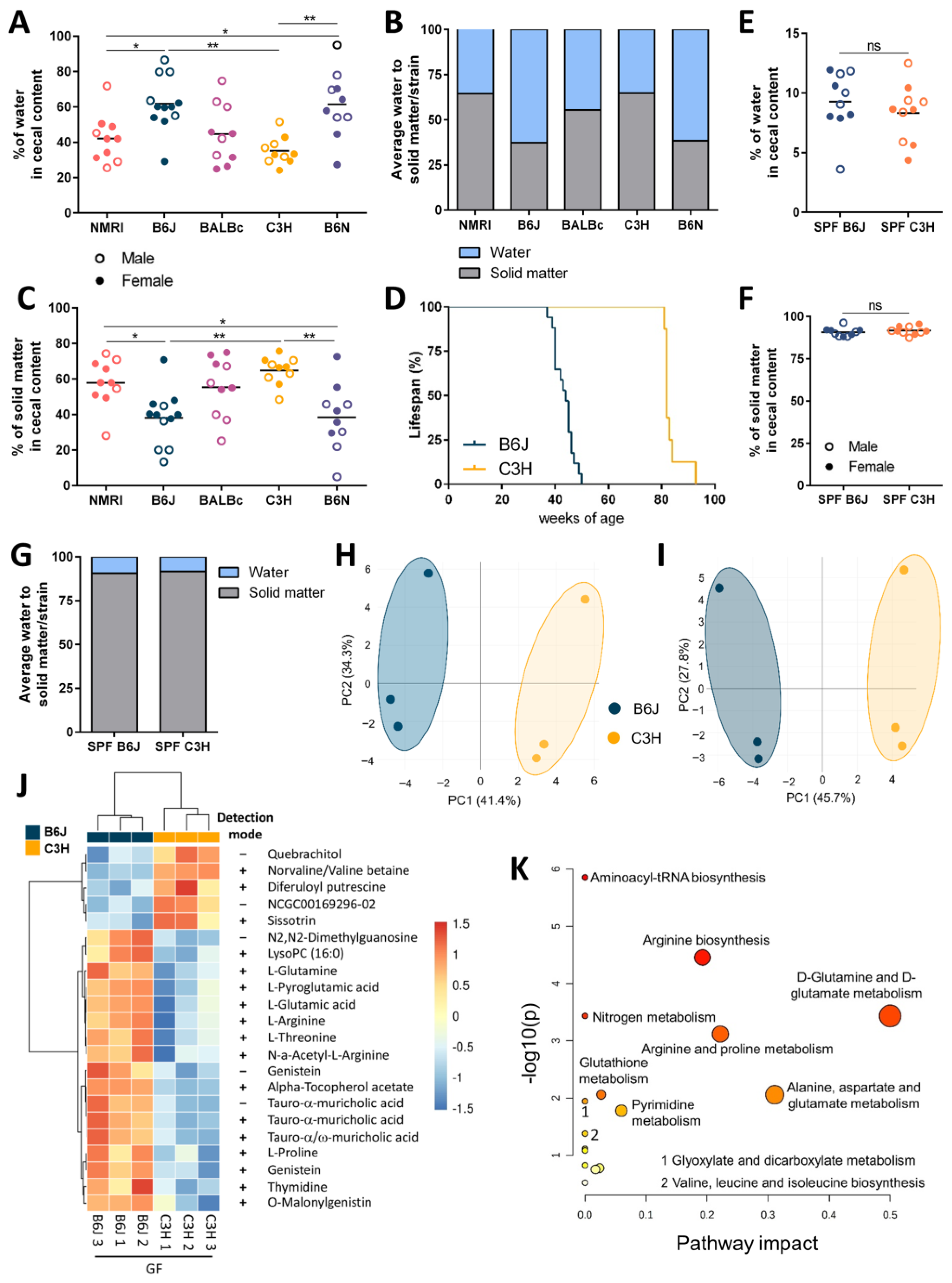
3.2. Host Genetic Background Shapes Distinct Metabolic Profiles in The Gf Mice Cecum
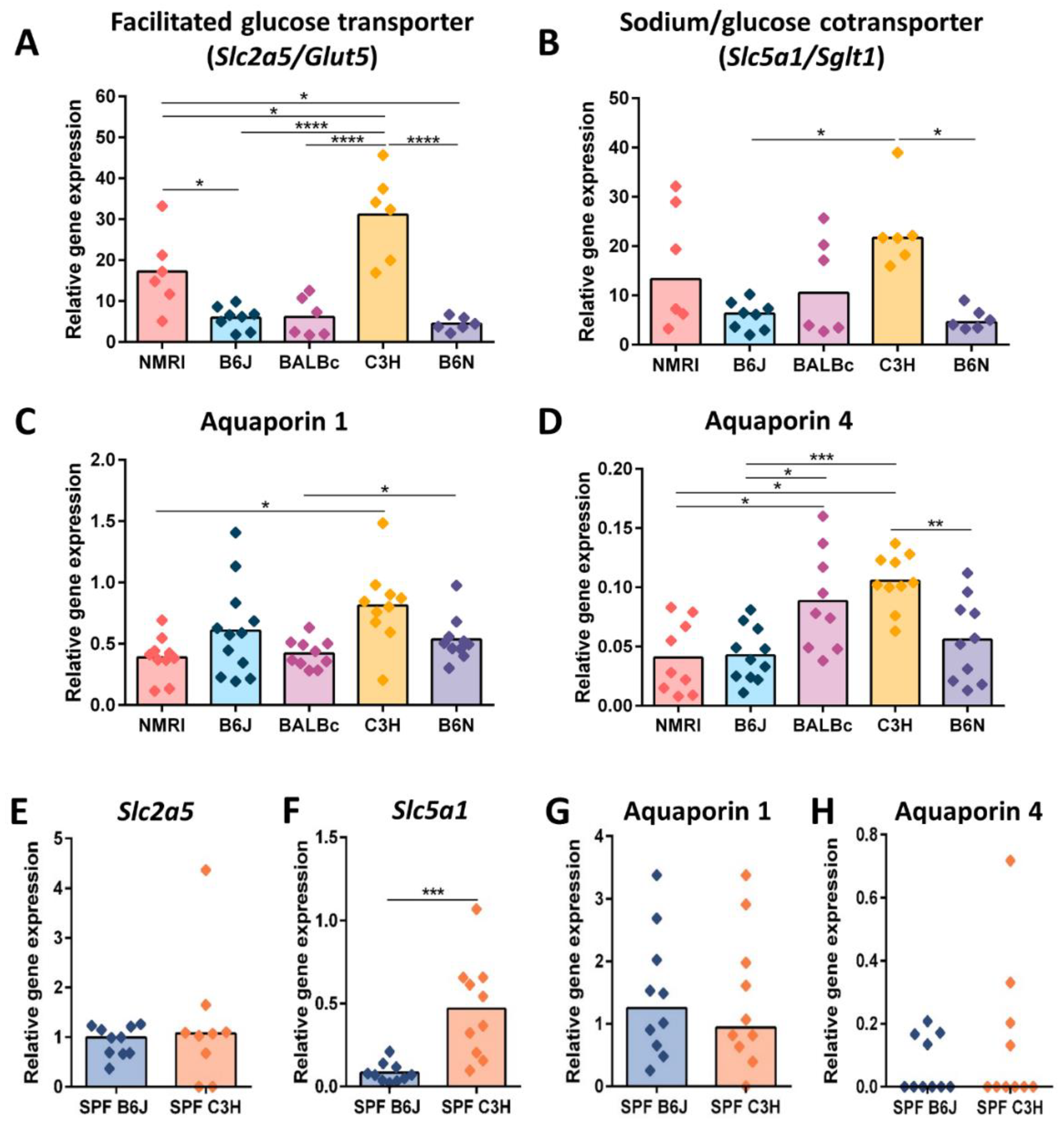
3.3. Reduced Cecal Size Correlates with Higher Water Transportation across the Epithelial Barrier
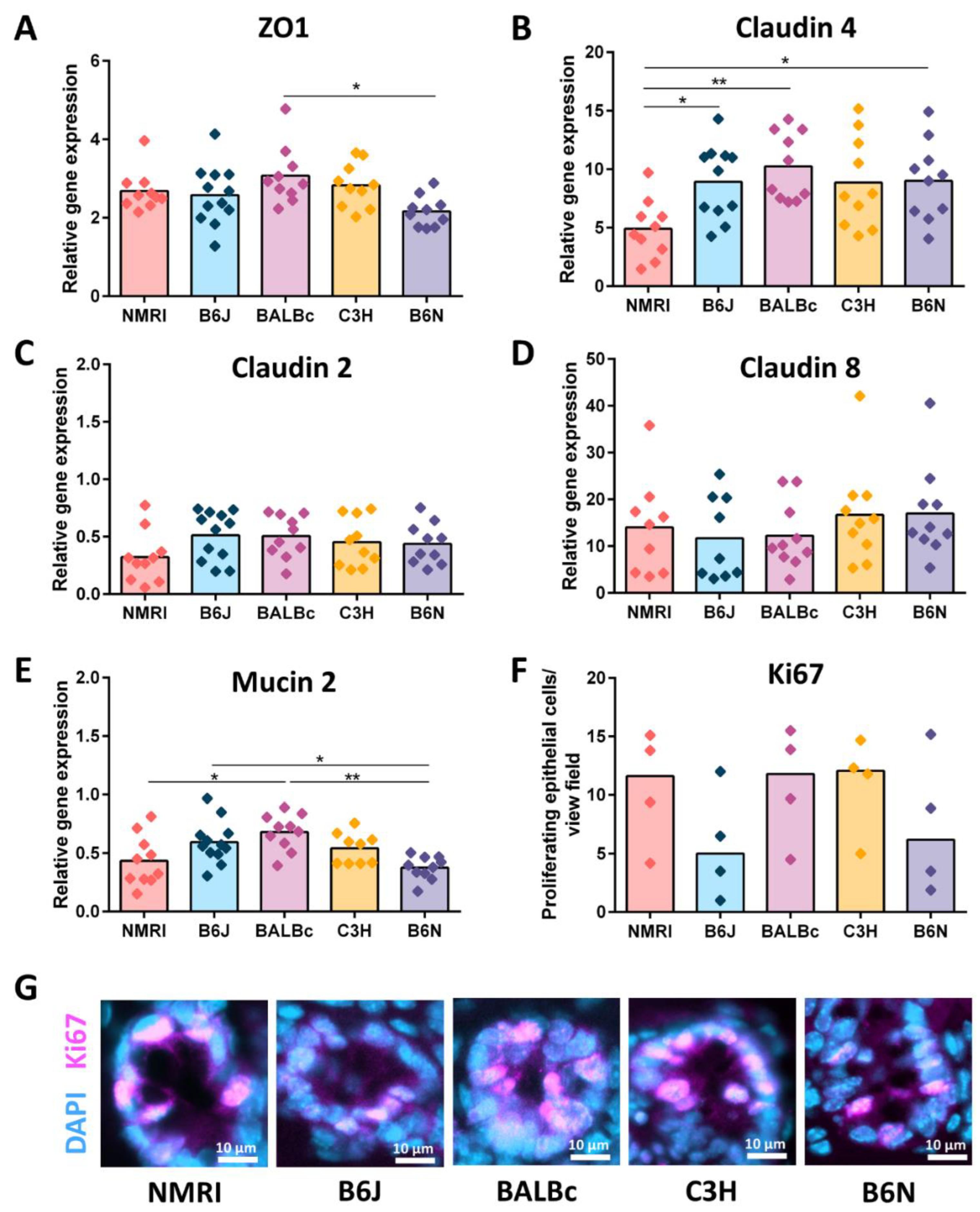
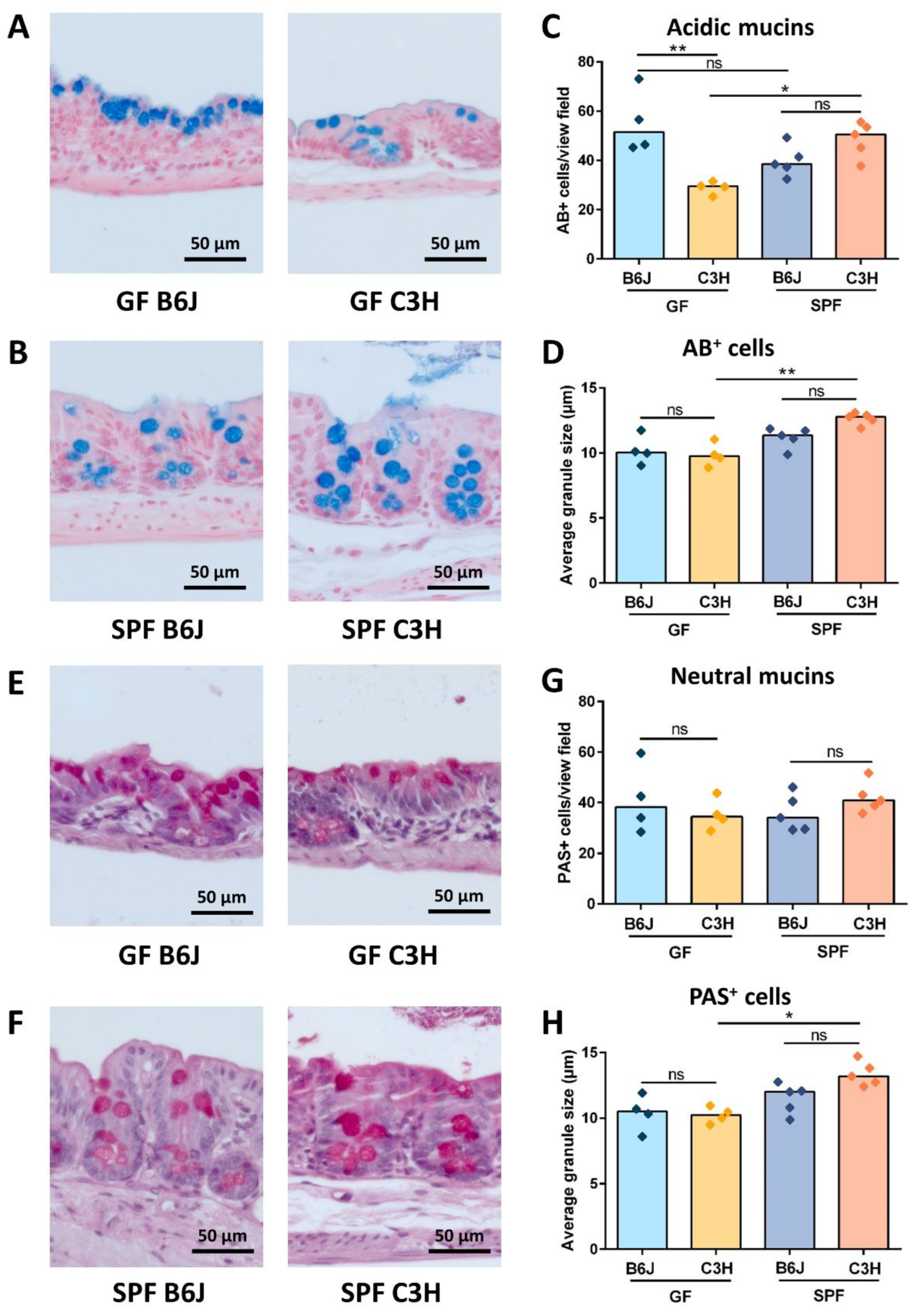
3.4. GF B6J Display Increased Production of Acidic Mucins in the Gut
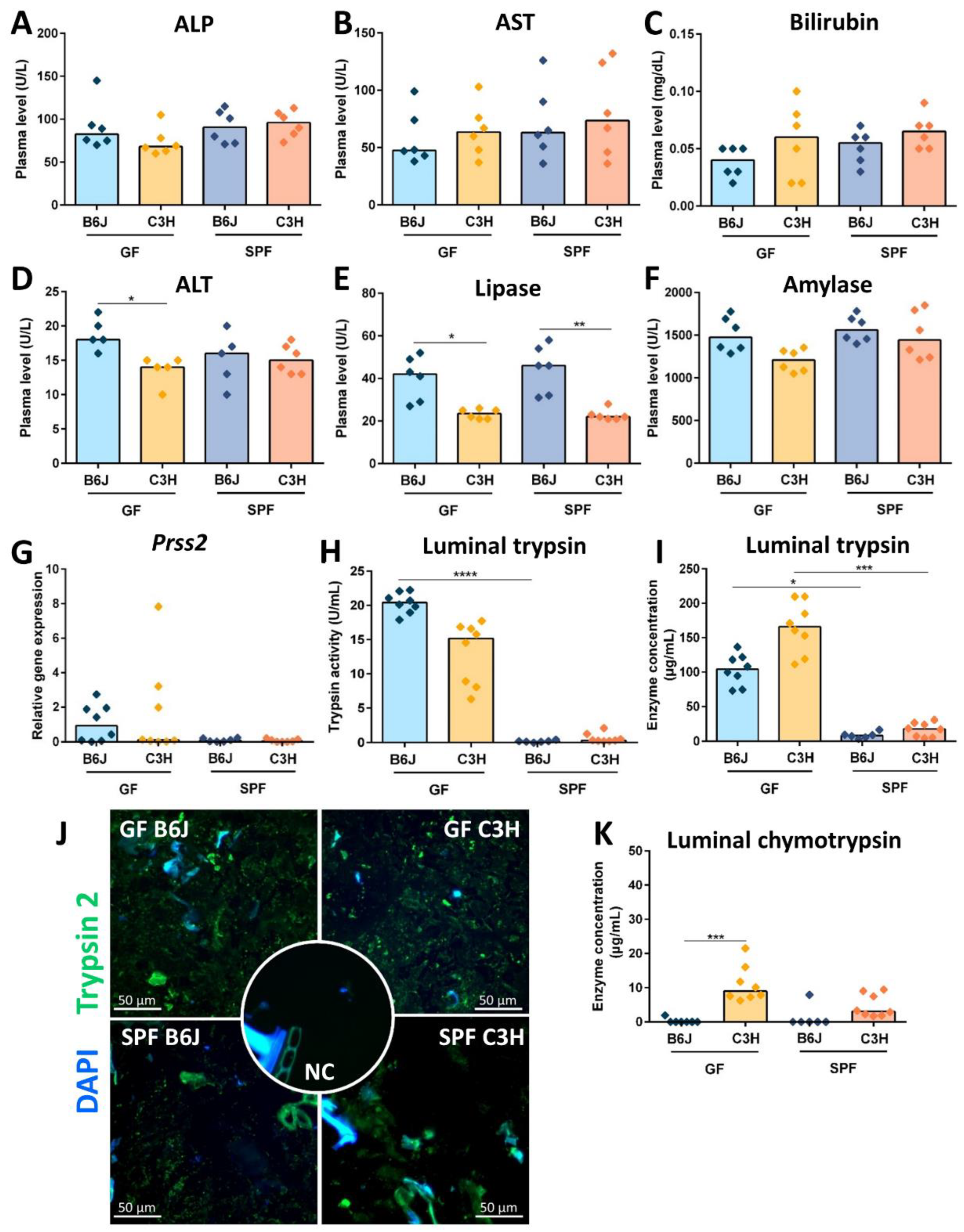
3.5. Reduced Cecal Size in Gf C3h Mice Is Independent of Increased Digestive Enzyme Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolsega, S.; Bleich, A.; Basic, M. Synthetic Microbiomes on the Rise—Application in Deciphering the Role of Microbes in Host Health and Disease. Nutrients 2021, 13, 4173. [Google Scholar] [CrossRef] [PubMed]
- Basic, M.; Bleich, A. Gnotobiotics: Past, present and future. Lab. Anim. 2019, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, G.H.; Thierfelder, H. Thierisches Leben ohne Bakterien im Verdauungskanal; De Gruyter: Berlin, Germany, 1896. [Google Scholar]
- Gustafsson, B. GErm-free rearing of rats. Acta Anat. 1946, 2, 376–391. [Google Scholar] [CrossRef]
- Reyniers, J.A.; Trexler, P.C.; Ervin, R.F. Rearing germ-free albino rats. Lobund Rep. 1946, 1, 1–84. [Google Scholar]
- Rahija, R.J. Gnotobiotics. In The Mouse in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2007; pp. 217–233. [Google Scholar]
