Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature
Abstract
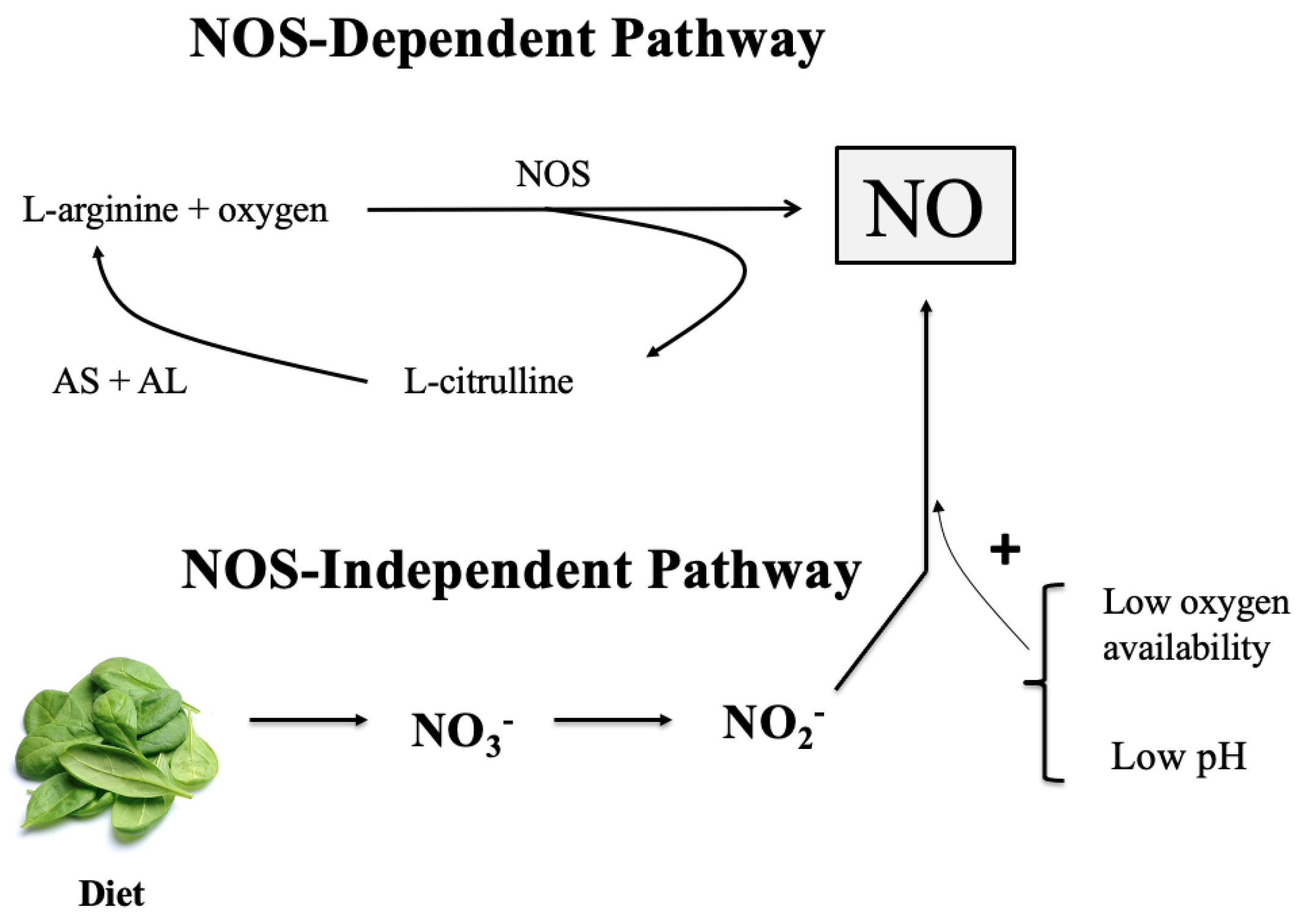
:1. Introduction
2. Nitric Oxide Production Pathways
3. Effect of L-arginine Supplementation on Strength Performance
| Study | Subjects | Design | Intervention | Performance Tests | Main Findings |
|---|---|---|---|---|---|
| Aguiar et al. (2016) [33] | 20 physically active older women (71.6 ± 5.9 y; 61.9 ± 8.6 kg) | Randomized, double-blind, placebo-controlled study | 8 g L-arginine vs. placebo; 80 min prior | 3 sets of 8 maximal isokinetic leg extensions at 60°·s−1 Maximal unilateral isometric force at 60° of knee flexion Sit–stand, tandem gait, and timed up and go functional tests | ↔ isokinetic strength ↔ isometric strength ↔ functional tests ↔ femoral artery vasodilation |
| Álvares et al. (2012) [30] | 15 recreationally trained men (26.3 ± 4.9 y; 79.2 ± 13.4 kg) | Randomized, double-blind, placebo-controlled study | 6 g L-arginine vs. placebo; 80 min prior | 3 sets of 10 maximal isokinetic elbow extensions at 60°·s−1 | ↔ isokinetic strength ↔ NOx |
| Andrade et al. (2018) [44] | 20 recreationally active men and women (23.0 ± 4.0 y; 71.2 ± 8.3 kg) | Randomized, double-blind, placebo-controlled study | 6 g L-arginine vs. placebo; 60 min prior | 3 sets of 8–12 leg press and hack squat (70% 1RM) 1 set of leg press (60% 1RM) to failure performed at 24, 48, and 72 h post | ↔ repetitions to failure ↔ surface EMG ↔ creatine kinase ↔ lactate ↔ testosterone:cortisol ratio ↔ muscle soreness |
| Campbell et al. (2006) [40] | 35 resistance trained men (38.9 ± 5.8 y; 86.0 ± 13.7 kg) | Randomized, double-blind, placebo-controlled study | 12 g L-arginine α-ketoglutarate (1:1 ratio) vs. placebo; daily for 8 weeks during periodized resistance training | 1RM bench press Isokinetic leg extension endurance test 30 s WAnT Aerobic capacity test Body composition | ↑ 1RM bench press ↔ isokinetic endurance ↑ peak WAnT power ↔ aerobic capacity ↔ body composition |
| Fahs et al. (2009) [34] | 18 healthy men (24.2 ± 0.7 y; 86.7 ± 4.9 kg) | Randomized, double-blind, placebo-controlled, crossover study | 7 g L-arginine vs. placebo; 30 min prior | 4 sets of 5 bench press (80% 1RM) 4 sets of 10 biceps curl (70% 1RM) | ↔ forearm blood flow ↔ arterial stiffness |
| Greer & Jones (2011) [39] | 12 resistance-trained men (22.6 ± 3.8 y; 12.1 ± 4.1% body fat) | Randomized, double-blind, placebo-controlled, crossover study | 3.7 g L-arginine α-ketoglutarate vs. placebo; 4 h and 30 min prior | 3 sets of chin-ups, reverse chin-ups, and push-ups to failure | ↓ repetitions to failure |
| Liu et al. (2009) [31] | 10 elite judo athletes (20.2 ± 0.6 y; 73.3 ± 2.1 kg) | Randomized, double-blind, placebo-controlled, crossover study | 6 g L-arginine vs. placebo; 3 days (60 min prior) | Intermittent anaerobic exercise test | ↔ peak power ↔ average power ↑ arginine concentrations ↔ NO x↔ lactate ↔ ammonia |
| Meirellas & Matsuura (2018) [32] | 12 recreationally resistance-trained men (27 ± 3 y; 77 ± 8 kg) | Randomized, double-blind, placebo-controlled, crossover study | 6 g L-arginine vs. placebo; 60 min prior | 3 sets of bench press (70% 1RM) to failure 3 sets knee extensions (80% 1RM) to failure | ↔ repetitions to failure ↔ NOx |
| Olek et al. (2010) [37] | 6 physically active men (23.2 ± 0.5 y; 84.0 ± 2.5 kg) | Randomized, double-blind, placebo-controlled, crossover study | 6 g L-arginine vs. placebo; 60 min prior | 3 30 s WAnT | ↔ WAnT performance ↔ lactate ↔ ammonia |
| Santos et al. (2002) [36] | 12 inactive men (23.8 ± 3.5 y; 75.8 ± 12.1 kg) | Non-randomized, non-placebo- controlled study | 3 g L-arginine for 15 days; 60 min prior | 15 maximal isokinetic leg extensions at 180°·s−1 | ↓ work fatigue index |
| Tang et al. (2011) [35] | 8 recreationally active men (22.1 ± 2.6 y; 76.6 ± 6.2 kg) | Randomized, double-blind, crossover study | 10 g of essential amino acids with 10 g of L-arginine vs. isonitrogenous control; post-exercise | Unilateral seated leg press and knee extension exercises | ↔ NO synthesis ↔ muscle blood flow ↔ muscle protein synthesis |
| Wax et al. (2013) [38] | 19 recreationally active men (19.4 ± 1.3 y; 79.2 ± 10.6 kg) | Randomized, double-blind, placebo-controlled, crossover study | 3 g L-arginine α-ketoglutarate vs. placebo; 45 min prior | 1RM bench press and leg press 1 set of bench press and leg press (60% 1RM) to failure | ↔ 1RM strength ↔ repetitions to fatigue |
4. Effect of L-citrulline Supplementation on Strength Performance
| Study | Subjects | Design | Intervention | Performance Tests | Main Findings |
|---|---|---|---|---|---|
| Chappell et al. (2018) [67] | 15 recreationally resistance-trained men and women (23.7 ± 2.4 y; 75.2 ± 13.7 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 10 sets of concentric only single leg knee extensions (70% peak force) to failure Isokinetic leg extension test before and after protocol | ↔ repetitions to failure ↔ isometric force ↔ blood lactate |
| Chappell et al. (2020) [68] | 19 recreationally active men and women (25.7 ± 7.7 y; 75.3 ± 13.7 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 10 sets of up to 10 barbell curls (80% 1RM) to failure | ↔ repetitions to failure ↔ blood lactate |
| Cutrufello et al. (2015) [57] | 22 recreationally trained men (20.6 ± 1.2 y; 78.7 ± 9.9 kg) and women (21.0 ± 1.3 y; 65.5 ± 10.9 kg) | Randomized, double-blind, placebo-controlled, crossover study | 710 mL watermelon juice (~1.0 g L-citrulline) vs. 6 g L-citrulline vs. placebo; 60 min prior | FMD of brachial artery 5 sets of chest press (80% 1RM) to failure | ↔ repetitions to failure ↔ FMD |