- Coates, M.E. Gnotobiotic animals in research: Their uses and limitations. Lab. Anim. 1975, 9, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wostmann, B.S. The germfree animal in nutritional studies. Annu. Rev. Nutr. 1981, 1, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ellis, M.L.; Dowell, A.E.; Kumar, R.; Morrow, C.D.; Schoeb, T.R.; Knight, J. Response of Germfree Mice to Colonization by Oxalobacter formigenes and Altered Schaedler Flora. Appl. Environ. Microbiol. 2016, 82, 6952–6960. [Google Scholar] [CrossRef] [Green Version]
- Whary, M.T.; Wang, C.; Ruff, C.F.; DiVincenzo, M.J.; Labriola, C.; Ge, L.; Feng, Y.; Ge, Z.; Bakthavatchalu, V.; Muthupalani, S.; et al. Effects of Colonization of Gnotobiotic Swiss Webster Mice with Helicobacter bilis. Comp. Med. 2020, 70, 216–232. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Östman, S.; Rask, C.; Wold, A.E.; Hultkrantz, S.; Telemo, E. Impaired regulatory T cell function in germ-free mice. Eur. J. Immunol. 2006, 36, 2336–2346. [Google Scholar] [CrossRef]
- Wostmann, B.; Bruckner-Kardoss, E. Development of cecal distention in germ-free baby rats. Am. J. Physiol. Content 1959, 197, 1345–1346. [Google Scholar] [CrossRef] [Green Version]
- Wostmann, B.; Bruckner-Kardoss, E. Cecal Enlargement in Germ-Free Animals. Nutr. Rev. 1960, 18, 313–314. [Google Scholar] [CrossRef]
- Gordon, H.A. A substance acting on smooth muscle in intestinal contents of germfree animals. Ann. N. Y. Acad. Sci. 1967, 147, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, G.; Lindstedt, S.; Gustafsson, B.E. Mucus in intestinal contents of germfree rats. J. Exp. Med. 1965, 121, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, G.R.; Trexler, P.C. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut 1971, 12, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Niimi, K.; Takahashi, E. New system to examine the activity and water and food intake of germ-free mice in a sealed positive-pressure cage. Heliyon 2019, 5, e02176. [Google Scholar] [CrossRef]
- Paik, J.; Pershutkina, O.; Meeker, S.; Yi, J.J.; Dowling, S.; Hsu, C.; Hajjar, A.M.; Maggio-Price, L.; Beck, D.A.C. Potential for using a hermetically-sealed, positive-pressured isocage system for studies involving germ-free mice outside a flexible-film isolator. Gut Microbes 2015, 6, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Schaedler, R.W.; Dubos, R.; Costello, R. Association of germfree mice with bacteria isolated from normal mice. J. Exp. Med. 1965, 122, 77–82. [Google Scholar] [CrossRef]
- Courtney, C.L. Cecal Torsion in Rodents Associated with Chronic Administration of Clinafloxacin. Toxicol. Pathol. 2000, 28, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Djurickovic, S.M.; Ediger, R.D.; Hong, C.C. Volvulus at the i1eocaecal junction in germfree mice. Lab. Anim. 1978, 12, 219–220. [Google Scholar] [CrossRef] [PubMed]
- FELASA Working Group on Revision of Guidelines for Health Monitoring of Rodents and Rabbits; Mähler, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar] [CrossRef]