| Farney et al. (2019) [69] | 12 recreationally trained men and women (24.0 ± 3.9 y) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo vs. control (no drink); 60 min prior | 3 rounds of squats, lunge jumps, squat jumps, and lateral jumps with weighted vest (40 lb. for men; 20 lb. for women) Isokinetic leg extension test performed before and after protocol | ↔ total work ↔ peak power ↔ peak torque ↔ fatigue rate ↔ blood lactate |
| Fick et al. (2021) [70] | 18 recreationally trained men (24.0 ± 5.0 y; 83.0 ± 14.0 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 7 days (60 min prior) | 30 min cycling test at 50-65% max power 50 maximal isokinetic leg extensions at 180°·s−1 | ↔ peak power ↔ peak torque ↔ fatigue rate |
| Gills et al. (2021) [62] | 19 recreationally trained women (23.5 ± 3.1 y; 61.9 ± 8.4 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 5 maximal isokinetic leg extensions at 60°·s−1 50 maximal isokinetic leg extensions at 180°·s−1 | ↑ total work during 5 repetition protocol ↔ performance during 50 repetition protocol |
| Glenn et al. (2016) [61] | 17 Masters female tennis athletes (51 ± 9 y; 66.6 ± 9.5 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | Maximal isometric hand-grip strength VJ assessment 30-sec WAnT | ↑ grip strength ↔ VJ power ↑ peak WAnT power ↑ explosive WAnT power ↔ anaerobic capacity |
| Glenn et al. (2017) [63] | 15 resistance-trained women (23 ± 3 y; 67.1 ± 7.0 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 6 sets of bench press (80% 1RM) to failure 6 sets of leg press (80% 1RM) to failure | ↑ bench press repetitions to failure ↑ leg press repetitions to failure ↓ RPE |
| Gonzalez et al. (2018) [59] | 12 recreationally resistance-trained men (21.4 ± 1.6 y; 85.0 ± 12.4 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 40 min prior | 5 sets of bench press (75% 1RM) to failure | ↔ repetitions to failure ↔ RPE ↔ triceps muscle thickness ↔ peak and mean power ↔ subjective measures of energy, focus, fatigue, and “muscle pump” |
| Gonzalez et al. (2022) [60] | 15 resistance-trained men (22.4 ± 2.9 y; 82.7 ± 11.2 kg) | Randomized, double-blind, placebo-controlled, crossover study | Watermelon juice concentrate (~2.2 g L-citrulline) vs. placebo; 7 days (60 min prior) | IMTP 2 sets of 2 “explosive” bench press repetitions (75% 1RM) 5 sets of bench press (75% 1RM) to failure | ↔ IMTP peak force ↔ bench press mean/peak power ↔ repetitions to failure ↔ RPE ↔ muscle oxygenation ↔ brachial artery diameter ↔ subjective measures of energy, focus, fatigue, and “muscle pump” |
| Hwang et al. (2018) [72] | 50 resistance-trained men (18–35 y) | Randomized, double-blind, placebo-controlled study | 2 g CitMal·d−1 vs. placebo; daily for 8 weeks during periodized resistance training (60 min prior) | Bench press 1RM Leg press 1RM Body composition | ↔ bench press 1RM ↔ leg press 1RM ↔ body composition |
| Martínez-Sánchez et al. (2017) [71] | 19 resistance-trained men (23.9 ± 3.7 y; 75.2 ± 7.6 kg) | Randomized, double-blind, placebo-controlled, crossover study | Watermelon juice (~0.5 g L-citrulline) vs. watermelon juice enriched with L-citrulline (~3.3 g L-citrulline) vs. placebo; 60 min prior | 8 sets of 8RM barbell half squats Isokinetic knee extension test performed before and after protocol | ↔ power during squats ↔ force during squats ↔ peak torque ↓ RPE (enriched only) ↓ muscle soreness at 24- and 48-h post (enriched only) |
| Perez-Guisado & Jakeman (2010) [64] | 41 resistance-trained men (29.8 ± 7.64 y; 81.12 ± 17.43 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 4 sets of bench press (80% 1RM) to failure 4 sets of incline bench press (80% 1RM) to failure 4 sets of incline fly (60% 1RM) to failure 4 sets of bench press (80% 1RM) to failure | ↑ repetitions to failure during bench press exercise ↓ muscle soreness at 24- and 48-h post |
| Trexler et al. (2019) [58] | 27 recreationally active men (22.0 ± 4.0 y; 78.9 ± 12.5 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 120 min prior | 5 sets of 30 maximal isokinetic concentric knee extensions at 180°·s−1 | ↔ peak and average torque ↔ total work ↔ blood lactate ↔ femoral artery diameter ↔ vastus lateralis cross-sectional area |
| Wax et al. (2015) [65] | 12 resistance-trained men (22.1 ± 1.4 y; 84.8 ± 10.9 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 5 sets of leg press, hack squats, and leg extensions (60% 1RM) to failure | ↑ leg press repetitions to failure ↑ hack squat repetitions to failure ↑ leg extension repetitions to failure ↔ blood lactate |
| Wax et al. (2016) [66] | 14 resistance-trained males (23.3 ± 1.5 y; 87.8 ± 9.1 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 g CitMal vs. placebo; 60 min prior | 3 sets of chin-ups, reverse chin-ups, and push-ups (bodyweight) to failure | ↑ chin-up repetitions to failure ↑ reverse chin-up repetitions to failure ↑ push-up repetitions to failure ↔ blood lactate |
5. Effect of Nitrate Supplementation on Strength Performance
6. Synergistic Effects of Multiple Precursors on Strength Performance
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harty, P.S.; Zabriskie, H.A.; Erickson, J.L.; Molling, P.E.; Kerksick, C.M.; Jagim, A.R. Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: A brief review. J. Int. Soc. Sport. Nutr. 2018, 15, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, C.; Jones, A.M.; Bailey, S.J. Dietary Supplementation in Sport and Exercise: Evidence, Safety and Ergogenic Benefits; Hoffman, J.R., Ed.; Routledge Press: London, UK, 2019. [Google Scholar]
- Bailey, S.J.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. Eur. J. Sport Sci. 2012, 12, 309–320. [Google Scholar] [CrossRef]
- Figueroa, A.; Jaime, S.J.; Morita, M.; Gonzales, J.U.; Moinard, C. L-citrulline supports vascular and muscular benefits of exercise training in older adults. Exerc. Sport Sci. Rev. 2020, 48, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.O.; Drummond, L.R.; Rodrigues, Q.T.; Machado, F.S.; Pires, W.; Wanner, S.P.; Coimbra, C.C. Nitrate supplementation improves physical performance specifically in non-athletes during prolonged open-ended tests: A systematic review and meta-analysis. Br. J. Nutr. 2018, 119, 636–657. [Google Scholar] [CrossRef]