- Nicklas, W.; Keubler, L.; Bleich, A. Maintaining and Monitoring the Defined Microbiota Status of Gnotobiotic Rodents. ILAR J. 2015, 56, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Robinson, P.G.; Smith, P.A.; Elliot, R.B. A simple method for the quantitative determination of stool trypsin and chymotrypsin. Clin. Chim. Acta 1975, 62, 225–229. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Marinelli, R.A.; LaRusso, N.F. Water transport by epithelia of the digestive tract. Gastroenterology 2002, 122, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Gan, L.; Lv, L.; Mei, Z. The regulatory roles of aquaporins in the digestive system. Genes Dis. 2020, 8, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Corring, T.; Juste, C.; Simoes-Nunes, C. Digestive enzymes in the germ-free animal. Reprod. Nutr. Dev. (1980) 1981, 21, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbitt, N.; Kimura, S.; Isse, K.; Specht, S.; Chedwick, L.; Rosborough, B.R.; Lunz, J.G.; Murase, N.; Yokota, S.; Demetris, A.J. Gut Bacteria Drive Kupffer Cell Expansion via MAMP-Mediated ICAM-1 Induction on Sinusoidal Endothelium and Influence Preservation-Reperfusion Injury after Orthotopic Liver Transplantation. Am. J. Pathol. 2013, 182, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Sun, N.N.; Li, L.L.; Zhu, W.W.; Xiu, J.; Shen, Y.; Xu, Q. Hepatic progenitor cell activation is induced by the depletion of the gut microbiome in mice. Microbiologyopen 2019, 8, e873. [Google Scholar] [CrossRef]
- LaGamma, E.F.; Hu, F.; Cruz, F.P.; Bouchev, P.; Nankova, B.B. Bacteria—Derived short chain fatty acids restore sympathoadrenal responsiveness to hypoglycemia after antibiotic-induced gut microbiota depletion. Neurobiol. Stress 2021, 15, 100376. [Google Scholar] [CrossRef]