- Cholewa, J.; Trexler, E.; Lima-Soares, F.; de Araújo Pessôa, K.; Sousa-Silva, R.; Santos, A.M.; Zhi, X.; Nicastro, H.; Cabido, C.E.T.; de Freitas, M.C. Effects of dietary sports supplements on metabolite accumulation, vasodilation and cellular swelling in relation to muscle hypertrophy: A focus on “secondary” physiological determinants. Nutrition 2019, 60, 241–251. [Google Scholar] [CrossRef]
- Vårvik, F.T.; Bjørnsen, T.; Gonzalez, A.M. Acute Effect of Citrulline Malate on Repetition Performance During Strength Training: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 350–358. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Contreras, B. The muscle pump: Potential mechanisms and applications for enhancing hypertrophic adaptations. Strength Cond. J. 2014, 36, 21–25. [Google Scholar] [CrossRef]
- Hirono, T.; Ikezoe, T.; Taniguchi, M.; Tanaka, H.; Saeki, J.; Yagi, M.; Umehara, J.; Ichihashi, N. Relationship between muscle swelling and hypertrophy induced by resistance training. J. Strength Cond. Res. 2022, 36, 359–364. [Google Scholar] [CrossRef]
- Goron, A.; Lamarche, F.; Blanchet, S.; Delangle, P.; Schlattner, U.; Fontaine, E.; Moinard, C. Citrulline stimulates muscle protein synthesis, by reallocating ATP consumption to muscle protein synthesis. J. Cachexia Sarcopenia Muscle 2019, 10, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Le Plénier, S.; Goron, A.; Sotiropoulos, A.; Archambault, E.; Guihenneuc, C.; Walrand, S.; Salles, J.; Jourdan, M.; Neveux, N.; Cynober, L. Citrulline directly modulates muscle protein synthesis via the PI3K/MAPK/4E-BP1 pathway in a malnourished state: Evidence from in vivo, ex vivo, and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E27–E36. [Google Scholar] [CrossRef]
- Anderson, J.E. A role for nitric oxide in muscle repair: Nitric oxide–mediated activation of muscle satellite cells. Mol. Biol. Cell 2000, 11, 1859–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.W.; Smith, J.D.; Criswell, D.S. Involvement of nitric oxide synthase in skeletal muscle adaptation to chronic overload. J. Appl. Physiol. 2002, 92, 2005–2011. [Google Scholar] [CrossRef] [Green Version]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S. The nitrate-nitrite-nitric oxide pathway on healthy ageing: A review of pre-clinical and clinical data on the impact of dietary nitrate in the elderly. Front. Aging 2021, 2, 778467. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, N. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef] [PubMed]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Modin, A.; Björne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J. Nitrite-derived nitric oxide: A possible mediator of ‘acidic–metabolic’vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef]
- Morris Jr, S.M. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Besco, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sport. Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Trexler, E.T. Effects of citrulline supplementation on exercise performance in humans: A review of the current literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.I.; La Bounty, P.M.; Roberts, M. The ergogenic potential of arginine. J. Int. Soc. Sport. Nutr. 2004, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvares, T.S.; Meirelles, C.M.; Bhambhani, Y.N.; Paschoalin, V.M.; Gomes, P.S. L-Arginine as a potential ergogenic aidin healthy subjects. Sport. Med. 2011, 41, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Endogenous flux of nitric oxide: Citrulline is preferred to Arginine. Acta Physiol. 2021, 231, e13572. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef]
- Shiraseb, F.; Asbaghi, O.; Bagheri, R.; Wong, A.; Figueroa, A.; Mirzaei, K. Effect of l-Arginine Supplementation on Blood Pressure in Adults: A Systematic Review and Dose–Response Meta-analysis of Randomized Clinical Trials. Adv. Nutr. 2022, 13, 1226–1242. [Google Scholar] [CrossRef]
- Álvares, T.S.; Conte, C.A., Jr.; Paschoalin, V.M.F.; Silva, J.T.; Meirelles, C.d.M.; Bhambhani, Y.N.; Gomes, P.S.C. Acute l-arginine supplementation increases muscle blood volume but not strength performance. Appl. Physiol. Nutr. Metab. 2012, 37, 115–126. [Google Scholar] [CrossRef]
- Liu, T.-H.; Wu, C.-L.; Chiang, C.-W.; Lo, Y.-W.; Tseng, H.-F.; Chang, C.-K. No effect of short-term arginine supplementation on nitric oxide production, metabolism and performance in intermittent exercise in athletes. J. Nutr. Biochem. 2009, 20, 462–468. [Google Scholar] [CrossRef]
- Meirelles, C.M.; Matsuura, C. Acute supplementation of L-arginine affects neither strength performance nor nitric oxide production. J. Sport. Med. Phys. Fit. 2016, 58, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.; Balvedi, M.C.W.; Buzzachera, C.F.; Altimari, L.R.; Lozovoy, M.A.B.; Bigliassi, M.; Januário, R.S.B.; Pereira, R.M.; Sanches, V.C.; da Silva, D.K. L-arginine supplementation does not enhance blood flow and muscle performance in healthy and physically active older women. Eur. J. Nutr. 2016, 55, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Fahs, C.A.; Heffernan, K.S.; Fernhall, B. Hemodynamic and vascular response to resistance exercise with L-arginine. Med. Sci. Sport. Exerc. 2009, 41, 773–779. [Google Scholar] [CrossRef]
- Tang, J.E.; Lysecki, P.J.; Manolakos, J.J.; MacDonald, M.J.; Tarnopolsky, M.A.; Phillips, S.M. Bolus arginine supplementation affects neither muscle blood flow nor muscle protein synthesis in young men at rest or after resistance exercise. J. Nutr. 2011, 141, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.; Pacheco, M.; Martins, R.; Villaverde, A.; Giana, H.; Baptista, F.; Zangaro, R. Study of the effect of oral administration of L-arginine on muscular performance in healthy volunteers: An isokinetic study. Isokinet. Exerc. Sci. 2002, 10, 153–158. [Google Scholar] [CrossRef]
- Olek, R.; Ziemann, E.; Grzywacz, T.; Kujach, S.; Luszczyk, M.; Antosiewicz, J.; Laskowski, R. A single oral intake of arginine does not affect performance during repeated Wingate anaerobic test. J. Sport. Med. Phys. Fit. 2010, 50, 52. [Google Scholar]
- Wax, B.; Mayo, J.J.; Hilton, L.A.; Mareio, H.C.; Miller, J.D.; Webb, H.E.; Lyons, B. Acute ingestion of L-arginine alpha-ketoglutarate fails to improve muscular strength and endurance in ROTC cadets. Int. J. Exerc. Sci. 2013, 6, 2. [Google Scholar]
- Greer, B.K.; Jones, B.T. Acute arginine supplementation fails to improve muscle endurance or affect blood pressure responses to resistance training. J. Strength Cond. Res. 2011, 25, 1789–1794. [Google Scholar] [CrossRef]
- Campbell, B.; Roberts, M.; Kerksick, C.; Wilborn, C.; Marcello, B.; Taylor, L.; Nassar, E.; Leutholtz, B.; Bowden, R.; Rasmussen, C. Pharmacokinetics, safety, and effects on exercise performance of L-arginine α-ketoglutarate in trained adult men. Nutrition 2006, 22, 872–881. [Google Scholar] [CrossRef]
- Pasa, C.; de Oliveira, R.G.; da Rosa Lima, T.; Kommers, M.J.; Figueiredo, K.R.F.V.; Fett, W.C.R.; Fett, C.A. Effectiveness of acute L-arginine supplementation on physical performance in strength training: A systematic review and meta-analysis. F1000Research 2022, 10, 1072. [Google Scholar] [CrossRef]
- McRae, M.P. Therapeutic benefits of l-arginine: An umbrella review of meta-analyses. J. Chiropr. Med. 2016, 15, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sport. Nutr. 2018, 15, 38. [Google Scholar]
- Andrade, W.B.; Jacinto, J.L.; da Silva, D.K.; Roveratti, M.C.; Estoche, J.M.; Oliveira, D.B.; Balvedi, M.C.W.; da Silva, R.A.; Aguiar, A.F. l-Arginine supplementation does not improve muscle function during recovery from resistance exercise. Appl. Physiol. Nutr. Metab. 2018, 43, 928–936. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. l-Citrulline: A non-essential amino acid with important roles in human health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline supplementation: Impact on cardiometabolic health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef] [Green Version]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef] [Green Version]
- Rashid, J.; Kumar, S.S.; Job, K.M.; Liu, X.; Fike, C.D.; Sherwin, C.M. Therapeutic potential of citrulline as an arginine supplement: A clinical pharmacology review. Pediatr. Drugs 2020, 22, 279–293. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Jagim, A.R.; Harty, P.S.; Camic, C.L. Common ingredient profiles of multi-ingredient pre-workout supplements. Nutrients 2019, 11, 254. [Google Scholar] [CrossRef] [Green Version]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.-E.; Cozzone, P. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sport. Med. 2002, 36, 282–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, L.A.; Sparks, S.A.; McNaughton, L.R.; Higgins, M.F.; Newbury, J.W.; Trexler, E.; Faghy, M.A.; Bridge, C.A. A critical review of citrulline malate supplementation and exercise performance. Eur. J. Appl. Physiol. 2021, 121, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Persky, A.M.; Ryan, E.D.; Schwartz, T.A.; Stoner, L.; Smith-Ryan, A.E. Acute effects of citrulline supplementation on high-intensity strength and power performance: A systematic review and meta-analysis. Sport. Med. 2019, 49, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M.; Gills, J.; Gray, M. Acute effects of Nitrosigine® and citrulline malate on vasodilation in young adults. J. Int. Soc. Sport. Nutr. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The effect of l-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J. Sport. Sci. 2015, 33, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Keith, D.S.; Schwartz, T.A.; Ryan, E.D.; Stoner, L.; Persky, A.M.; Smith-Ryan, A.E. Effects of citrulline malate and beetroot juice supplementation on blood flow, energy metabolism, and performance during maximum effort leg extension exercise. J. Strength Cond. Res. 2019, 33, 2321–2329. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Spitz, R.W.; Ghigiarelli, J.J.; Sell, K.M.; Mangine, G.T. Acute effect of citrulline malate supplementation on upper-body resistance exercise performance in recreationally resistance-trained men. J. Strength Cond. Res. 2018, 32, 3088–3094. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Pinzone, A.G.; Lipes, S.E.; Mangine, G.T.; Townsend, J.R.; Allerton, T.D.; Sell, K.M.; Ghigiarelli, J.J. Effect of watermelon supplementation on exercise performance, muscle oxygenation, and vessel diameter in resistance-trained men. Eur. J. Appl. Physiol. 2022, 1–12. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Jensen, A.; Stone, M.S.; Vincenzo, J.L. Acute citrulline-malate supplementation improves maximal strength and anaerobic power in female, masters athletes tennis players. Eur. J. Sport Sci. 2016, 16, 1095–1103. [Google Scholar] [CrossRef]
- Gills, J.L.; Spliker, B.; Glenn, J.M.; Szymanski, D.; Romer, B.; Lu, H.-C.; Gray, M. Acute Citrulline-Malate Supplementation Increases Total Work in Short Lower-Body Isokinetic Tasks for Recreationally Active Females During Menstruation. J. Strength Cond. Res. 2021. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W.; Moyen, N.E. Acute citrulline malate supplementation improves upper-and lower-body submaximal weightlifting exercise performance in resistance-trained females. Eur. J. Nutr. 2017, 56, 775–784. [Google Scholar] [CrossRef]
- Pérez-Guisado, J.; Jakeman, P.M. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J. Strength Cond. Res. 2010, 24, 1215–1222. [Google Scholar] [CrossRef]