- Wiseman, R.F.; Gordon, H.A. A Bioactive Substance in the Caecum of Germ-Free Animals: Reduced Levels of a Bioactive Substance in the Caecal Content of Gnotobiotic Rats Mono-associated with Salmonella typhimurium. Nature 1965, 205, 572–573. [Google Scholar] [CrossRef]
- Donowitz, M.; Binder, H.J. Mechanism of fluid and electrolyte secretion in the germ-free rat cecum. Dig. Dis. Sci. 1979, 24, 551–559. [Google Scholar] [CrossRef]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Oku, T. Reversible cecal and colonic enlargement induced by dietary fiber in rats. Nutr. Res. 1995, 15, 1355–1366. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F. Coupling between Na+, sugar, and water transport across the intestine. Ann. N. Y. Acad. Sci. 2006, 915, 54–66. [Google Scholar] [CrossRef]
- Loo, D.D.F.; Zeuthen, T.; Chandy, G.; Wright, E.M. Cotransport of water by the Na+/glucose cotransporter. Proc. Natl. Acad. Sci. USA 1996, 93, 13367–13370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeschke, K.; Uhlich, E.; Kinne, R. Stimulation of sodium transport and Na+-K+-ATPase activity in the hypertrophying rat cecum. Pflugers Arch. 1974, 346, 233–249. [Google Scholar] [CrossRef]
- Schreiner, J.; Weber, M.; Loeschke, K. Sodium Chloride Transport of Normal and Dietary Enlarged Rat Cecum in vitro. Digestion 1998, 59, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman, J.A.; Samji, F.N.; Li, Y.; Deng, W.; Lin, A.; Finlay, B.B. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cells Microbiol. 2006, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kuramoto, H.; Kadowaki, M. Downregulation in aquaporin 4 and aquaporin 8 expression of the colon associated with the induction of allergic diarrhea in a mouse model of food allergy. Life Sci. 2007, 81, 115–120. [Google Scholar] [CrossRef]
- Hardin, J.A.; Wallace, L.E.; Wong, J.F.K.; O’Loughlin, E.V.; Urbanski, S.J.; Gall, D.G.; Macnaughton, W.K.; Beck, P.L. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res. 2004, 318, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yang, M.; Ou, Z.; Li, D.; Geng, L.; Chen, P.; Chen, H.; Gong, S. Involvement of aquaporins in a mouse model of rotavirus diarrhea. Virol. Sin. 2014, 29, 211–217. [Google Scholar] [CrossRef]
- Loeschke, K.; Gordon, H.A. Water Movement Across the Cecal Wall of the Germfree Rat. Exp. Biol. Med. 1970, 133, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of PCA (2-Pyrrolidone-5-Carboxylic Acid) and Its Salts as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 5S–11S. [Google Scholar] [CrossRef]
- Hofmann, A.F. The Syndrome of Ileal Disease and the Broken Enterohepatic Circulation: Cholerheic Enteropathy. Gastroenterology 1967, 52, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Heubi, J.E.; Balistreri, W.F.; Fondacaro, J.D.; Partin, J.C.; Schubert, W.K. Primary Bile Acid Malabsorption: Defective In Vitro Ileal Active Bile Acid Transport. Gastroenterology 1982, 83, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Mekada, K.; Yoshiki, A. Substrains matter in phenotyping of C57BL/6 mice. Exp. Anim. 2021, 70, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.P.; Rathkolb, B.; Oestereicher, M.A.; Lengger, C.J.; Moerth, C.; Micklich, K.; Fuchs, H.; Gailus-Durner, V.; Wolf, E.; de Angelis, M.H. Clinical Chemistry Reference Intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus musculus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 375–386. [Google Scholar] [PubMed]
- Noblet, J.; Gilbert, H.; Jaguelin-Peyraud, Y.; Lebrun, T. Evidence of genetic variability for digestive efficiency in the growing pig fed a fibrous diet. Animal 2013, 7, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P.; Procopio, J.; Lima, M.M.R.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate? Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef]
- Matthews, D.M.; Laster, L. Kinetics of intestinal active transport of five neutral amino acids. Am. J. Physiol. Content 1965, 208, 593–600. [Google Scholar] [CrossRef]
- Whitt, D.D.; Demoss, R.D. Effect of Microflora on the Free Amino Acid Distribution in Various Regions of the Mouse Gastrointestinal Tract. Appl. Environ. Microbiol. 1975, 30, 609–615. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [Green Version]
- Sheahan, D.G.; Jervis, H.R. Comparative histochemistry of gastrointestinal mucosubstances. Am. J. Anat. 1976, 146, 103–131. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Watanabe, E.; Kawashima, Y.; Plichta, D.R.; Wang, Z.; Ujike, M.; Ang, Q.Y.; Wu, R.; Furuichi, M.; Takeshita, K.; et al. Identification of trypsin-degrading commensals in the large intestine. Nature 2022, 609, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.A.; Bruckner-Kardoss, E.; Wostmann, B.S. Aging in Germ-free Mice: Life Tables and Lesions Observed at Natural Death. J. Gerontol. 1966, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolsega, S.; Smoczek, A.; Meng, C.; Kleigrewe, K.; Scheele, T.; Meller, S.; Glage, S.; Volk, H.A.; Bleich, A.; Basic, M. The Genetic Background Is Shaping Cecal Enlargement in the Absence of Intestinal Microbiota. Nutrients 2023, 15, 636. https://doi.org/10.3390/nu15030636
Bolsega S, Smoczek A, Meng C, Kleigrewe K, Scheele T, Meller S, Glage S, Volk HA, Bleich A, Basic M. The Genetic Background Is Shaping Cecal Enlargement in the Absence of Intestinal Microbiota. Nutrients. 2023; 15(3):636. https://doi.org/10.3390/nu15030636
Chicago/Turabian StyleBolsega, Silvia, Anna Smoczek, Chen Meng, Karin Kleigrewe, Tim Scheele, Sebastian Meller, Silke Glage, Holger A. Volk, André Bleich, and Marijana Basic. 2023. "The Genetic Background Is Shaping Cecal Enlargement in the Absence of Intestinal Microbiota" Nutrients 15, no. 3: 636. https://doi.org/10.3390/nu15030636
APA StyleBolsega, S., Smoczek, A., Meng, C., Kleigrewe, K., Scheele, T., Meller, S., Glage, S., Volk, H. A., Bleich, A., & Basic, M. (2023). The Genetic Background Is Shaping Cecal Enlargement in the Absence of Intestinal Microbiota. Nutrients, 15(3), 636. https://doi.org/10.3390/nu15030636