- Wax, B.; Kavazis, A.N.; Weldon, K.; Sperlak, J. Effects of supplemental citrulline malate ingestion during repeated bouts of lower-body exercise in advanced weightlifters. J. Strength Cond. Res. 2015, 29, 786–792. [Google Scholar] [CrossRef]
- Wax, B.; Kavazis, A.N.; Luckett, W. Effects of supplemental citrulline-malate ingestion on blood lactate, cardiovascular dynamics, and resistance exercise performance in trained males. J. Diet. Suppl. 2016, 13, 269–282. [Google Scholar] [CrossRef]
- Chappell, A.J.; Allwood, D.M.; Johns, R.; Brown, S.; Sultana, K.; Anand, A.; Simper, T. Citrulline malate supplementation does not improve German Volume Training performance or reduce muscle soreness in moderately trained males and females. J. Int. Soc. Sport. Nutr. 2018, 15, 42. [Google Scholar] [CrossRef]
- Chappell, A.J.; Allwood, D.M.; Simper, T.N. Citrulline malate fails to improve German volume training performance in healthy young men and women. J. Diet. Suppl. 2020, 17, 249–260. [Google Scholar] [CrossRef]
- Farney, T.M.; Bliss, M.V.; Hearon, C.M.; Salazar, D.A. The effect of citrulline malate supplementation on muscle fatigue among healthy participants. J. Strength Cond. Res. 2019, 33, 2464–2470. [Google Scholar] [CrossRef]
- Fick, A.N.; Kowalsky, R.J.; Stone, M.S.; Hearon, C.M.; Farney, T.M. Acute and Chronic Citrulline Malate Supplementation on Muscle Contractile Properties and Fatigue Rate of the Quadriceps. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 490–496. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Alacid, F.; Rubio-Arias, J.A.; Fernandez-Lobato, B.; Ramos-Campo, D.J.; Aguayo, E. Consumption of watermelon juice enriched in L-citrulline and pomegranate ellagitannins enhanced metabolism during physical exercise. J. Agric. Food Chem. 2017, 65, 4395–4404. [Google Scholar] [CrossRef]
- Hwang, P.; Morales Marroquín, F.E.; Gann, J.; Andre, T.; McKinley-Barnard, S.; Kim, C.; Morita, M.; Willoughby, D.S. Eight weeks of resistance training in conjunction with glutathione and L-Citrulline supplementation increases lean mass and has no adverse effects on blood clinical safety markers in resistance-trained males. J. Int. Soc. Sport. Nutr. 2018, 15, 30. [Google Scholar] [CrossRef] [Green Version]
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.-M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef]
- Nyakayiru, J.; van Loon, L.J.; Verdijk, L.B. Could intramuscular storage of dietary nitrate contribute to its ergogenic effect? A mini-review. Free Radic. Biol. Med. 2020, 152, 295–300. [Google Scholar] [CrossRef]
- Govoni, M.; Jansson, E.Å.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [Green Version]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [Green Version]
- Porcelli, S.; Pugliese, L.; Rejc, E.; Pavei, G.; Bonato, M.; Montorsi, M.; La Torre, A.; Rasica, L.; Marzorati, M. Effects of a Short-Term High-Nitrate Diet on Exercise Performance. Nutrients 2016, 8, 534. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, J.; Cai, H.; Zhang, J.; Yi, J.; Niu, Y.; Xi, H.; Peng, X.; Guo, L. Effect of inorganic nitrate supplementation on blood pressure in older adults: A systematic review and meta-analysis. Nitric Oxide 2021, 113, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Boissiere, J.; Pawlak-Chaouch, M.; Cuvelier, G.; Gamelin, F.X. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide 2015, 49, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.C.; Racine, M.L.; Hearon Jr, C.M.; Kunkel, M.; Luckasen, G.J.; Larson, D.G.; Allen, J.D.; Dinenno, F.A. Acute ingestion of dietary nitrate increases muscle blood flow via local vasodilation during handgrip exercise in young adults. Physiol. Rep. 2018, 6, e13572. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Morgado, M.; Conte-Junior, C.A.; Alvares, T.S. Acute effect of dietary nitrate on forearm muscle oxygenation, blood volume and strength in older adults: A randomized clinical trial. PLoS ONE 2017, 12, e0188893. [Google Scholar] [CrossRef]
- Haynes, J.T.; Townsend, J.R.; Aziz, M.A.; Jones, M.D.; Littlefield, L.A.; Ruiz, M.D.; Johnson, K.D.; Gonzalez, A.M. Impact of Red Spinach Extract Supplementation on Bench Press Performance, Muscle Oxygenation, and Cognitive Function in Resistance-Trained Males. Sports 2021, 9, 77. [Google Scholar] [CrossRef]
- Tan, R.; Pennell, A.; Price, K.M.; Karl, S.T.; Seekamp-Hicks, N.G.; Paniagua, K.K.; Weiderman, G.D.; Powell, J.P.; Sharabidze, L.K.; Lincoln, I.G. Effects of Dietary Nitrate Supplementation on Performance and Muscle Oxygenation during Resistance Exercise in Men. Nutrients 2022, 14, 3703. [Google Scholar] [CrossRef] [PubMed]
- Husmann, F.; Bruhn, S.; Mittlmeier, T.; Zschorlich, V.; Behrens, M. Dietary Nitrate Supplementation Improves Exercise Tolerance by Reducing Muscle Fatigue and Perceptual Responses. Front Physiol 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.; Weitzberg, E.; Lundberg, J.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, D.; Townsend, J.R.; Vantrease, W.C.; Marshall, A.C.; Henry, R.N.; Heffington, S.H.; Johnson, K.D. Acute beetroot juice administration improves peak isometric force production in adolescent males. Appl. Physiol. Nutr. Metab. 2018, 43, 816–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, S.J.; Baur, D.A.; Spicer, M.T.; Vukovich, M.D.; Ormsbee, M.J. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J. Int. Soc. Sport. Nutr. 2016, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Hoogervorst, D.; Peelen, H.B.; De Niet, M.; Verdijk, L.B.; Van Loon, L.J.; van Dijk, J.-W. The impact of beetroot juice supplementation on muscular endurance, maximal strength and countermovement jump performance. Eur. J. Sport Sci. 2021, 21, 871–878. [Google Scholar] [CrossRef]
- Haider, G.; Folland, J.P. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med. Sci. Sport. Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef]
- Mosher, S.L.; Sparks, S.A.; Williams, E.L.; Bentley, D.J.; Mc Naughton, L.R. Ingestion of a nitric oxide enhancing supplement improves resistance exercise performance. J. Strength Cond. Res. 2016, 30, 3520–3524. [Google Scholar] [CrossRef] [Green Version]
- Ranchal-Sanchez, A.; Diaz-Bernier, V.M.; De La Florida-Villagran, C.A.; Llorente-Cantarero, F.J.; Campos-Perez, J.; Jurado-Castro, J.M. Acute effects of beetroot juice supplements on resistance training: A randomized double-blind crossover. Nutrients 2020, 12, 1912. [Google Scholar] [CrossRef]
- Williams, T.D.; Martin, M.P.; Mintz, J.A.; Rogers, R.R.; Ballmann, C.G. Effect of Acute Beetroot Juice Supplementation on Bench Press Power, Velocity, and Repetition Volume. J. Strength Con. Res. 2020, 34, 924–928. [Google Scholar] [CrossRef]
- Jurado-Castro, J.M.; Campos-Perez, J.; Ranchal-Sanchez, A.; Durán-López, N.; Domínguez, R. Acute Effects of Beetroot Juice Supplements on Lower-Body Strength in Female Athletes: Double-Blind Crossover Randomized Trial. Sport. Health 2022, 19417381221083590. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, A.; Castillo, D.; Raya-González, J.; Domínguez, R.; Bailey, S.J. Beetroot juice supplementation increases concentric and eccentric muscle power output. Original investigation. J. Sci. Med. Sport 2021, 24, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Castaño, M.V.; Sánchez-Nuño, S.; Molina-Raya, L.; Carbonell, T.; Maté-Muñoz, J.L.; Pleguezuelos-Cobo, E.; Serra-Payá, N. Circulating nitrate-nitrite reduces oxygen uptake for improving resistance exercise performance after rest time in well-trained CrossFit athletes. Sci. Rep. 2022, 12, 1–11. [Google Scholar]
- Flanagan, S.D.; Looney, D.P.; Miller, M.J.; DuPont, W.H.; Pryor, L.; Creighton, B.C.; Sterczala, A.J.; Szivak, T.K.; Hooper, D.R.; Maresh, C.M. The effects of nitrate-rich supplementation on neuromuscular efficiency during heavy resistance exercise. J. Am. Coll. Nutr. 2016, 35, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Hart, T.L.; Haynes, J.T., IV; Woods, C.A.; Toy, A.M.; Pihera, B.C.; Aziz, M.A.; Zimmerman, G.A.; Jones, M.D.; Vantrease, W.C. Influence of Dietary Nitrate Supplementation on Physical Performance and Body Composition Following Offseason Training in Division I Athletes. J. Diet Suppl. 2021, 1–16. [Google Scholar] [CrossRef]
- Esen, O.; Dobbin, N.; Callaghan, M.J. The effect of dietary nitrate on the contractile properties of human skeletal muscle: A systematic review and meta-analysis. J. Am. Nutr. Assoc. 2022, 1–12. [Google Scholar] [CrossRef]
- Coggan, A.R.; Baranauskas, M.N.; Hinrichs, R.J.; Liu, Z.; Carter, S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sport. Nutr. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Jones, L.; Bailey, S.J.; Rowland, S.N.; Alsharif, N.; Shannon, O.M.; Clifford, T. The effect of nitrate-rich beetroot juice on markers of exercise-induced muscle damage: A systematic review and meta-analysis of human intervention trials. J. Diet. Suppl. 2022, 19, 749–771. [Google Scholar] [CrossRef]
- Tan, R.; Cano, L.; Lago-Rodríguez, Á.; Domínguez, R. The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 762. [Google Scholar] [CrossRef]
- Alvares, T.S.; Oliveira, G.V.d.; Volino-Souza, M.; Conte-Junior, C.A.; Murias, J.M. Effect of dietary nitrate ingestion on muscular performance: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 5284–5306. [Google Scholar] [CrossRef]
- San Juan, A.F.; Dominguez, R.; Lago-Rodríguez, Á.; Montoya, J.J.; Tan, R.; Bailey, S.J. Effects of dietary nitrate supplementation on weightlifting exercise performance in healthy adults: A systematic review. Nutrients 2020, 12, 2227. [Google Scholar] [CrossRef]
- Silva, K.V.C.; Costa, B.D.; Gomes, A.C.; Saunders, B.; Mota, J.F. Factors that Moderate the Effect of Nitrate Ingestion On Exercise Performance in Adults: A Systematic Review With Meta-Analyses and Meta-Regressions. Adv. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tillin, N.; Moudy, S.; Nourse, K.; Tyler, C. Nitrate supplement benefits contractile forces in fatigued but not unfatigued muscle. Med. Sci. Sport Exerc. 2018, 50, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morita, M.; Hayashi, T.; Kamimura, A. The effects on plasma L-arginine levels of combined oral L-citrulline and L-arginine supplementation in healthy males. Biosci. Biotechnol. Biochem. 2017, 81, 372–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, I.; Sakuraba, K.; Horiike, T.; Kishi, T.; Yabe, J.; Suzuki, T.; Morita, M.; Nishimura, A.; Suzuki, Y. A combination of oral L-citrulline and L-arginine improved 10-min full-power cycling test performance in male collegiate soccer players: A randomized crossover trial. Eur. J. Appl. Physiol. 2019, 119, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roux-Mallouf, T.; Vibert, F.; Doutreleau, S.; Verges, S. Effect of acute nitrate and citrulline supplementation on muscle microvascular response to ischemia–reperfusion in healthy humans. Appl. Physiol. Nutr. Metab. 2017, 42, 901–908. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Laurent, J.; Besset, D.; Marillier, M.; Larribaut, J.; Belaidi, E.; Corne, C.; Doutreleau, S.; Verges, S. Effects of acute nitric oxide precursor intake on peripheral and central fatigue during knee extensions in healthy men. Exp. Physiol. 2019, 104, 1100–1114. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Pelen, F.; Vallejo, A.; Halimaoui, I.; Doutreleau, S.; Verges, S. Effect of chronic nitrate and citrulline supplementation on vascular function and exercise performance in older individuals. Aging 2019, 11, 3315. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Vallejo, A.; Pelen, F.; Halimaoui, I.; Doutreleau, S.; Verges, S. Synergetic Effect of NO Precursor Supplementation and Exercise Training. Med. Sci. Sport. Exerc. 2020, 52, 2437–2447. [Google Scholar] [CrossRef]
- Burgos, J.; Viribay, A.; Fernández-Lázaro, D.; Calleja-González, J.; González-Santos, J.; Mielgo-Ayuso, J. Combined Effects of Citrulline Plus Nitrate-Rich Beetroot Extract Co-Supplementation on Maximal and Endurance-Strength and Aerobic Power in Trained Male Triathletes: A Randomized Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 14, 40. [Google Scholar] [CrossRef]
| Study | Subjects | Design | Intervention | Performance Tests | Main Findings |
|---|---|---|---|---|---|
| Bender et al. (2018) [94] | 12 healthy, active male adolescents (16.8 ± 1.0 y; 74.8 ± 12.5 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (800 mg/~12.9 mmol nitrates) vs. nitrate depleted placebo; 150 min prior | IMTP 4 20 s WAnT | ↑ IMTP peak force ↔ WAnT |
| Coggan et al. (2015) [93] | 12 healthy men and women (36 ± 10 y; 26.1 ± 4.1 kg·m2) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (11.2 mmol nitrates) vs. nitrate depleted placebo; 120 min prior | Isokinetic knee extensions at 0, 90, 180, 270, & 360° s−1 50-contraction fatigue test at 180° s−1 | ↑ maximal knee extensor velocity ↑ maximal knee power velocity ↑ peak torque at 360° s−1 ↔ 50-contraction fatigue test |
| Flanagan et al. (2016) [104] | 14 resistance-trained males (21.1 ± 0.9 y; 77.6 ± 4.3 kg) | Randomized, double-blind, placebo-controlled, crossover study | Nitrate-rich bar (35.2 mg nitrates) vs. nitrate-poor bar; 3 days | Dynamic box squat pyramid protocol (60-90% 1RM ascending and descending load by 10%) Box squat MVIC with EMG | ↔ box squat repetitions to failure ↑ mean peak EMG amplitude |
| Garnacho-Castaño et al. (2022) [103] | 11 trained male CrossFit athletes (29.2 ± 3.7 y; 78.9 ± 5.4 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (~800 mg/12.8 mmol nitrates) vs. placebo; 180 min prior | 90 s of wall-balls (10 kg) and 60 s of back squat (50% 1RM) separated by 3 min rest, followed by 90 s of wall-balls (10 kg) and 60 s of back squat (50% 1RM) without rest between the two exercises | ↑ back squat repetitions completed in 1st round ↔ wall-balls repetitions completed in 1st round ↔ back squat or wall-balls completed in 2nd round |
| Haynes et al. (2021) [86] | 10 resistance-trained males (22.6 ± 3.2 y; 88.3 ± 7.8 kg) | Randomized, double-blind, placebo-controlled, crossover study | Red spinach extract (180 mg nitrates) vs. maltodextrin placebo; 7 days (60 min prior) | 5 sets of bench press (75% 1RM) to failure | ↔ repetitions to failure ↔ velocity & power during bench press |
| Haider & Folland (2014) [97] | 19 healthy untrained males (21 ± 3 y; 73 ± 10 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (9.7 mmol) vs. placebo; 7 days (150 min prior) | Surface EMG during voluntary and involuntary knee extension MVIC at 110° & 120° | ↔ maximal and explosive voluntary force ↑ maximal evoked force ↑ twitch peak force |
| Jonvik et al. (2021) [96] | 15 recreationally active males (25 ± 4 y; 81 ± 10 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (985 mg nitrates) vs. nitrate depleted placebo; 6 days (180 min prior) | CMJ Knee extension MVIC at 30° & 60° Isokinetic knee extension/flexion at 60°,120°,180°, & 300°·s−1 30 isokinetic knee extensions at 180°·s−1 | ↔ CMJ jump ↔ isometric strength ↑ isokinetic knee flexion power at 60°·s−1 ↔ isokinetic knee flexion power at 120°,180°, and 300°·s−1 ↔ isokinetic knee extension power at all velocities ↔ total workload and fatigue index for 30 isokinetic repetitions |
| Jurado-Castro et al. (2022) [101] | 14 physically active women (25.4 ± 4.0 y; 57.0 ± 5.4 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (400 mg/6.4 mmol nitrates) vs. nitrate depleted placebo; 120 min prior | CMJ Back squat mean and peak power and velocity at 50% and 70% 1RM 3 sets of back squat, leg press, and leg extension (75% 1RM) to failure | ↑ CMJ height ↑ mean/peak velocity at 50% 1RM ↑ mean/peak power at 50% 1RM ↑ repetitions to failure |
| Kramer et al. (2016) [95] | 12 male CrossFit athletes (23 ± 5 y; 82.7 ± 13.5 kg) | Randomized, double-blind, placebo-controlled, crossover study | 8 mmol potassium nitrate vs. nitrate-free potassium chloride; 6 days | Day 1: 2 sets of 5 isokinetic knee extension/flexion at 60°·s−1 and 180°·s−1 30-s WAnT Day 2: CrossFit workout (“Grace” protocol) | ↔ Isokinetic leg performance ↔ Isometric leg performance ↑ peak WAnT power ↔ CrossFit performance |
| Mosher et al. (2016) [98] | 12 recreationally trained males (21 ± 2 y; 82.5 ± 9.8 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (6.4 mmol nitrate) vs. nitrate depleted placebo; 6 days (150 min prior) | 3 sets of bench press (60% 1RM) to failure | ↑ repetitions to failure ↑ total weight lifted |
| Porcelli et al. (2016) [81] | 7 recreationally active males (25 ± 2 y; 66.3 ± 6.0 kg) | Randomized, crossover study | High-nitrate diet (~8.2 mmol·d−1) vs. control diet (~2.9 mmol·d−1); 6 days | Knee extension MVIC Isometric knee extensions (75% max voluntary torque) to failure | ↔ MVIC ↑ muscle work during isometric knee extensions |
| Ranchal-Sanchez et al. (2020) [99] | 12 recreationally trained males (24 ± 3 y; 73 ± 9.2 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (400 mg nitrates) vs. nitrate depleted placebo; 120 min prior | 3 sets of the back squat and bench press (60%, 70%, and 80% 1RM) to failure | ↑ total repetitions to failure ↑ back squat repetitions to failure ↔ bench press repetitions to failure ↔ maximum power & velocity for back squat and bench press |
| Rodríguez-Fernández et al. (2021) [102] | 18 healthy, active adult males (22.8 ± 4.9 y; 74.4 ± 9.6 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (800 mg nitrates) vs. nitrate depleted placebo; 150 min prior | 4 sets of 8 maximal half-squats on flywheel device at inertial loads of 0.025, 0.050, 0.075, & 0.100 kg·m−2 | ↑ mean/peak power during concentric and eccentric contractions at all inertial loads |
| Tan et al. (2022) [87] | 14 recreationally active males (22 ± 5 y; 84 ± 17 kg) | Randomized, double-blind, placebo-controlled, cross-over study | BRJ (11.8 mmol nitrates) vs. nitrate depleted placebo; 4 days | 2 sets of 2 “explosive” back squat and bench press repetitions (70% 1RM) 1 set of back squat and bench press (60% 1RM) to failure | ↔ back squat mean/peak power ↔ bench press mean/peak power ↑ bench press repetitions to failure ↔ quadricep & pectoralis tissue saturation index |
| Tillin et al. (2018) [113] | 17 recreationally active males (23 ± 4 y; 74.0 ± 9.6 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (800 mg/~12.9 mmol nitrate) vs. nitrate depleted placebo; 7 days (150 min prior) | Knee extensions MVIC and involuntary tetanic contractions at 10, 20, 50, and 100 Hz in unfatigued and fatigued state (following 60 MVICs) | ↔ knee extension performance in unfatigued state ↓ fatigue during the 60 MVICs ↑ knee extension performance in fatigued state (lower decline in tetanic force) |
| Townsend et al. (2021) [105] | 16 Division I male baseball athletes (20.5 ± 1.7 y; 90.4 ± 10.5 kg) | Randomized, double-blind, placebo-controlled, parallel study | Red spinach extract (180 mg nitrates) vs. placebo; daily for 11 weeks during offseason training (~30 min prior) | 1RM bench press 30 s WAnT Body composition Muscle thickness of RF and VL via ultrasound | ↔ 1RM Bench Press ↔ WAnT ↔ Body Composition ↔ Muscle Thickness (RF, VL) |
| Trexler et al. (2019) [58] | 27 recreationally active males (22.0 ± 4.0 y; 78.9 ± 12.5 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (400 mg nitrates) vs. placebo; 120 min prior | 5 sets of 30 maximal isokinetic concentric knee extensions at 180°·s−1 | ↔ peak and average torque ↔ total work ↔ blood lactate ↔ femoral artery diameter ↔ vastus lateralis cross-sectional area |
| Williams et al. (2020) [100] | 11 resistance-trained males (22.1 ± 2.4 y; 89.3 ± 10.3 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (400 mg nitrate) vs. nitrate depleted placebo; 120 min prior | 2 sets of 2 “explosive” bench press repetitions (70% 1RM) 3 sets of bench press (70% 1RM) to failure | ↑ mean power & velocity ↑ repetitions to failure |
| Study | Subjects | Design | Intervention | Performance Tests | Main Findings |
|---|---|---|---|---|---|
| L-citrulline/L-arginine + nitrates | |||||
| Burgos et al. (2022) [120] | 32 male endurance athletes (32.2 ± 4.9 y; 22.6 ± 1.8 kg·m2) | Randomized, double-blind, placebo-controlled trial | 3 g L-citrulline vs. 300 mg nitrates vs. 3 g L-citrulline + 300 mg nitrates vs. placebo; 9 weeks | Horizontal jump test Handgrip dynamometer test 1 min abdominal test | ↔ horizontal jump distance * ↔ handgrip strength ↔ abdominal crunch repetitions * |
| Le Roux-Mallouf et al. (2017) [116] | 14 healthy, physically active men and women (27.8 ± 7.5 y; 64.3 ± 9.1 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ containing 1200 mg nitrates + 6 g L-citrulline vs. placebo; 90 min prior | Thigh ischemia-reperfusion test | ↑ post-occlusive hemoglobin/oxyhemoglobin response suggesting enhanced vasodilation |
| Le Roux-Mallouf, Laurent, et al. (2019) [117] | 15 healthy, physically active men (28 ± 6 y; 73 ± 6 kg) | Randomized, double-blind, placebo-controlled, crossover study | BRJ (520 mg nitrate) vs. BRJ (520 mg nitrate) + 6 g L-citrulline vs. BRJ (520 mg nitrate + 6 g L-arginine; 90 min prior | Thigh ischemia-reperfusion test Isometric knee extension repetitions-to-failure (5 s on-4 s off at 45% of MVC) | ↑ post-occlusive hemoglobin/oxyhemoglobin response suggesting enhanced vasodilation (nitrate and nitrate/citrulline group) ↔ knee extension repetitions |
| Le Roux-Mallouf, Pelen, et al. (2019) [118] | 24 healthy older adult men and women (64 ± 2 y; 73.5 ±6.1 kg) | Randomized, double-blind, placebo-controlled trial | 6 g L-citrulline + 520 mg nitrate vs. placebo; 4 weeks | Thigh ischemia-reperfusion test Incremental isometric knee extension test | ↔ ischemia-reperfusion test ↔ knee extension performance |
| Le Roux-Mallouf et al. (2020) [119] | 24 healthy men and women (26 ± 3 y; 76.4 ± 5.1 kg) | Randomized, double-blind, placebo-controlled trial | 6 g L-citrulline + 520 mg nitrate vs. placebo; 8 weeks | Thigh ischemia-reperfusion test Incremental isometric knee extension test | ↔ ischemia-reperfusion test ↑ maximal knee extensor strength ↔ knee extension performance * |
| L-citrulline + L-arginine | |||||
| Suzuki et al. (2019) [115] | 20 male collegiate soccer players (19.0 ± 0.2 y; 65.4 ± 0.1 kg) | Randomized, double-blind, placebo-controlled, crossover study | 1.2 g L-citrulline + 1.2 g L-arginine vs. placebo; 7 days (60 min prior) | 10 min full-power cycling test | ↑ mean power output ↔ peak pedaling speed ↑ subjective “leg muscle soreness” ↑ subjective “ease of pedaling” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, A.M.; Townsend, J.R.; Pinzone, A.G.; Hoffman, J.R. Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature. Nutrients 2023, 15, 660. https://doi.org/10.3390/nu15030660
Gonzalez AM, Townsend JR, Pinzone AG, Hoffman JR. Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature. Nutrients. 2023; 15(3):660. https://doi.org/10.3390/nu15030660
Chicago/Turabian StyleGonzalez, Adam M., Jeremy R. Townsend, Anthony G. Pinzone, and Jay R. Hoffman. 2023. "Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature" Nutrients 15, no. 3: 660. https://doi.org/10.3390/nu15030660
APA StyleGonzalez, A. M., Townsend, J. R., Pinzone, A. G., & Hoffman, J. R. (2023). Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature. Nutrients, 15(3), 660. https://doi.org/10.3390/nu15030660






